Evaluating the Nutrient Contents and Nutritive Value of Taif’s Rose (Rosa damascena Mill var. trigintipetala) Waste to Be Used as Animal Forage or Soil Organic Fertilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological and Biomass Characteristics
2.2. Plant Chemical Analyses
2.3. Soil Characteristics
2.4. Multivariate Analysis
2.5. Statistical Analysis
3. Results
3.1. Morphological and Growth Characteristics
3.2. Soil Characteristics and Their Relationships with the Plant Variables
3.3. Inorganic and Organic Nutrient Contents
3.4. Nutritive Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Dong, F.; Chen, M.; Zhu, J.; Tan, J.; Fu, X.; Wang, Y.; Chen, S. Advances in recycling and utilization of agricultural wastes in China: Based on environmental risk, crucial pathways, influencing factors, policy mechanism. Procedia Environ. Sci. 2016, 31, 12–17. [Google Scholar] [CrossRef]
- Varma, V.S.; Yadav, J.; Das, S.; Kalamdhad, A.S. Potential of waste carbide sludge addition on earthworm growth and organic matter degradation during vermicomposting of agricultural wastes. Ecol. Eng. 2015, 83, 90–95. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, S.; Zhai, L.M.; Zhang, J.Z.; Ren, T.Z.; Fan, B.Q.; Liu, H.B. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefinery 2021, 1–24. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Din, Z.U.; Sabah, N.U.; Jamil, M.A.; Mujtaba, M.A.; Abid, A. The potential of sustainable biogas production from biomass waste for power generation in Pakistan. J. Clean. Prod. 2021, 307, 127250. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef]
- Torkashvand, M.A.; Alidoust, M.; Mahboub Khomami, A. The reuse of peanut organic wastes as a growth medium for ornamental plants. Int. J. Recycl. Org. Waste Agric. 2015, 4, 85–94. [Google Scholar] [CrossRef]
- Duan, Y.; Mehariya, S.; Kumar, A.; Singh, E.; Yang, J.; Kumar, S.; Li, H.; Kumar Awasthi, M. Apple orchard waste recycling and valorization of valuable product—A review. Bioengineered 2021, 12, 476–495. [Google Scholar] [CrossRef]
- Sharma, B.; Vaish, B.; Singh, U.K.; Singh, P.; Singh, R.P. Recycling of organic wastes in agriculture: An environmental perspective. Int. J. Environ. Res. 2019, 13, 409–429. [Google Scholar] [CrossRef]
- Galal, T.M.; Al-Yasi, H.M.; Fawzy, M.A.; Abdelkader, T.G.; Hamza, R.Z.; Eid, E.M.; Ali, E.F. Evaluation of the Phytochemical and Pharmacological Potential of Taif’s Rose (Rosa damascena Mill var. trigintipetala) for Possible Recycling of Pruning Wastes. Life 2022, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Yasi, H.M.; Ali, E.F.; Fawzy, M.A.; Abdelkader, T.G.; Galal, T.M. Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigintipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals 2022, 12, 460. [Google Scholar] [CrossRef]
- Ali, E.F.; Al-Yasi, H.M.; Issa, A.A.; Hessini, K.; Hassan, F.A.S. Ginger extract and fulvic acid foliar applications as novel practical approaches to improve the growth and productivity of damask rose. Plants 2022, 11, 412. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Baser, K.H.C.; Akterian, S.G.; Fidan, H.N.; Stoyanova, A.S. Chemical composition, sensory evaluation and antimicrobial activity of Taif rose (Rosa damascena Mill.) essential oils. Bulg. Chem. Commun. 2020, 460. [Google Scholar]
- Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The efficacies of 1-methylcyclopropene and chitosan nanoparticles in preserving the postharvest quality of damask rose and their underlying biochemical and physiological mechanisms. Biology 2022, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.F.; Alyasi, H.M.; Hassan, F.A.; Alamer, K.H.; Hessini, K.; Attia, H.; Elshazly, S. Effect of the Pruning System and P-Fertilizer on Growth and Productivity of Rosa damascena mill. Egypt. J. Bot. 2021, 61, 565–578. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Hassan, F.; Al-Yasi, H.; Ali, E.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt-stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2020, 101, 157–165. [Google Scholar] [CrossRef]
- El-Sharnouby, M.E.; Montaser, M.M.; Abdallah, S.M. Oil and Flower Production in Rosa damascena trigintipetala Dieck under Salinity Stress in Taif Region, Saudi Arabia. Sustainability 2021, 13, 4547. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded moisture deficit effect on secondary metabolites, antioxidant, and inhibitory enzyme activities in leaf extracts of Rosa damascena Mill. var. trigintipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Dragoev, S.; Vlahova-Vangelova, D.; Balev, D.; Bozhilov, D.; Dagnon, S. Valorization of waste by-products of rose oil production as feedstuff phytonutrients. Bulg. J. Agric. Sci. 2020, 27, 209–219. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants, and Water; Department of Soil, Plant Nutrition, University of California Press: Berkeley, CA, USA, 1961. [Google Scholar]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: London, UK, 1989. [Google Scholar]
- Le Houérou, H.N. Chemical composition and nutritive value of browse in tropical West Africa. In Browse in Africa, the Current State of Knowledge; Le Houérou, H.N., Ed.; ILCA: Addis Ababa, Ethiopia, 1980; pp. 261–289. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 8th ed.; NRC (National Research Council): Ottawa, ON, Canada, 2001. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); Agriculture Handbook No. 379; ARSUSDA: Washington, DC, USA, 1970. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal performance. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Blaxter, K.L. The Energy Metabolism of Ruminants, 2nd ed.; Charles Thomas Publisher: Spring Field, IL, USA, 1968. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Harlow, Pearson: London, UK, 2011. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Beef Cattle, 7th ed.; updated 2000; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Maff, R. The Analysis of Agricultural Materials, 3rd ed.; HMSO: London, UK, 1986; p. 427. [Google Scholar]
- Leps, J.; Šmilauer, P. Multivariate Analysis of Ecological Data using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual for User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4.0); Microcomputer Power: Ithaca, NY, USA, 1998; p. 352. [Google Scholar]
- Ter Braak, C.J. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 69–77. [Google Scholar] [CrossRef]
- SPSS. IBM SPSS statistics version 21.0. Copyright of IBM and other(s); IBM Corp.: Armonk, NY, USA, 2012.
- Idrovo-Novillo, J.; Gavilanes-Terán, I.; Veloz-Mayorga, N.; Erazo-Arrieta, R.; Paredes, C. Closing the cycle for the cut rose industry by the reuse of its organic wastes: A case study in Ecuador. J. Clean. Prod. 2019, 220, 910–918. [Google Scholar] [CrossRef]
- Galal, T.M.; Gharib, F.A.; Al-Yasi, H.M.; Al-Mutairi, K.A.; Mansour, K.H.; Eid, E.M. Nutrient Remediation Efficiency of the Sedge Plant (Cyperus alopecuroides Rottb.) to Restore Eutrophic Freshwater Ecosystems. Sustainability 2022, 14, 2823. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C., Jr. Current approaches and future trends in compost quality criteria for agronomic, environmental, and human health benefits. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, NY, USA, 2017; Volume 144, pp. 143–233. [Google Scholar]
- Khater, E.S.G. Some physical and chemical properties of compost. Int. J. Waste Resour. 2015, 5, 172. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Chang, K.H.; Wu, R.Y.; Chuang, K.C.; Hsieh, T.F.; Chung, R.S. Effects of chemical and organic fertilizers on the growth, flower quality and nutrient uptake of Anthurium andreanum, cultivated for cut flower production. Sci. Hortic. 2010, 125, 434–441. [Google Scholar] [CrossRef]
- Naeem, M.; Ansari, A.; Gill, S. (Eds.) V Role of Organic Fertilizers in Improving Soil Fertility. In Contaminants in Agriculture; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Vasileva, V.; Naydenova, Y.; Stoycheva, I. Nutritive value of forage biomass from sainfoin mixtures. Saudi J. Biol. Sci. 2019, 26, 942–949. [Google Scholar] [CrossRef]
- Heneidy, S.Z.; Halmy, M.W. The nutritive value and role of Panicum turgidum Forssk. in the arid ecosystems of the Egyptian desert. Acta Bot. Croat. 2009, 68, 127–146. [Google Scholar]
- A.O.A.C. Official Methods of Analysis. Association of Official Analytical Chemists, 14th ed.; A.O.A.C.: Arlington, TX, USA, 1984. [Google Scholar]
- MAFF (Ministry of Agriculture, Fisheries, and Food). Energy Allowances and Feeding Systems for Ruminants; Technical Bulletin 33; Her Majesty’s Stationary Office: London, UK, 1975. [Google Scholar]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Principles of Animal Nutrition; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2018. [Google Scholar]
- Krachunov, I. Estimation of energy feeding value of forages for ruminants II. Energy prediction through crude fiber content. J. Mt. Agric. Balk. 2007, 10, 122–134. [Google Scholar]
- El-Kady, H.F. Seasonal variation in phytomass and nutrient status of Phragmites australis along the watercourses in the Middle Delta region. Taekholmia 2000, 20, 123–138. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Galal, T.M.; Komi, T.M. Nutrients and heavy metals accumulation in the aboveground biomass of two perennial grasses along the watercourses of Nile Delta, Egypt. In Journal of Botany, 3rd International Congress 17–18 April 2013; Helwan University: Cairo, Egypt, 2013; pp. 201–218. [Google Scholar]
- Norton, B.W. Differences between species in forage quality. In Nutritional Limits to Animal Production from Pastures; Hacker, J.B., Ed.; CAB: Farnham Royal, UK, 1982; pp. 98–110. [Google Scholar]
- Čop, J.; Vidrih, M.; Hacin, J. Influence of cutting regime and fertilizer application on the botanical composition, yield and nutritive value of herbage of wet grasslands in Central Europe. Grass Forage Sci. 2009, 64, 454–465. [Google Scholar] [CrossRef]
- Mohammed, G.; Trolard, F.; Bourrié, G.; Gillon, M.; Tronc, D.; Charron, F. A long- term data sequence (1960–2013) to analyse the sustainability of hay quality in irrigated permanent grasslands under climate change. Am. J. Agric. For. 2016, 4, 140–151. [Google Scholar]
- Dindová, A.; Hakl, J.; Hrevušová, Z.; Nerušil, P. Relationships between long-term fertilization management and forage nutritive value in grasslands. Agric. Ecosyst. Environ. 2019, 279, 139–148. [Google Scholar] [CrossRef]
- Farahat, E.; Fahmy, G.; Farrag, H.; Mahmoud, W.; Awad, H. Potential Nutritional Value of the Macrophyte Vossia cuspidata (Roxb.) Griff. (Poaceae) in a River Nile System, Egypt. Egypt. J. Bot. 2021, 6, 867–877. [Google Scholar] [CrossRef]
- Galal, T.M.; Gharib, F.A.; Al-Yasi, H.M.; Mansour, K.H.; Hassan, M.M. Evaluation of the nutrient status and forage quality of the hippo grass (Vossia cuspidata (Roxb.) Griff.) along Ismailia canal, Egypt. J. Freshw. Ecol. 2021, 36, 63–76. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, B.; Liu, J. Effects of the dietary non-fiber carbohydrates content on lactation performance, rumen fermentation, and nitrogen utilization in mid-lactation dairy cows receiving corn stover. J. Anim. Sci. Biotechnol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Galal, T.M.; El-Komi, T. Evaluation of the nutrient status of some hydrophytes in the watercourses of Nile Delta, Egypt. J. Bot. 2009, 2009, 1–11. [Google Scholar] [CrossRef]
- Batajoo, K.K.; Shaver, R.D. Impact of nonfiber carbohydrate on intake, digestion, and milk production by dairy cows. J. Dairy Sci. 1994, 77, 1580–1587. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Akins, M.S.; Cavadini, J.S.; Jokela, W.E. Net effects of nitrogen fertilization on the nutritive value and digestibility of oat forages. J. Dairy Sci. 2017, 100, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Heneidy, S.Z. Palatability and nutritive value of some common plant species from the Aqaba Gulf area of Sinai, Egypt. J. Arid. Environ. 1996, 34, 115–123. [Google Scholar] [CrossRef]
- Blümmel, M.; Updahyay, S.R.; Gautam, N.; Barma, N.C.D.; Abdul Hakim, M.; Hussain, M.; Joshi, A.K. Comparative assessment of food-fodder traits in a wide range of wheat germplasm for diverse biophysical target domains in South Asia. Field Crops Res. 2019, 236, 68–74. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Salem, A.Z.M.; Lopez, S.; Kholif, A.M. Moringa oleifera leaf meal as a protein source in lactating goat’s diets: Feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin. Res. 2015, 129, 29–137. [Google Scholar] [CrossRef]
- Martínez-Martínez, R.; López-Ortiz, S.; Ortega-Cerrilla, M.E.; Soriano-Robles, R.; Herrera-Haro, J.G.; López-Collado, J.; Ortega-Jiménez, E. Preference, consumption and weight gain of sheep supplemented with multinutritional blocks made with fodder tree leaves. Livest. Sci. 2012, 149, 185–189. [Google Scholar] [CrossRef]
- Nelson, M.L.; Finley, J.W.; Scarnecchia, D.L.; Parish, S.M. Diet and forage quality of intermediate wheatgrass managed under continuous and short-duration grazing. J. Range Manag. 1989, 42, 474–477. [Google Scholar] [CrossRef]
- Coleman, S.W.; Rao, S.C.; Volesky, J.D.; Phillips, W.A. Growth and nutritive value of perennial C3 grasses in the southern Great Plains. Crop Sci. 2010, 50, 1070–1078. [Google Scholar] [CrossRef]
- Favre, J.R.; Castiblanco, T.M.; Combs, D.K.; Wattiaux, M.A.; Picasso, V.D. Forage nutritive value and predicted fiber digestibility of Kernza intermediate wheatgrass in monoculture and in mixture with red clover during the first production year. Anim. Feed. Sci. Technol. 2019, 258, 114298. [Google Scholar] [CrossRef]
- Cherian, G. A Guide to the Principles of Animal Nutrition; Open textbook library, Oregon State Open Educational Resources: Corvallis, IL, USA, 2020; p. 163. [Google Scholar]
- Shoukry, M.M. An Actual Vision about the Availability of the Utilization of Water Hyacinth in Feeding Ruminants; National Symposium on Water Hyacinth, Assiut University: Assiut, Egypt, 1992; pp. 75–92. [Google Scholar]
- Al-Sodany, Y.M.; El-Sheikh, M.A.; Baraka, D.M.; Shaltout, K.H. Element’s accumulation and nutritive value of Phragmites australis (Cav.) Trin, ex Steudel in Lake Burullus: A Ramsar site, Egypt. Catrina 2012, 8, 51–63. [Google Scholar]
- Galal, T.M.; Abu Alhmad, M.F.; Al-Yasi, H.M. Nutrient sequestration potential of water primrose Ludwigia stolinefera (Guill. & Perr.) P.H. Raven: A strategy for restoring wetland eutrophication. Saudi J. Biol. Sci. 2021, 28, 2438–2446. [Google Scholar] [PubMed]
- Gill, K.S.; Omokanye, A. Spring triticale varieties forage yield, nutrients composition, and suitability for beef cattle production. J. Agric. Sci. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Heneidy, S.Z. Browsing and nutritive value of the most common range species in Matruh area, a coastal Mediterranean region, Egypt. Ecol. Mediterr. 2002, 28, 39–49. [Google Scholar] [CrossRef]
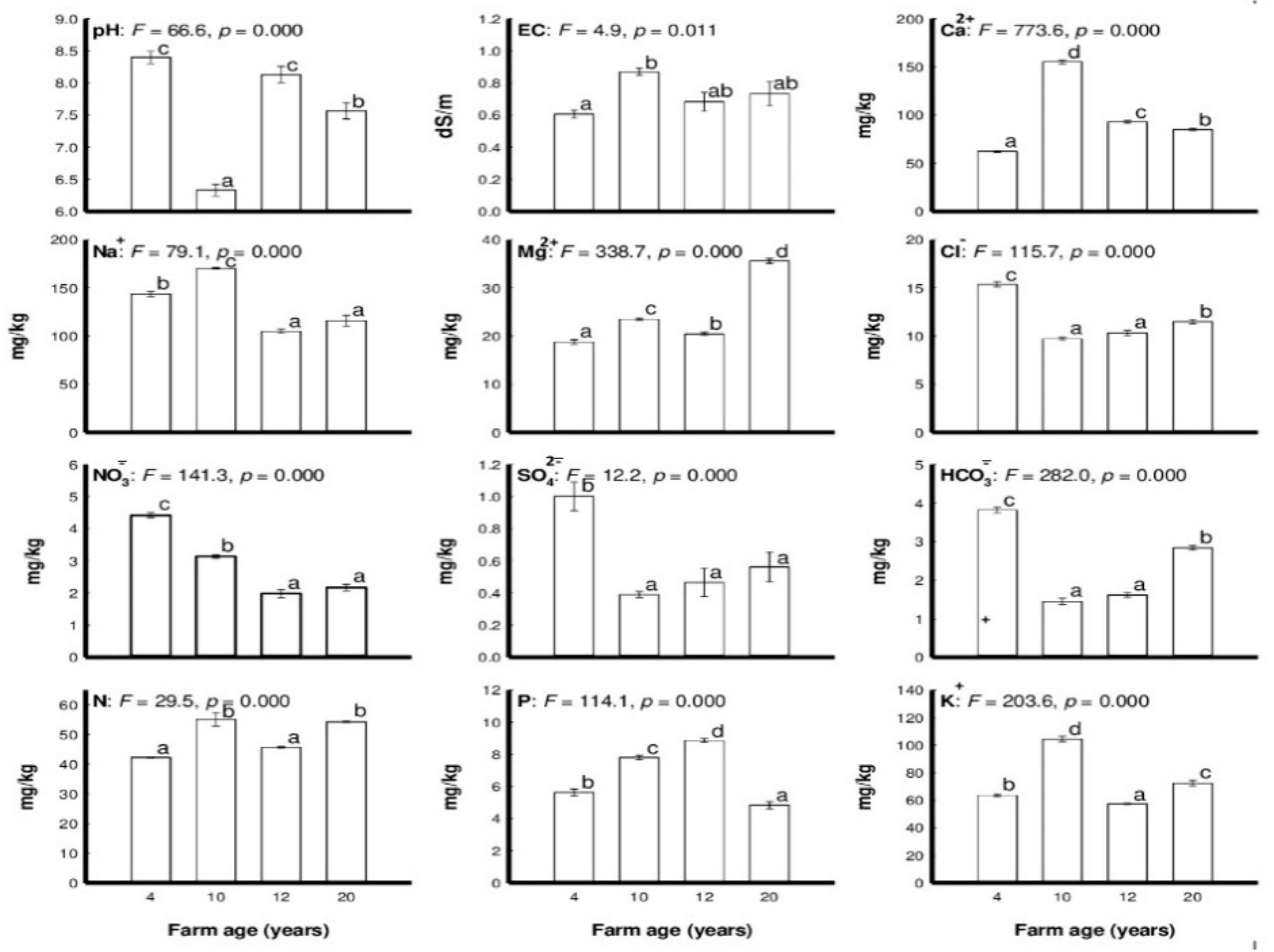
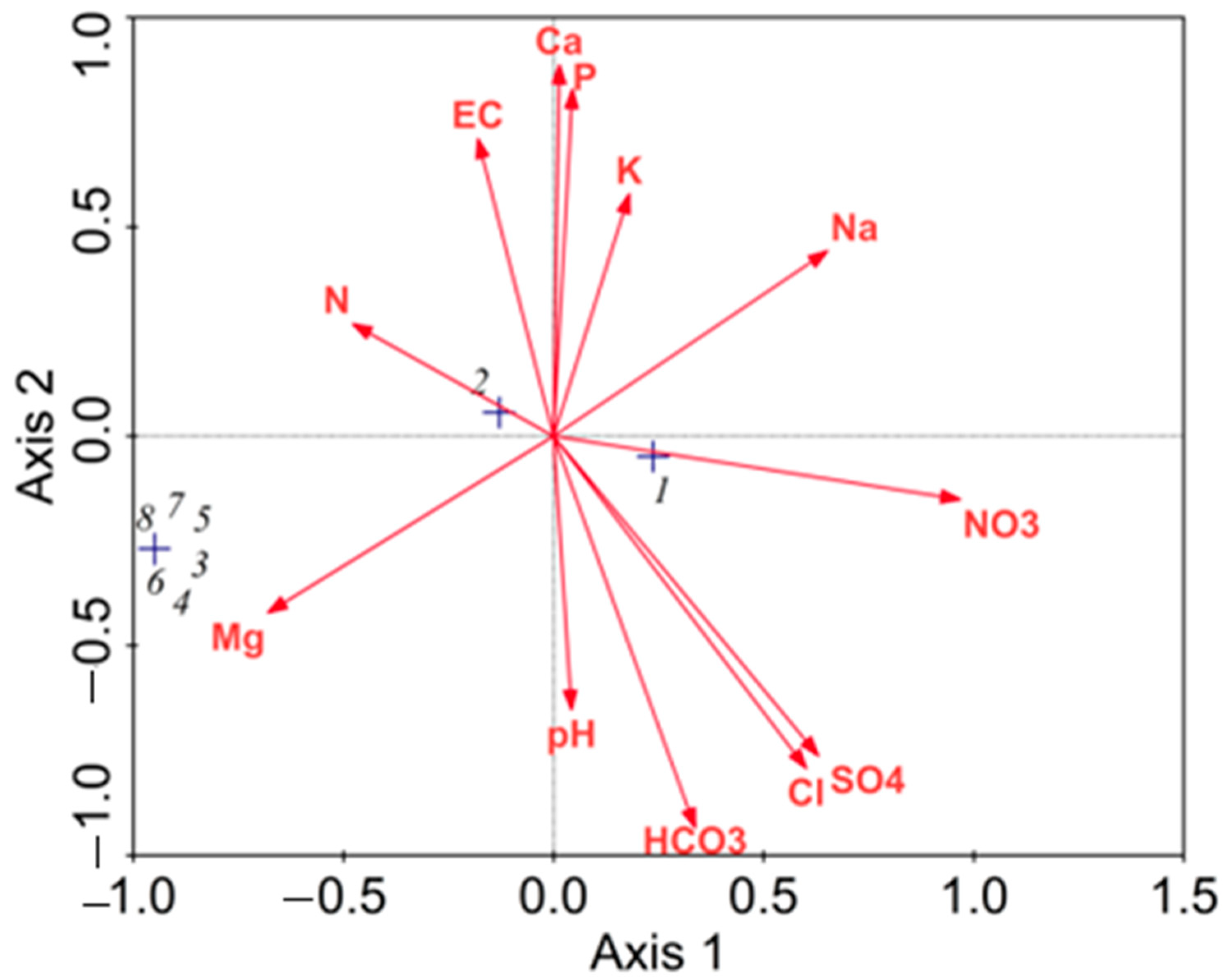
| Variable | Farm No. (Age, Years) | F-Value | |||
|---|---|---|---|---|---|
| 1 (4 Years) | 2 (10 Years) | 3 (12 Years) | 4 (20 Years) | ||
| Height (cm) | 110.50 ± 3.70 a | 148.70 ± 3.70 b | 155.30 ± 4.00 b | 184.20 ± 3.10 c | 69.8 *** |
| Diameter (cm) | 112.70 ± 3.90 a | 182.90 ± 2.60 b | 204.50 ± 5.70 c | 243.50 ± 3.10 d | 186.4 *** |
| Stem biomass (kg ind.−1) | 0.89 ± 0.08 a | 1.67 ± 0.07 b | 2.49 ± 0.11 c | 3.52 ± 0.13 d | 128.5 *** |
| Leaf biomass (kg ind.−1) | 0.30 ± 0.03 a | 0.56 ± 0.02 b | 0.83 ± 0.04 c | 1.17 ± 0.04 d | 128.5 *** |
| AGB (kg ind.−1) | 1.19 ± 0.10 a | 2.22 ± 0.09 b | 3.32 ± 0.15 c | 4.69 ± 0.17 d | 128.5 *** |
| Stem biomass (t ha−1) | 0.99 ± 0.08 a | 1.85 ± 0.08 b | 2.77 ± 0.12 c | 3.91 ± 0.14 d | 128.5 *** |
| Leaf biomass (t ha−1) | 0.33 ± 0.03 a | 0.62 ± 0.03 b | 0.92 ± 0.04 c | 1.30 ± 0.05 d | 128.5 *** |
| AGB (t ha−1) | 1.32 ± 0.11 a | 2.47 ± 0.11 b | 3.69 ± 0.16 c | 5.21 ± 0.19 d | 128.5 *** |
| Soil Characteristic | Axis 1 | Axis 2 |
|---|---|---|
| pH | 0.0424 | –0.6525 |
| EC (dS m−1) | –0.1797 | 0.7102 |
| Cl− (mg kg−1) | 0.6106 | –0.7924 |
| NO3− (mg kg−1) | 0.9681 | –0.1516 |
| SO42− (mg kg−1) | 0.6303 | –0.7626 |
| HCO3− (mg kg−1) | 0.3367 | –0.9351 |
| Total N (mg kg−1) | –0.4778 | 0.2682 |
| Total P (mg kg−1) | 0.0451 | 0.8280 |
| K+ (mg kg−1) | 0.1805 | 0.5779 |
| Ca2+ (mg kg−1) | 0.0140 | 0.8868 |
| Mg2+ (mg kg−1) | –0.6800 | –0.4213 |
| Na+ (mg kg−1) | 0.6225 | 0.4416 |
| Element | Organ | Farm No. (Age, Years) | FFarm | FOrgan | FFarm×Organ | |||
|---|---|---|---|---|---|---|---|---|
| 1 (4 Years) | 2 (10 Years) | 3 (12 Years) | 4 (20 Years) | |||||
| N (mg kg−1) | Leaf | 154.30 ± 7.99 | 42.20 ± 1.48 | 92.00 ± 2.65 | 103.50 ± 4.15 | 84.6 *** | 163.8 *** | 94.4 *** |
| Stem | 161.30 ± 6.61 | 172.33 ± 3.70 | 85.07 ± 2.72 | 136.20 ± 2.62 | ||||
| P (mg kg−1) | Leaf | 6.10 ± 0.11 | 6.70 ± 0.24 | 7.17 ± 0.09 | 6.33 ± 0.09 | 68.4 *** | 0.2 ns | 65.7 *** |
| Stem | 6.33 ± 0.16 | 9.40 ± 0.29 | 5.47 ± 0.15 | 5.33 ± 0.09 | ||||
| K (mg kg−1) | Leaf | 94.70 ± 1.66 | 103.43 ± 4.25 | 112.47 ± 3.90 | 95.57 ± 3.14 | 139.0 *** | 412.9 *** | 92.5 *** |
| Stem | 91.87 ± 1.57 | 174.67 ± 4.71 | 277.30 ± 8.52 | 154.87 ± 8.01 | ||||
| Ca (mg kg−1) | Leaf | 152.30 ± 5.58 | 171.47 ± 1.64 | 165.63 ± 8.99 | 206.30 ± 7.99 | 44.6 *** | 4.1 * | 70.1 *** |
| Stem | 150.83 ± 0.72 | 125.03 ± 4.68 | 251.93 ± 7.99 | 134.03 ± 3.41 | ||||
| Mg (mg kg−1) | Leaf | 114.10 ± 1.94 | 20.57 ± 0.26 | 122.27 ± 2.31 | 84.77 ± 1.05 | 974.9 *** | 78.2 *** | 50.3 *** |
| Stem | 111.87 ± 1.88 | 56.17 ± 1.81 | 123.57 ± 1.86 | 92.87 ± 1.69 | ||||
| Na (mg kg−1) | Leaf | 170.70 ± 2.98 | 186.23 ± 3.76 | 173.03 ± 3.23 | 140.60 ± 3.29 | 77.7 *** | 216.7 *** | 307.3 *** |
| Stem | 142.53 ± 4.04 | 68.67 ± 1.28 | 128.00 ± 2.14 | 205.40 ± 2.54 | ||||
| Element | Organ | Farm Age (Years) | FFarm | FOrgan | FFarm×Organ | |||
|---|---|---|---|---|---|---|---|---|
| 1 (4 Years) | 2 (10 Years) | 3 (12 Years) | 4 (20 Years) | |||||
| ADF (%) | Leaf | 18.20 ± 0.53 | 26.40 ± 1.24 | 31.60 ± 1.02 | 15.30 ± 0.83 | 40.1 *** | 4.2 * | 82.8 *** |
| Stem | 17.33 ± 0.40 | 25.13 ± 0.59 | 21.60 ± 0.61 | 32.60 ± 1.39 | ||||
| ADL (%) | Leaf | 2.67 ± 0.02 | 2.83 ± 0.09 | 7.63 ± 0.09 | 2.57 ± 0.06 | 690.9 *** | 697.7 *** | 391.1 *** |
| Stem | 1.87 ± 0.09 | 2.37 ± 0.09 | 3.03 ± 0.09 | 2.70 ± 0.04 | ||||
| NDF (%) | Leaf | 36.13 ± 1.04 | 45.10 ± 1.68 | 44.10 ± 0.79 | 27.50 ± 0.90 | 24.3 *** | 5.8* | 52.5 *** |
| Stem | 33.27 ± 0.38 | 36.10 ± 0.68 | 36.10 ± 1.04 | 40.57 ± 0.95 | ||||
| Fat (%) | Leaf | 0.79 ± 0.02 | 0.57 ± 0.01 | 0.24 ± 0.01 | 0.41 ± 0.01 | 198.5 *** | 1627.5 *** | 166.8 *** |
| Stem | 0.11 ± 0.01 | 0.23 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | ||||
| Crude fiber (%) | Leaf | 28.83 ± 1.52 | 28.27 ± 0.71 | 13.27 ± 0.71 | 17.07 ± 0.63 | 27.4 *** | 161.3 *** | 230.1 *** |
| Stem | 19.43 ± 1.08 | 17.43 ± 0.61 | 42.57 ± 1.02 | 45.90 ± 1.61 | ||||
| Ash (%) | Leaf | 14.07 ± 0.42 | 11.63 ± 0.82 | 8.03 ± 0.22 | 12.93 ± 0.58 | 4.7 ** | 147.8 *** | 49.2 *** |
| Stem | 6.03 ± 0.28 | 5.17 ± 0.15 | 11.07 ± 0.79 | 7.10 ± 0.28 | ||||
| Total Protein (%) | Leaf | 9.65 ± 0.49 | 2.63 ± 0.09 | 5.75 ± 0.16 | 6.48 ± 0.25 | 87.6 *** | 166.4 *** | 95.8 *** |
| Stem | 10.08 ± 0.40 | 10.71 ± 0.24 | 5.31 ± 0.17 | 8.52 ± 0.17 | ||||
| NFE (%) | Leaf | 46.67 ± 0.61 | 56.91 ± 0.34 | 72.71 ± 0.66 | 63.11 ± 0.29 | 44.4 *** | 117.6 *** | 331.1 *** |
| Stem | 64.34 ± 0.97 | 66.47 ± 0.25 | 40.94 ± 1.97 | 38.37 ± 1.18 | ||||
| Element | Organ | Farm No. (Age, Years) | FFarm | FOrgan | FFarm×Organ | |||
|---|---|---|---|---|---|---|---|---|
| 4 | 10 | 12 | 20 | |||||
| DCP (%) | Leaf | 5.44 ± 0.46 | 1.08 ± 0.08 | 1.82 ± 0.15 | 2.50 ± 0.23 | 81.8 *** | 98.8 *** | 48.7 *** |
| Stem | 5.85 ± 0.37 | 6.43 ± 0.22 | 1.42 ± 0.16 | 4.40 ± 0.16 | ||||
| TDN (%) | Leaf | 55.97 ± 0.34 | 60.85 ± 0.06 | 58.34 ± 0.11 | 57.95 ± 0.17 | 78.5 *** | 222.5 *** | 96.5 *** |
| Stem | 55.13 ± 0.30 | 54.77 ± 0.18 | 58.56 ± 0.13 | 56.25 ± 0.13 | ||||
| DE (Mcal kg−1) | Leaf | 2.31 ± 0.02 | 2.44 ± 0.02 | 3.22 ± 0.02 | 2.74 ± 0.02 | 35.1 *** | 9.2 ** | 418.9 *** |
| Stem | 3.02 ± 0.06 | 3.15 ± 0.01 | 2.07 ± 0.05 | 2.18 ± 0.02 | ||||
| ME (Mcal kg−1) | Leaf | 1.89 ± 0.02 | 1.99 ± 0.02 | 2.64 ± 0.02 | 2.25 ± 0.01 | 35.1 *** | 9.2 ** | 418.9 *** |
| Stem | 2.48 ± 0.05 | 2.58 ± 0.01 | 1.69 ± 0.04 | 1.79 ± 0.02 | ||||
| NE (Mcal kg−1) | Leaf | 0.95 ± 0.01 | 0.99 ± 0.01 | 1.32 ± 0.01 | 1.12 ± 0.01 | 35.1 *** | 9.2 ** | 418.9 *** |
| Stem | 1.24 ± 0.02 | 1.29 ± 0.01 | 0.85 ± 0.02 | 0.90 ± 0.01 | ||||
| GE (Mcal kg−1) | Leaf | 388.83 ± 1.94 | 385.15 ± 3.66 | 391.72 ± 1.75 | 377.02 ± 2.29 | 2.0 ns | 324.4 *** | 28.9 *** |
| Stem | 411.09 ± 0.91 | 414.76 ± 0.71 | 400.35 ± 2.15 | 424.25 ± 2.09 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Galal, T.; Ali, E.F.; Eid, E.M.; Al-Yasi, H.M.; Magrashi, A.; Althobaiti, F.; Farahat, E.A. Evaluating the Nutrient Contents and Nutritive Value of Taif’s Rose (Rosa damascena Mill var. trigintipetala) Waste to Be Used as Animal Forage or Soil Organic Fertilizers. Agriculture 2022, 12, 1481. https://doi.org/10.3390/agriculture12091481
M. Galal T, Ali EF, Eid EM, Al-Yasi HM, Magrashi A, Althobaiti F, Farahat EA. Evaluating the Nutrient Contents and Nutritive Value of Taif’s Rose (Rosa damascena Mill var. trigintipetala) Waste to Be Used as Animal Forage or Soil Organic Fertilizers. Agriculture. 2022; 12(9):1481. https://doi.org/10.3390/agriculture12091481
Chicago/Turabian StyleM. Galal, Tarek, Esmat F. Ali, Ebrahem M. Eid, Hatim M. Al-Yasi, Ali Magrashi, Fayez Althobaiti, and Emad A. Farahat. 2022. "Evaluating the Nutrient Contents and Nutritive Value of Taif’s Rose (Rosa damascena Mill var. trigintipetala) Waste to Be Used as Animal Forage or Soil Organic Fertilizers" Agriculture 12, no. 9: 1481. https://doi.org/10.3390/agriculture12091481






