Abstract
In recent years, consumers have increasingly demanded nutritious, healthy, and fresh-like food products with high organoleptic quality. Watermelon is rich in water, which is 92% mandatory for body functioning, and contains several vitamins, amino acids, antioxidants, carotenoids, and lycopenes with various health benefits. The present study examines the combined effect of ultrasound (US) and microwave (MW) on the physico-chemical and phytochemicals of watermelon juice during storage (up to 120 days). Sonication was employed for different time intervals, particularly from 2 to 8 min at 20 kHz frequency and 525 W power, while microwave was applied at two different time intervals (1 min 50 s and 2 min) at 1000 W power and a frequency of 2450 MHz. The product was stored at 4 °C up to 120 days for further examination. Our results revealed that treatment T5 (10 min ultrasound & 1 min 50 s microwave) manifested the maximum cloud value (3.00), acidity (0.15%), vitamin C content (202.67 mg/100 mL), phenolics (852.57 mgGAE/100 mL), flavonoids (1970.9 µg CE/100 mL), and total antioxidant activity (8650.3 µg equivalent of ascorbic acid/mL of juice). Sonication in combination with microwave proved to be an efficient technique for increasing the antioxidant potential of watermelon juice. Thus, US and MW treatments may be incorporated for enhancing the phytochemical release and shelf life of watermelon juice.
1. Introduction
Fruits and vegetables are rich in antioxidants, minerals, and fiber and, due to their bioactive chemicals, contribute to disease protection []. Watermelon (Citrulluslanatus) belongs to the family Cucurbitaceae, has a connotation with cucumber, pumpkin, and gourds, and is therefore botanically considered as fruit. It is believed that watermelon was first cultivated in Egypt around 5000 years ago []. The consumption per capita of watermelon in Asia is practically three times that of the rest of the world [,]. This fruit contains on average 92% water [,,] and, in desert regions, is treasured as a substitute source of water. It is also known as a fruit loaded with nutrients [] but low in calories and devoid of cholesterol. It is a rich source of vitamin A, vitamin C, vitamin B (especially B1 and B6), amino acids, carotenoids, lycopenes, and minerals like magnesium and potassium [,]. Watermelon also contains phenolic compounds in abundance.
Nowadays people are demanding healthy and nutritious food []. Despite this, watermelon is sometimes a neglected fruit in agriculture-based developing countries. As a result, most watermelon is wasted during the months of surplus production, particularly June and July. The food industry in various developing countries has not yet become involved in processing watermelon juice for marketing. Watermelons appear on the market for a very short span during the summer season and are usually used during hot days to quench thirst. In recent years, watermelon juice has gained widespread popularity due to its sensory, physical, and nutritional benefits []. As the temperature (>30 °C) remains high in Punjab for nearly 8 months, it would be fitting for watermelon juice to be available for most of the time throughout the year.
Thermal processing of watermelon juice should not be carried out simply to avoid nutritional losses and changes in taste. Equally important is the fact that HMF is undesirable in food since it is harmful to human health, and its presence and concentration are also significant quality criteria for food processing [,]. Although thermal processing ensures safe food with longer stability, it leads to the loss of heat-sensitive nutrients. Similarly, chemical preservatives extend the shelf life of food but can contribute to a variety of health concerns in humans, including neurological dysfunction, asthma, hyperactivity, hypersensitivity, dermatitis, allergies, and gastrointestinal and respiratory difficulties [].
Novel processing techniques, particularly ultrasound, ozone, high-pressure processing, and irradiations, are currently popular because they deliver food with improved functionalities, high quality, and a “fresh-like” physical appearance [,,]. Such kinds of processed juice can be packed and then marketed. On the other hand, nonthermal technologies may result in incomplete inactivation of the indigenous enzymes of different foods and a further reduction in the microbial load with improved qualitative parameters [,]. The combined use of nonthermal processing techniques for fruits and vegetable juices has been shown to improve the abovementioned quality, safety, and shelf stability.
Less processing time, a lower energy requirement, and a higher output are among the benefits of ultrasound []. Moreover, sonication also results in a significant increase in vitamin C, phenolic compounds, total antioxidant capacity, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, and a reduction in the microbial population [,]. Microwaves are described as electromagnetic waves with frequencies and wavelength ranging from 300 MHz to 300 GHz and 1 mm to 1 m, respectively []. Microwaving boosts the quality and safety of foods by inactivating microorganisms and enzymes in a short period of time, thereby preserving the foods’ nutritional value—e.g., the retention of vitamins—to a greater extent. The effect of microwave heating on the bioactive components and chemical contents (volatile organic compounds and fatty acids) of an orange juice–milk beverage was investigated. The microwave-treated orange juice–milk beverage demonstrated a lower browning index and greater amounts of ascorbic acid, carotenoids, total phenolics, and antioxidants, according to the study’s findings [].
In our review of the literature, we found no indication that ultrasonic or microwave treatment of watermelon juice was conducted. Additionally, to avoid post-harvest losses and ensure year-round availability, watermelon juice was preserved using nonthermal processing technologies such as sonication and microwave. These techniques also serve as an alternative to chemical preservatives by mitigating their adverse effects. The application of novel technologies resulted in healthy juice with a long shelf life. Hence, based on the above-mentioned benefits of using novel processing techniques, this study aimed at evaluating the synergistic effects of microwave and ultrasonication treatments on the physicochemical characteristics, phytochemicals, and shelf stability of watermelon juice stored at a refrigerated temperature.
2. Materials and Methods
2.1. Collection of Raw Materials
Thirty fully ripened high-quality sugar baby watermelons were randomly obtained from the local fruit-and-vegetable market. Other raw materials such as glass bottles and potassium-meta bisulphite (KMS) were also procured from the market. To eliminate dirt and dust, the fruits were sorted and cleaned.
2.2. Chemicals and Reagents
All the chemicals used in the study were of analytical grade, were purchased from Sigma Aldrich (Seelze, Germany), and were available in the local market.
2.3. Preparation of the Watermelon Juice
The peel, seeds, matured portions, and stem of the watermelon were removed. The peeled watermelons were put into an electric blender (MJ-M176P, Panasonic Manufacturing, Berhad, Malaysia) to extract the juice, then the juice was further strained through a double layer of muslin cloth to remove the fiber content and seeds. The juice was then poured into sterilized glass bottles and capped tightly as per reported method []. The juice was stored at a refrigerated temperature (4 °C) for further analysis.
2.4. Ultrasound Treatment
The prepared juice was subjected to US treatment using an ultrasound processor type UP400S (HielscherUltrasonics GmbH Hielscher, Oderstr. 53. D-14513 Teltow, Germany) with a probe of 0.5 inch and a power output of 400 W (see treatment plan in Table 1). Sonication for a working juice sample (200 mL confined in a 500 mL beaker) was performed at 20 kHz frequency, 525 W power, and 70% amplitude level by keeping the sample probe at 25 mm [,,]. The juice samples were given sonication treatment for 2, 4, 6, 8, and 10 min. Using an HI 9063 thermocouple (Hanna Instruments Ltd., Leighton Buzzard, UK), the ultrasonic intensity was measured to be 2 W/cm2. By allowing cold water to flow through a jacketed vessel, a constant temperature of 20 °C was maintained, and all the procedures were carried out in the dark to prevent light from interfering with the samples. The ultrasonic parameters were established by revising prior studies and conducting preliminary experiments [,]. The US treatment was performed three times to make triplicate samples for each treatment.

Table 1.
Treatment plan for the watermelon juice.
2.5. Microwave Treatment
After sonication, the juice was treated with a domestic microwave processor (Model No: DW-131A, Dawlance, Lahore, Pakistan) operating at 1000 W power and a frequency of 2450 MHz at two different time intervals (1 min 50 s and 2 min) (see the treatment plan in Table 1). In sterile beakers, samples of watermelon juice (200 mL) were microwaved (500 mL). Immediately after pasteurization, the product was transferred and packaged in pre-sterilized 250 mL plastic bottles that were immersed in ice-cold water to prevent shrinking of the bottles and to rapidly cool the product, as the temperature after 1 min and 50 s of pasteurization is 90 ± 2 °C. The juice was stored at 4 ± 2 °C [,,].
2.6. Chemical Preservation
To compare the juice with the control and other treatments, KMS was introduced in treatment T0+ []. The treatment plan is described in Table 1.
2.7. Storage
The sonicated and microwaved juice was stored at a refrigerated temperature (4 °C) in pre-sterilized glass bottles for further analysis.
2.8. Physicochemical Analysis
The total soluble solids (TSS measured in °B) were determined using a digital refractometer (Model HI-96801 Hanna, Germany), and the pH of the watermelon juice was observed using a pH meter (Lutron PH-209B, Lutron Electronic Co., Ltd., Tokyo, Japan) by following the method 981.12 of AOAC [,]. The acidity of the samples was evaluated using the standard titration technique through the method 942.15 of AOAC [].
2.9. Determination of Vitamin C (Ascorbic Acid)
The dye (2, 6-dichlorophenol-indophenol (C12H6Cl2NNaO2·2H2O)) titration reduction method 967.21 was used for the determination of ascorbic acid content []. The acquired results are expressed as milligrams of ascorbic acid per 100 mL of the sample, and the calculation was performed as described below:
Ascorbic acid mg/100 mL = (titer × dye factor × concentration × 100)/(extract aliquot used for estimation × volume of sample use for estimation)
2.10. Determination of Cloud Value
The cloud value of the samples was evaluated through the procedure reported by Versteeg et al. []. Centrifugation of all the samples was carried out at 5000 rpm for 20 min, and later absorbance was taken on a spectrophotometer (HettichRotofix32 A, Tuttlingen, Germany) at a wavelength of 660 nm.
2.11. Determination of Total Flavonoids
The total flavonoids (TF) of the watermelon juice were determined according to Saeeduddin et al. []. 1.5 mL aliquot of diluted watermelon juice was added into a 75 µL of sodium nitrite (5%) solution. After vortexing for 1 min, 150 µL of an aluminum chloride (10%) solution was added. Then after adding 0.5 mL of 1 M NaOH, absorbance was measured at 510 nm using a spectrophotometer (Halo DB-20, UV-VIS double beam, Diepoldsau, Switzerland). The results were calculated in µg of catechin equivalent (CE) per 100 mL of juice.
2.12. Determination of Total Phenolic Contents
The total phenolics were determined by using the Folin–Ciocalteu reagent method with some modifications []. 1 mL aliquot of diluted watermelon juice was mixed with 1 mL of the Folin–Ciocalteu reagent (10%). After vortexed, 2 mL of sodium carbonate (20%) solution was added into the mixture. After incubation for 60 min at 30 °C in the dark, the absorbance was measured at 760 nm using a spectrophotometer (Halo DB-20, UV-VIS double beam). The results were calculated in mg gallic acid equivalent (GAE) per 100 mL of juice.
2.13. Determination of Total Antioxidant Activity
The total antioxidant activity of the watermelon juice was determined using the method described by Prieto et al. []. 1 mL of diluted watermelon juice was mixed with 4 mL of reagent (0.6 M sulphuric acid, 28 M sodium phosphate, and 4 M ammonium molybdate) solution. After incubating the mixture (95 min at 90 °C), the absorbance was measured at 695 nm using a spectrophotometer. The results were calculated in µg equivalent of ascorbic acid per 100 mL of juice.
2.14. Determination of Viscosity
The viscosity of the juice samples was monitored according to the method described by Nindo et al. [] by using a rotatory viscometer (Rheomat RM 100, Lamy Rheology, Champagne-au-Mont-d’Or, France) equipped with a precision cylindrical spindle (R-2) rotating adapter. The viscosity of the juices at various dilutions was measured using a concentric cylinder geometry (stator inner radius 15 mm, rotor outer radius 14 mm, cylinder immersed height 42 mm, and gap 5920 µm). Approximately 19mLof juice was added to the rheometer cup. The RM100 features a Peltier plate for precise temperature regulation within the gap.
2.15. Statistical Analysis
Statistical analysis was performed through Minitab statistical software version 16 (Minitab Inc., State College, PA, USA), using the two-way ANOVA and Tukey’s tests for pairwise comparison in analysis of variance at the level of p < 0.05 by Steel et al. []
3. Results and Discussion
3.1. Total Soluble Solids of Watermelon Juice
Variations in the TSS of the watermelon juice (Table 2) indicated highly significant differences among treatments during storage conditions. The mean values of the TSS of the watermelon juice during storage indicated that the TSS of all the treated samples increased significantly during the storage period up to 120 days. The observed increase in the TSS throughout storage was from 7.87 to 12.50 °B. The highest TSS value was found in T1 and T2 (10.03 °B and 10.00 °B), while the lowest value was observed in T0− (7.87 °B) at the start of the study. The TSS of treatment T0+ (chemically preserved juice) increased up to 90 days and then decreased. Treatment T1 and T3 also showed an increasing trend in TSS during storage. This increase in TSS during storage might be attributed to polysaccharide breakdown into monosaccharaides and oligosaccharides. In a study of carrot and grapes juice blendreported by Nadeem et al. [], a significant (p < 0.05) increment (from 12.5 to 13.02 °B) in the TSS was also observed due to sonication. Similarly, in another study, it was also reported that the TSS of thermo-sonicated pear juice increased with the increase in the storage period []. The same findings were also reported by Walkling–Ribeiro et al. []. They claimed that different processing methods increased the amount of TSS in various fruit juices.

Table 2.
pH, acidity, total soluble solids (TSS measured in °B), cloud value, and vitamin C (mg/100 mL of juice) of the watermelon juice during storage.
3.2. Titratable Acidity of Watermelon Juice
The mean acidity values during storage of all the treated samples increased significantly from 0 to 120 days (Table 2). The highest acidity values were observed in T5 (0.15%) and T4 (0.14%), while T0− (0.06%) showed the lowest value without storage. The maximum acidity value was obtained at 120 days in T0- and in T5 and the minimum value (0.16%) after 120 days in treatment T1 and T2, respectively. The overall increase in acidity was observed in T0− from 0.06–0.19% in 120 days. With the increase in ultrasonication time, the acidity values increased, because ultrasound induction generates heat. The results were inconsistent with the findings of Nadeem et al. [], who reported an increasing trend in the acidity of phalsa juice treated through the sonication technique. Malik et al. [] reported an increase in the acidity of microwave-treated lemon cordial after storage and hypothesized that the increase in acidity is due to increased production of organic acids during anaerobic fermentation. Another study stated that the acidity of pineapple juice increased during storage, which could be attributed to fermentation and spoilage leading to conversion of sugars into acids, carbon dioxide, or alcohol [].
3.3. pH of Watermelon Juice
The mean pH values of the watermelon juice indicated that the pH of all the treated samples dropped significantly during storage from 0 to 120 days. The drop in pH is linked with the rise in acidity, which could assist in the shelf-life extension of watermelon juice []. The highest pH values were found in T2 (6.12), followed by T0−(5.90), and the least value was detected in T0−(5.68) at start of the storage period. The maximum and minimum pH values were obtained at 0 day (6.12) and 120 days (3.95), respectively, as shown in Table 2. It has been reported that the overall reduction in pH was from 5.68 to 3.95 in the microwave-treated lemon cordial []. They asserted that the acidic compounds, such as lactic acid and acetic acid, accumulated because of microbial activity during the natural fermentation process, which was the primary reason for the decrease in pH values. Due to conceivable biochemical reactions, ethanol is made during storage, and this is the principal reason for pH degeneration. In liquids, the application of high-power sonication creates free radicals such as H+ and OH−. These radicals are formed by the decomposition of water inside the cavities []. The results concerning the increase in pH are consistent with the previous results of ultrasonicated carrot blends [,].
3.4. Cloud Value of Watermelon Juice
Cloud value plays an important role in developing the color and flavor of fruit juices. The cloud stability of fruit juices is a pictorial quality parameter for consumers []. The mean cloud value of all the treated samples varied significantly during storage from 0 to 120 days, as shown in Table 2. The highest cloud value was detected in T3 and T5 (3.00), and the lowest was detected in T0− (1.10), and T0+ (1.19). The maximum (3.00) and minimum mean cloud values (0.76) were obtained at 0 day and 120 days, respectively. Cloud values considerably increased (p < 0.05) in grapefruit juice due to sonication treatments. The increased cloud value may be due to the high-pressure gradient formed by cavitation during sonication treatment, which results in the colloidal dissolution, dispersion, and degradation of larger molecules to smaller ones, thus making the juice properly homogenized and more consistent [].
3.5. Vitamin C Content (Ascorbic Acid) of Watermelon Juice
The mean values of the vitamin C content of the watermelon juice indicated that vitamin C, of all treated samples, increased significantly (Table 2). The highest vitamin C value was found in T5 (202.67 mg/100 mL of juice) and the least in T4 (140.00 mg/100 mL of juice). During storage, an overall decrease in vitamin C content was observed. The maximum decrease was observed in T0− (175.00 to 51.67 mg/100 mL). Ascorbic acid is a form of vitamin C []. In grapefruit juice, sonication treatment resulted in a significant increase (p < 0.05) in ascorbic acid content. Samples that were sonicated for 90 and 60 min showed higher values of ascorbic acid as compared to the control sample and to a sample sonicated for 30 min. Sonication is directly responsible for the rise in ascorbic acid in grapefruit juice; it depletes the dissolved oxygen in the juice through cavitation without the addition of heat []. In comparison to the control, samples treated with sonication and microwave retained more ascorbic acid. The retention of ascorbic acid during sonication treatment is most likely due to the removal of the dissolved oxygen required for ascorbic acid content breakdown during storage [].
3.6. Total Phenolic Contentof the Watermelon Juice
The mean values of the TP of the watermelon juice indicated that the phenolic content of all the treated samples decreased significantly during storage from 0 to 120 days (Figure 1). The highest TP value was found in T5 (852.57 mg of GAE/100 mL of juice), and the lowest value in T0− (143.10 mg of GAE/100 mL of juice) followed by T0+ (158.60 mg of GAE/100 mL of juice), correspondingly. The plant’s secondary metabolites, such as phenolic compounds, are responsible for color and flavor improvement in fruit juices. Higher concentration of phenolics could be attributed to the inactivation of polyphenol oxidaze enzymes, which employ these chemicals as a substrate and cause their breakdown during storage. The effect of sonication on the phenolic contents of apple juice was studied by Abid et al. []. Fruit juice samples were sonicated for 30, 60, and 90 min in a bath-type sonicator. The TP contents increased as the sonication time was extended but decreased as the storage period progressed. The impact of sonication on the phenolic content of carrot juice was studied by Jabbar et al. []. The carrot juice was given sonication and heat treatment at various temperatures. The phenolic content of the carrot juice samples was found to be considerably higher in the sonicated samples than in the control samples.
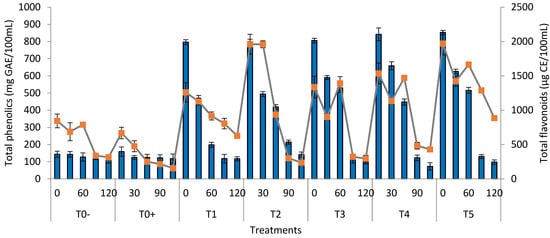
Figure 1.
Phytochemicals (total phenolics and flavonoids) of watermelon juice (T0− = control (watermelon juice with no preservative and treatment); T0+ = control (watermelon juice preserved with KMS); T1 = 2 min ultrasound & 2 min microwave; T2 = 4 min ultrasound & 1 min 50 s microwave; T3 = 6 min ultrasound & 1 min 50 s microwave; T4 = 8 min ultrasound & 1 min 50 s microwave; T5 = 10 min ultrasound & 1 min 50 s) during storage.
3.7. Total Flavonoid Contents of the Watermelon Juice
The mean values of the TF of the watermelon juice indicated that the flavonoid content of all treated samples decreased significantly during storage from 0 to 120 days (Figure 1). The overall decline in the TF content during storage was reported to be from 1960.2 to 236.3 µg CE/100 mL of juice. The highest TF value was found in T5 (1970.9 µg CE/100 mL of juice), followed by T2 (1960.2 µg CE/100 mL of juice) and T0+ (1668.4 µg CE/100 mL of juice), whereas the lowest value was found in T0− (1260.3 µg CE/100 mL of juice). A higher concentration of flavonoids could be attributed to the inactivation of PPO enzymes, which employ these chemicals as a substrate and cause their breakdown during storage. The effect of sonication on the flavonoid concentration of apple juice was studied by Abid et al. []. They sonicated the apple juice samples for 30, 60, and 90 min. It was observed that as the sonication period was extended, the TF content increased. When compared to non-sonicated juice samples, the TF concentration increased from 486Ig GAE/g to 600Ig GAE/g. Malik et al. [] asserted that the TF content of the microwave-treated lemon cordial decreased during storage and that the increased production of free radicals was responsible for the decrease in flavonoid content. The effect of thermo-sonication on the phytochemical characteristics of carrot juice was studied by Jabbar et al. []. In comparison to the control, the juice was treated to ultrasonic processing for 30 and 60 min at 20 °C. The total flavonoid content increased when the temperature increased from 30 to 60 °C. The flavonoid content of the juice samples increased from 344.76 ± 0.05 (catechin equivalent lg/g) to 544.56 ± 0.07 (catechin equivalent lg/g).
3.8. Total Antioxidant Activity of the Watermelon Juice
The mean values of the total antioxidant activity (TAC) of the watermelon juice indicated that antioxidants of all the treated samples varied significantly during storage from 0 to 120 days (Figure 2). The overall reduction in total antioxidants in the watermelon juice during storage was from 6139.2 to 229.5 µg, the equivalent of ascorbic acid/mL of juice in the control treatment. The highest TAC value was found in T5 (8650.3) and T4 (7106.3 µg equivalent of ascorbic acid/mL of juice), and the least value in T0− (6139.2 µg equivalent of ascorbic acid/mL of juice). Depreciation was observed in 120 days of storage. The increased TAC in the treated juice may be attributed to the fact that a combined ultrasound and microwave treatment inhibits the enzymes responsible for triggering oxidation. In the study by Abid et al. [], the apple juice samples were sonicated at different temperatures, such as 30, 60, and 90 min. During storage, the TAC of the sonicated juice samples decreased. Antioxidant activity increased from 324.57 mg ascorbic acid/g to 363.05 mg ascorbic acid/g in the sonicated samples. The increase in the polyphenolic and ascorbic content of the apple juice was responsible for the increase in the TAC. Because of the enhanced availability and extraction of these phytochemical components due to the creation of cavities during sonication, there will be more polyphenolic compounds if there is an increase in antioxidant chemicals []. According to Saeeduddin et al. [], when pear juice is exposed to ultrasound at 25 °C, its antioxidant capacity increases significantly. The amount of total phenolics and ascorbic acid in the sample determines the antioxidant activity. Ultrasound pasteurization is particularly successful at 65 °C, and it retains more antioxidant components than doestraditional pasteurization.
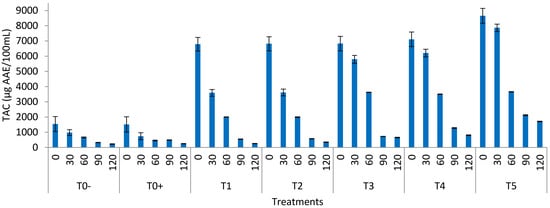
Figure 2.
Total antioxidant capacity (TAC) of the watermelon juice (T0− = control (watermelon juice with no preservative and treatment); T0+ = control (watermelon juice preserved with KMS); T1 = 2 min ultrasound & 2 min microwave; T2 = 4 min ultrasound & 1 min 50 s microwave; T3 = 6 min ultrasound & 1 min 50 s microwave; T4 = 8 min ultrasound & 1 min 50 s microwave; T5 = 10 min ultrasound & 1 min 50 s) during storage.
3.9. Viscosity of the Watermelon Juice
The viscosity of all the treated samples decreased significantly with the time passage from 0 to 120 days of storage. The maximum viscosity values were observed in T5 (44.19 mPa·s) and the minimum value in T0− (21.21 mPa·s). Within the storage mean, the greatest value of viscosity was obtained at 0 day (44.19), and the least value at 120 days (16.82 mPa·s) (Figure 3). The viscosity of processed as well as untreated watermelon juice decreased during the storage period. The considerable increase in viscosity in the treated samples may be ascribed to the decrease in the particle size or to the inactivation of the PME. Thermally and nonthermally treated juices keep the highest values because of the inactivation of enzymes []. The sonication treatments may promote the extraction of the bound forms of the macromolecules, which increases their concentration in the aqueous system, making the juice more viscous [].
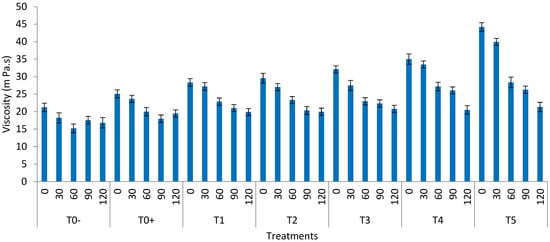
Figure 3.
Viscosity of the watermelon juice (T0− = control (watermelon juice with no preservative or treatment); T0+ = control (watermelon juice preserved with KMS); T1 = 2 min ultrasound & 2 min microwave; T2 = 4 min ultrasound & 1 min 50 s microwave; T3 = 6 min ultrasound & 1 min 50 s microwave; T4 = 8 min ultrasound & 1 min 50 s microwave; T5 = 10 min ultrasound & 1 min 50 s) during storage.
4. Conclusions
This study was conducted to evaluate the impact of ultrasonication and microwave treatment on the physico-chemical parameters of watermelon juice during 120 days of storage. The synergistic impact of ultrasound and of microwave on the watermelon juice progressively enhanced the TSS, cloud value, acidity, ascorbic acid, TP, TF, and TAC. Treatment T5 sonicated and microwaved for 10 min and 1 min and 50 s, respectively, showed maximum values for acidity (0.15%), cloud value (3.00), vitamin C content (202.67 mg/100 mL), TP (852.57 mgGAE/100 mL), TF (1970.9 µg CE/100 mL), and TAC (8650.3 µg equivalent of ascorbic acid/mL of juice). Moreover, the mentioned treatment also exhibited greater cloud stability and enhanced antioxidant potential. This study suggests that sonication coupled with microwave must be implemented commercially to process commercially unavailable watermelon juice, as these “green” techniques are the best alternates to chemical preservatives and conventional pasteurization practices. Additionally, combined ultrasound and microwave therapy has been demonstrated to be beneficial to the health of the individual, hence exerting a positive impact on consumer satisfaction and bioactive characteristics. New research is required to build models, such as surface response methods, for optimizing process factors during combined ultrasound–microwave procedures. In order to improve the quality of watermelon juice, it is advised that a combination of nonthermal food processing techniques be utilized to examine their synergistic influence on quality characteristics.
Author Contributions
Conceptualization, M.N. (Maham Navida), M.N. (Muhammad Nadeem), and T.M.Q.; Data curation, M.N. (Maham Navida), F.M., A.I. and M.S.; Formal analysis, M.N. (Maham Navida) and M.N. (Muhammad Nadeem); Funding acquisition, M.N. (Muhammad Nadeem), R.A.P., H.M.A.-D. and A.K.A.; Investigation, M.N. (Maham Navida), M.N. (Muhammad Nadeem), R.A.P., F.M., H.M.A.-D. and A.K.A.; Methodology, M.N. (Maham Navida), M.N. (Muhammad Nadeem), and T.M.Q.; Project administration, M.N. (Muhammad Nadeem); Resources, M.N. (Muhammad Nadeem) and M.S.; Software, M.N. (Maham Navida) and M.N. (Muhammad Nadeem); Supervision, M.N. (Muhammad Nadeem); Validation, M.N. (Maham Navida) and M.N. (Muhammad Nadeem); Visualization, R.A.P., M.S., H.M.A.-D. and A.K.A.; Writing—original draft, M.N. (Maham Navida), M.N. (Muhammad Nadeem), T.M.Q., F.M. and A.I.; Writing—review & editing, R.A.P., F.M., A.I., M.S., H.M.A.-D. and A.K.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this study by Grant Code: (22UQU4320141DSR36).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this study by Grant Code: (22UQU4320141DSR36). The authors are thankful to the University of Sargodha for its financial support of this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| KMS | Potassium-meta bisulphite |
| TSS | Total soluble solids |
| TF | Total flavonoid |
| TP | Total phenolic |
| TAC | Total antioxidant activity |
References
- Yıkmış, S. Sensory, Physicochemical, Microbiological and Bioactive Properties of Red Watermelon Juice and Yellow Watermelon Juice after Ultrasound Treatment. J. Food Meas. Charact. 2020, 14, 1417–1426. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, S.; Lemar, L.; Haytowitz, D.; Pehrsson, P.; Nickle, M.; Showell, B.; Thomas, R.; Exler, J.; Holden, J. USDA National Nutrient Database for Standard Reference, Release 21; United States Department of Agriculture: Washington, DC, USA, 2008. [Google Scholar]
- Li, X.; Yue, X.; Huang, Q.; Zhang, B. Effects of Wet-Media Milling on Multi-Scale Structures and in Vitro Digestion of Tapioca Starch and the Structure-Digestion Relationship. Carbohydr. Polym. 2022, 284, 119176. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Asfahan, H.M.; Sultan, M.; Askalany, A.A. A Novel Ejectors Integration with Two-Stages Adsorption Desalination: Away to Scavenge the Ambient Energy. Sustain. Energy Technol. Assess. 2021, 48, 101658. [Google Scholar] [CrossRef]
- Imran, M.A.; Xu, J.; Sultan, M.; Shamshiri, R.R.; Ahmed, N.; Javed, Q.; Asfahan, H.M.; Latif, Y.; Usman, M.; Ahmad, R. Free Discharge of Subsurface Drainage Effluent: An Alternate Design of the Surface Drain System in Pakistan. Sustainability 2021, 13, 4080. [Google Scholar] [CrossRef]
- Munyasya, A.N.; Koskei, K.; Zhou, R.; Liu, S.-T.; Indoshi, S.N.; Wang, W.; Zhang, X.-C.; Cheruiyot, W.K.; Mburu, D.M.; Nyende, A.B. Integrated On-Site & off-Site Rainwater-Harvesting System Boosts Rainfed Maize Production for Better Adaptation to Climate Change. Agric. Water Manag. 2022, 269, 107672. [Google Scholar]
- Jiang, G.; Ameer, K.; Kim, H.; Lee, E.-J.; Ramachandraiah, K.; Hong, G.-P. Strategies for Sustainable Substitution of Livestock Meat. Foods 2020, 9, 1227. [Google Scholar] [CrossRef]
- Bruton, B.D.; Fish, W.W.; Roberts, W.; Popham, T.W. The Influence of Rootstock Selection on Fruit Quality Attributes of Watermelon. Open Food Sci. J. 2009, 3, 5–34. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C. Effect of Thermosonication on Bioactive Compounds in Watermelon Juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Shahzad, T.; Ahmad, I.; Choudhry, S.; Saeed, M.K.; Khan, M.N. DPPH Free Radical Scavenging Activity of Tomato, Cherry Tomato Andwatermelon: Lycopene Extraction, Purification and Quantification. IJPPS 2014, 6, 223–228. [Google Scholar]
- Li, Z.; Yuan, Y.; Yao, Y.; Wei, X.; Yue, T.; Meng, J. Formation of 5-Hydroxymethylfurfural in Industrial-Scale Apple Juice Concentrate Processing. Food Control 2019, 102, 56–68. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of Fresh Watermelon Juice Aroma Characteristics of Five Varieties Based on Gas Chromatography-Olfactometry-Mass Spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mejia, L.R.; Cruz, C.; Berrone, P.; De Castro, J. The Bind That Ties: Socioemotional Wealth Preservation in Family Firms. Acad. Manag. Ann. 2011, 5, 653–707. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Gamlath, C.J.; Martin, G.J.O.; Hemar, Y.; Ashokkumar, M. Effect of Sonication, Microwaves and High-Pressure Processing on ACE-Inhibitory Activity and Antioxidant Potential of Cheddar Cheese during Ripening. Ultrason. Sonochem. 2020, 67, 105140. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Wang, P.-Y.; Xiong, X.-B.; Wang, Y.-B.; Zhou, R.; Tao, H.-Y.; Grace, U.A.; Wang, N.; Xiong, Y.-C. Environmental Risk of Multi-Year Polythene Film Mulching and Its Green Solution in Arid Irrigation Region. J. Hazard. Mater. 2022, 435, 128981. [Google Scholar] [CrossRef]
- Nadeem, M.; Ghaffar, A.; Hashim, M.M.; Murtaza, M.A.; Ranjha, M.M.A.N.; Mehmood, A.; Riaz, M.N. Sonication and Microwave Processing of Phalsa Drink: A Synergistic Approach. Int. J. Fruit Sci. 2021, 21, 993–1007. [Google Scholar] [CrossRef]
- Lee, H.-G.; Jo, Y.; Ameer, K.; Kwon, J.-H. Optimization of Green Extraction Methods for Cinnamic Acid and Cinnamaldehyde from Cinnamon (Cinnamomum Cassia) by Response Surface Methodology. Food Sci. Biotechnol. 2018, 27, 1607–1617. [Google Scholar] [CrossRef]
- Zenker, M.; Heinz, V.; Knorr, D. Application of Ultrasound-Assisted Thermal Processing for Preservation and Quality Retention of Liquid Foods. J. Food Prot. 2003, 66, 1642–1649. [Google Scholar] [CrossRef]
- Qureshi, T.M.; Nadeem, M.; Maken, F.; Tayyaba, A.; Majeed, H.; Munir, M. Influence of Ultrasound on the Functional Characteristics of Indigenous Varieties of Mango (Mangifera Indica L.). Ultrason. Sonochem. 2020, 64, 104987. [Google Scholar] [CrossRef]
- Ameer, K.; Bae, S.-W.; Jo, Y.; Lee, H.-G.; Ameer, A.; Kwon, J.-H. Optimization of Microwave-Assisted Extraction of Total Extract, Stevioside and Rebaudioside-A from Stevia Rebaudiana (Bertoni) Leaves, Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.P.; Cavalcanti, R.N.; Cardozo, F.T.S.; Couto, S.M.; Esmerino, A.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Pimentel, T.C.; Freitas, M.Q.; et al. Effects of Microwave Heating on the Chemical Composition and Bioactivity of Orange Juice-Milk Beverages. Food Chem. 2021, 345, 128746. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.; Abid, M.; Hu, B.; Wu, T.; Hashim, M.M.; Lei, S.; Zhu, X.; Zeng, X. Quality of Carrot Juice as Influenced by Blanching and Sonication Treatments. LWT-Food Sci. Technol. 2014, 55, 16–21. [Google Scholar] [CrossRef]
- Nadeem, M.; Tehreem, S.; Mudassar Ali Nawaz Ranjha, M.; Ahmad, A.; Din, A.; Mueen Ud Din, G.; Javeria, S.; Nadeem Riaz, M.; Siddeeg, A.; Ali Nawaz Ranjha, M.; et al. Probing of Ultrasonic Assisted Pasteurization (UAP) Effects on Physicochemical Profile and Storage Stability of Jambul (Syzygiumïcumini L.) Squash. Int. J. Food Prop. 2022, 25, 661–672. [Google Scholar] [CrossRef]
- Nadeem, M.; Modassar, M.; Ranjha, A.N.; Ameer, K.; Ainee, A.; Yasmin, Z.; Javaria, S.; Teferra, T.F. Effect of Sonication on the Functional Properties of Different Citrus Fruit Juices. Int. J. Fruit Sci. 2022, 22, 568–580. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. A Comparative Study on the Effect of Conventional Thermal Pasteurisation, Microwave and Ultrasound Treatments on the Antioxidant Activity of Five Fruit Juices. Food Sci. Technol. Int. 2015, 22, 288–301. [Google Scholar] [CrossRef]
- Malik, F.; Nadeem, M.; Ainee, A.; Kanwal, R.; Sultan, M.; Iqbal, A.; Mahmoud, S.F.; Alshehry, G.A.; Al-Jumayi, H.A.; Algarni, E.H. Quality Evaluation of Lemon Cordial Stored at Different Times with Microwave Heating (Pasteurization). Sustainability 2022, 14, 1953. [Google Scholar] [CrossRef]
- Sasikumar, R. Preparation of Therapeutic RTS Beverage from Aloe Vera Gel and Aonla Fruit Juice and Evaluation of Storage Stability. Asian J. Dairy Food Res. 2015, 34, 151–155. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2016.
- Versteeg, C.; Rombouts, F.M.; Spaansen, C.H.; Pilnik, W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J. Food Sci. 1980, 45, 969–971. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Awad, F.N.; Hu, B.; Lei, S.; Zeng, X. Quality Assessment of Pear Juice under Ultrasound and Commercial Pasteurization Processing Conditions. LWT-Food Sci. Technol. 2015, 64, 452–458. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Nindo, C.I.; Tang, J.; Powers, J.R.; Singh, P. Viscosity of Blueberry and Raspberry Juices for Processing Applications. J. Food Eng. 2005, 69, 343–350. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biometrical Approaches, 3rd ed.; McGraw Hill Book Co., Inc.: Singapore, MI, USA, 1997; pp. 204–227. [Google Scholar]
- Walkling-Ribeiro, M.; Noci, F.; Riener, J.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. The Impact of Thermosonication and Pulsed Electric Fields on Staphylococcus Aureus Inactivation and Selected Quality Parameters in Orange Juice. Food Bioprocess Technol. 2008, 2, 422. [Google Scholar] [CrossRef]
- Jan, A.; Masih, E.D. Development and Quality Evaluation of Pineapple Juice Blend with Carrot and Orange Juice. Int. J. Sci. Res. Publ. 2012, 2, 1–8. [Google Scholar]
- Bhardwaj, R.L.; Pandey, S. Juice Blends—A Way of Utilization of Under-Utilized Fruits, Vegetables, and Spices: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 563–570. [Google Scholar] [CrossRef]
- Cansino, N.C.; Carrera, G.P.; Rojas, Q.Z.; Olivares, L.D.; García, E.A.; Moreno, E.R. Ultrasound Processing on Green Cactus Pear (Opuntia Ficus Indica) Juice: Physical, Microbiological and Antioxidant Properties. J. Food Process. Technol. 2013, 4, 1000267. [Google Scholar] [CrossRef]
- Jingfei, G.; Vasantha, H.P.; Nutritional, R. Physicochemical and Microbial Quality of Ultrasound-Treated Apple-Carrot Juice Blends. Food Nutr. Sci. 2012, 3, 212–218. [Google Scholar]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication Enhances Polyphenolic Compounds, Sugars, Carotenoids and Mineral Elements of Apple Juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of Ultrasound Treatments on Quality of Grapefruit Juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of High-Intensity Pulsed Electric Field Processing Conditions on Lycopene, Vitamin C and Antioxidant Capacity of Watermelon Juice. Food Chem. 2009, 115, 1312–1319. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Color and Viscosity of Watermelon Juice Treated by High-Intensity Pulsed Electric Fields or Heat. Innov. Food Sci. Emerg. Technol. 2010, 11, 299–305. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).