Serendipita indica as a Plant Growth Promoter and Biocontrol Agent against Black Rot Disease in Cabbage Grown in a Phytotron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Condition and Treatments
2.2. Serendipita indica Inoculation
2.3. Detection of S. indica Colonisation in Cabbage Roots
2.4. Pathogen Preparation and Inoculation
2.4.1. Preparation of Xcc Inoculum
2.4.2. Colony Forming Unit (CFU) Enumeration
2.4.3. Pathogen Inoculation
2.5. In Vitro Test of S. indica with Alternaria brassicicola and Xanthomonas campestris
2.6. Morphological and Physiological Evaluation
2.7. Biochemical and Stress Biomarker Analyses
2.8. Disease Analyses
2.9. Statistical Analysis
3. Results
3.1. Morphological and Physiological Parameters
3.2. Effects of S. indica on Bio-Stress Parameters of Cabbage
4. Disease Analyses
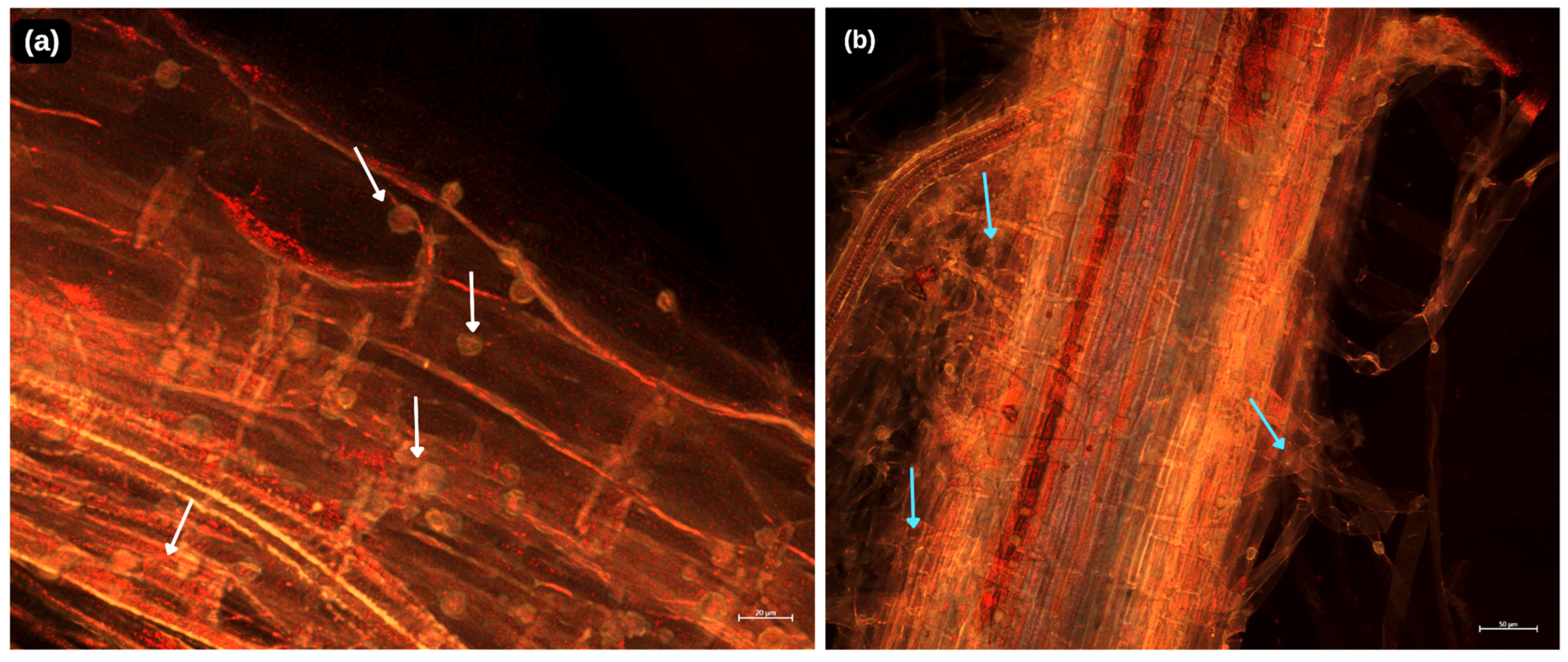
5. Root Colonization
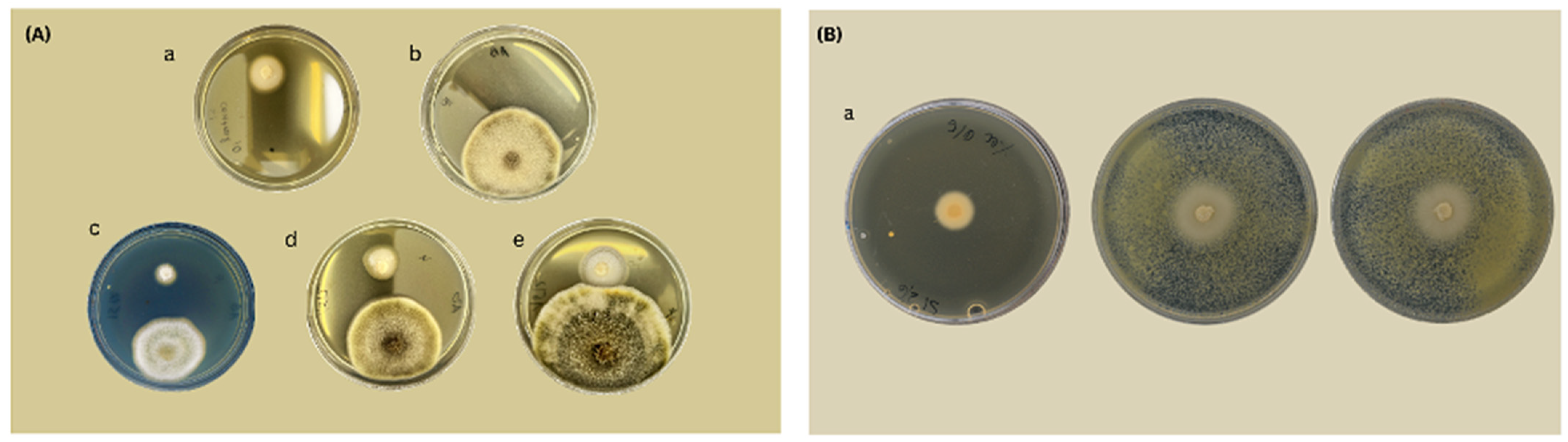
6. In Vitro Test
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naamala, J.; Smith, D.L. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy 2020, 10, 1179. [Google Scholar] [CrossRef]
- Liu, W.; Tan, M.; Qu, P.; Huo, C.; Liang, W.; Li, R.; Jia, Y.; Fan, X.; Cheng, C. Use of Piriformospora indica to Promote Growth of Strawberry Daughter Plants. Horticulturae 2022, 8, 370. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The Role of Beneficial Microorganisms in Soil Quality and Plant Health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Chiaranunt, P.; White, J.F. Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems. Plants 2023, 12, 400. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.; Hassel, A.; Kost, G.; Sarbhoy, A.; Franken, P. Piriformospora indica, gen. et sp. nov., a New Root-Colonizing Fungus. Mycologia 1998, 90, 896–903. [Google Scholar]
- Yan, C.; Rizwan, H.M.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root colonising Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defence shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Cheng, C. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, J.; Ren, X.; Yu, T.; Varma, A.; Lou, B.; Zheng, X. Effects of Piriformospora indica on the growth, fruit quality and interaction with Tomato yellow leaf curl virus in tomato cultivars susceptible and resistant to TYCLV. Plant Growth Regul. 2015, 76, 303–313. [Google Scholar] [CrossRef]
- Abdelaziz, E.M.; Sabra, M.; Ali, M. Colonising Lettuce (Lactuca sativa L.) with Rhizophagus irregularis and Piriformospora indica fungi enhance plant yield and quality in sand soil. Middle East J. Appl. Sci. 2018, 8, 1173–1180. [Google Scholar]
- Saleem, S.; Bytešníková, Z.; Richtera, L.; Pokluda, R. The Effects of Serendipita indica and Guanidine-Modified Nanomaterial on Growth and Development of Cabbage Seedlings and Black Spot Infestation. Agriculture 2021, 11, 1295. [Google Scholar] [CrossRef]
- Rokni, N.; Alizadeh, H.S.; Bazgir, E.; Darvishnia, M.; Mirzaei-Najafgholi, H. The tripartite consortium of Serendipita indica, Trichoderma simmonsii, and bell pepper (Capsicum annum). Biol. Control 2021, 158, 104608. [Google Scholar] [CrossRef]
- Attri, M.K.; Varma, A. Comparative Study of Growth of Piriformospora indica by using different Sources of Jaggery. J. Pure Appl. Microbiol. 2018, 12, 933–942. [Google Scholar] [CrossRef]
- Li, Q.; Kuo, Y.W.; Lin, K.H.; Huang, W.; Deng, C.; Yeh, K.W.; Chen, S.P. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Fakhro, A.; Andrade-Linares, D.R.; von Bargen, S.; Bandte, M.; Büttner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Roylawar, P.; Panda, S.; Kamble, A. Comparative analysis of BABA and Piriformospora indica mediated priming of defence-related genes in tomato against early blight. Physiol. Mol. Plant. Pathol. 2015, 91, 88–95. [Google Scholar] [CrossRef]
- Khalid, M.; Hui, N.; Rahman, S.U.; Hayat, K.; Huang, D. Suppression of clubroot (Plasmodiophora brassicae) development in Brassica campestris sp. chinensis L. via exogenous inoculation of Piriformospora indica. J. Radiat. Res. Appl. Sci. 2020, 13, 180–190. [Google Scholar] [CrossRef]
- Roylawar, P.; Khandagale, K.; Randive, P.; Shinde, B.; Murumkar, C.; Ade, A.; Singh, M.; Gawande, S.; Morelli, M. Piriformospora indica Primes Onion Response against Stemphylium Leaf Blight Disease. Pathogens 2021, 10, 1085. [Google Scholar] [CrossRef]
- Li, L.; Zhu, P.; Wang, X.; Zhang, Z. Phytoremediation effect of Medicago sativa colonised by Piriformospora indica in the phenanthrene and cadmium co-contaminated soil. BMC Biotechnol. 2020, 20, 20. [Google Scholar] [CrossRef]
- Mehri, M.; Ghabooli, M.; Movahedi, Z. Contribution of Serendipita indica on growth improvement, antioxidative capacity of Dracocephalum kotschyi, and its resistance against cadmium stress. Int. Microbiol. 2023, 26, 1–11. [Google Scholar] [CrossRef]
- Grahovac, J.; Pajčin, I.; Vlajkov, V.; Rončević, Z.; Dodić, J.; Cvetković, D.; Jokić, A. Xanthomonas campestris biocontrol agent: Selection, medium formulation and bioprocess kinetic analysis. Chem. Ind. Chem. Eng. Q. 2021, 27, 131–142. [Google Scholar] [CrossRef]
- Mácha, H.; Marešová, H.; Juříková, T.; Švecová, M.; Benada, O.; Škríba, A.; Baránek, M.; Novotný, Č.; Palyzová, A. Killing Effect of Bacillus velezensis FZB42 on a Xanthomonas campestris pv. campestris (Xcc) Strain Newly Isolated from Cabbage Brassica oleracea Convar. Capitata (L.): A Metabolomic Study. Microorganisms 2021, 9, 1410. [Google Scholar] [PubMed]
- Hakalová, E.; Čechová, J.; Tekielska, D.A.; Eichmeier, A.; Pothier, J.F. Combined effect of thyme and clove phenolic compounds on Xanthomonas campestris pv. campestris and biocontrol of black rot disease on cabbage seeds. Front. Microbiol. 2022, 13, 1007988. [Google Scholar]
- Pokluda, R.; Ragasová, L.; Jurica, M.; Kalisz, A.; Komorowska, M.; Niemiec, M.; Sekara, A. Effects of growth promoting microorganisms on tomato seedlings growing in different media conditions. PLoS ONE 2021, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Lin, C.C.; Ching, H.K. NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 1999, 216, 147–153. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Peňázová, E.; Kopta, T.; Jurica, M.; Pečenka, J.; Eichmeier, A.; Pokluda, R. Testing of inoculation methods and susceptibility testing of perspective cabbage breeding lines (Brassica oleracea convar. capitata) to the black rot disease caused by Xanthomonas campestris pv. campestris. Acta Univ. Agric. 2018, 66, 139–148. [Google Scholar] [CrossRef]
- Saleem, S.; Sekara, A.; Pokluda, R. Serendipita indica—A Review from Agricultural Point of View. Plants 2022, 11, 3417. [Google Scholar] [CrossRef]
- Aishwarya, M.; Dhanya, M.; Johnson, J.M.; Murugan, M.; Beena, R.; Paul, A. Individual and Combined Effects of Beneficial Fungal Root Endophytes Piriformospora Indica and Glomus Fasciculatum on Growth, Nutrient Uptake and IAA Production in Small Cardamom. J. Plant. Crops 2022, 50, 35–41. [Google Scholar] [CrossRef]
- Eliaspour, S.; Seyed Sharifi, R.; Shirkhani, A. Evaluation of interaction between Piriformospora indica, animal manure and NPK fertilizer on quantitative and qualitative yield and absorption of elements in sunflower. Food Sci. Nutr. 2020, 8, 2789–2797. [Google Scholar] [CrossRef]
- Bajaj, R.; Agarwal, A.; Rajpal, K.; Asthana, S.; Kumar, R.; Prasad, R.; Kharkwal, A.C.; Sherameti, I.; Oelmüller, R.; Varma, A. Co-cultivation of Curcuma longa with Piriformospora indica enhances the yield and active ingredients. Am. J. Curr. Microbiol. 2014, 2, 6–17. [Google Scholar]
- Del Barrio-Duque, A.; Ley, J.; Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S. Beneficial endophytic bacteria-Serendipita indica interaction for crop enhancement and resistance to phytopathogens. Front. Microbiol. 2019, 10, 2888. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.K.; Jangir, P.; Bishnoi, A.; Roy, S.; Ram, H.; Soni, P. Serendipita indica: Harnessing its versatile potential for food and nutritional security. Physiol. Mol. Plant Pathol. 2021, 116, 101708. [Google Scholar] [CrossRef]
- Hua, M.D.S.; Senthil Kumar, R.; Shyur, L.F.; Cheng, Y.B.; Tian, Z.; Oelmüller, R.; Yeh, K.W. Metabolomic compounds identified in Piriformospora indica-colonised Chinese cabbage roots delineate symbiotic functions of the interaction. Sci. Rep. 2017, 7, 9291. [Google Scholar] [CrossRef]
- Su, Z.Z.; Wang, T.; Shrivastava, N.; Chen, Y.Y.; Liu, X.; Sun, C.; Yin, Y.; Gao, Q.K.; Lou, B.G. Piriformospora indica promotes growth, seed yield and quality of Brassica napus L. Microbiol. Res. 2017, 199, 29–39. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Wang, B.; Qu, P.; Liu, J.; Zhang, Y.; Liu, W.; Tong, Z.; Deng, G. Influences of Serendipita indica and Dictyophorae echinovolvata on the Growth and Fusarium Wilt Disease Resistance of Banana. Biology 2022, 11, 393. [Google Scholar] [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Altschmied, L.; Varma, A.; Oelmüller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280, 26241–26247. [Google Scholar]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of banana to Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Li, L.; Guo, N.; Feng, Y.; Duan, M.; Li, C. Effect of Piriformospora indica-Induced Systemic Resistance and Basal Immunity Against Rhizoctonia cerealis and Fusarium graminearum in Wheat. Front. Plant Sci. 2022, 13, 836940. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Harrach, B.D.; Baltruschat, H.; Barna, B.; Fodor, J.K.; Ogel, K.H. The mutualistic fungus Piriformospora indica protects barley roots from a loss of antioxidant capacity caused by the necrotrophic pathogen Fusarium culmorum. Mol. Plant Microbe Interact. 2013, 26, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The Relationship between Phenolics and Flavonoids Production with Total Non-Structural Carbohydrate and Photosynthetic Rate in Labisia pumila Benth. under High CO2 and Nitrogen Fertilization. Molecules 2011, 16, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Rozy, M.; Evans, C.; Nicholas, K.; Joseph, G. Effects of different nitrogen forms on growth, phenolics, flavonoids and antioxidant activity in amaranth species. Trop. Plant Res. 2017, 4, 81–89. [Google Scholar]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef]
- Lakshmipriya, P.; Nath, V.S.; Veena, S.S.; Anith, K.N.; Sreekumar, J.; Jeeva, M.L. Piriformospora indica, a cultivable endophyte for growth promotion and disease management in Taro (Colocasia esculenta (L.). J. Root Crops 2016, 42, 107–114. [Google Scholar]
- Atri, C.; Akhatar, J.; Gupta, M.; Gupta, N.; Goyal, A.; Rana, K.; Kaur, R.; Mittal, M.; Sharma, A.; Singh, M.P.; et al. Molecular and genetic analysis of defensive responses of Brassica juncea–B. fruticulosa introgression lines to Sclerotinia infection. Sci. Rep. 2019, 9, 17089. [Google Scholar] [CrossRef]
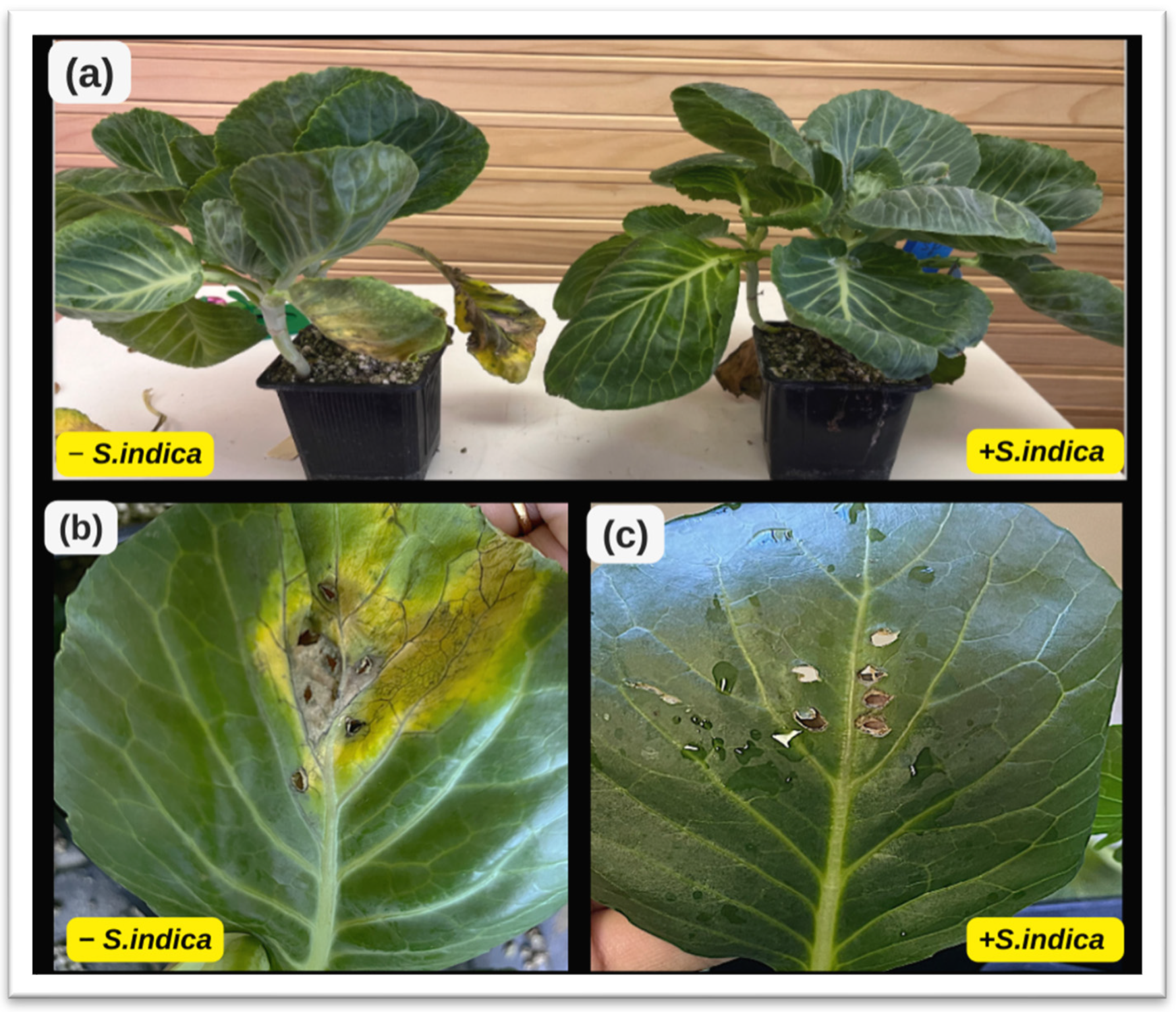
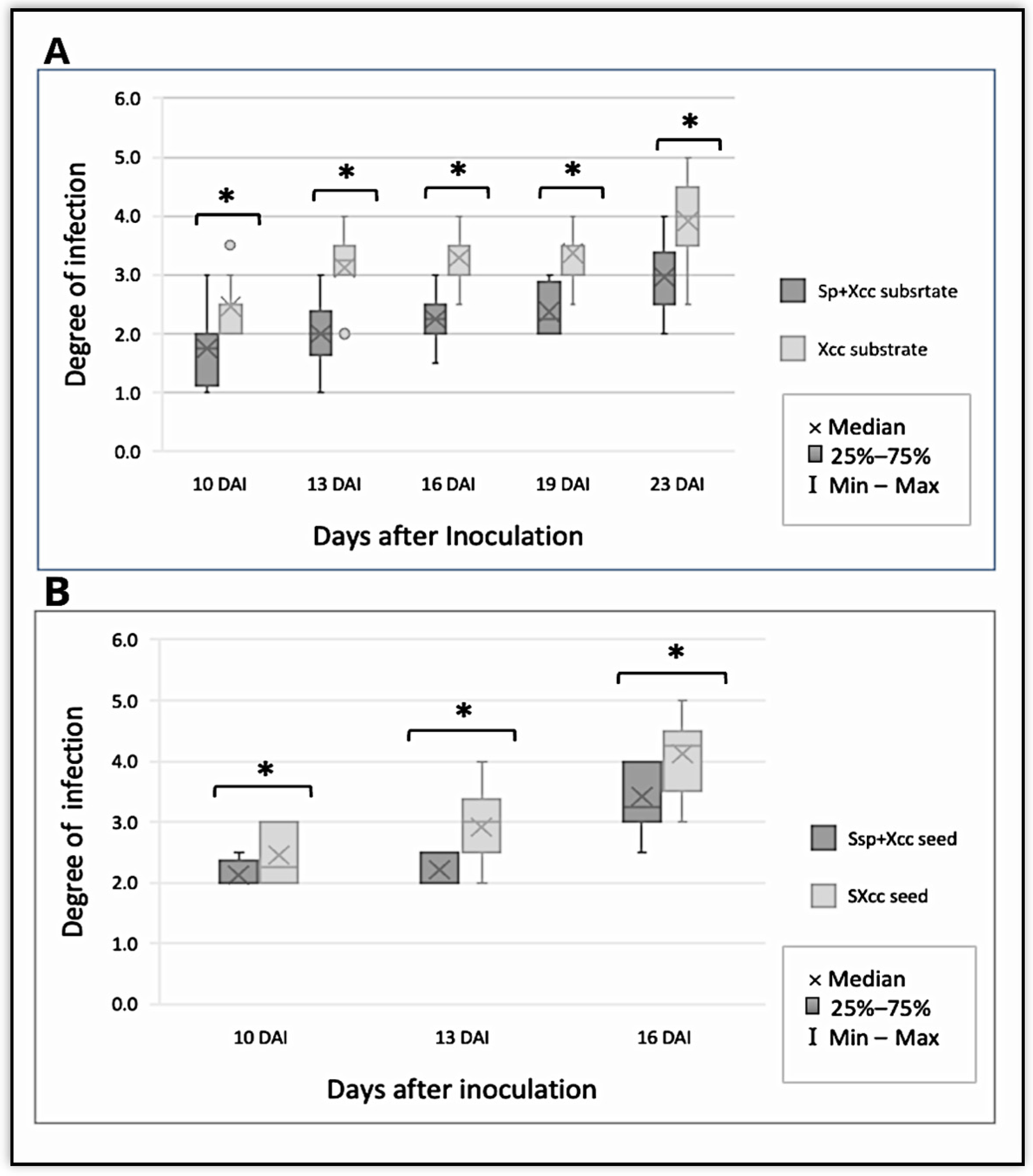
| Degree of infection | 1 | 2 | 3 | 4 | 5 |
| Affected surface | 0% | 10–24% | 25–50% | 51–75% | >75% |
| Treatment | Plant Height (mm) | Leaf Number | NDVI | Ft | Nitrogen % |
|---|---|---|---|---|---|
| Substrate treatment | |||||
| Sp | 193.91 (±4.35) a | 8.91 (±0.24) a | 0.64 (±0.003) a | 0.83 (±0.003) a | 4.71 (±0.15) a |
| C-H2O | 174.92 (±3.47) b | 8.37 (±0.26) ab | 0.61 (±0.005) b | 0.83 (±0.001) a | 4.08 (±0.10) c |
| Sp + Xcc | 202.76 (±1.83) a | 7.79 ( ±0.24) bc | 0.63 (±0.003) a | 0.82 (±0.002) b | 4.33 (±0.07) bc |
| Xcc | 181.92 (±2.67) b | 7.62 (±0.18) c | 0.59 (±0.007) c | 0.80 (±0.006) c | 4.57 (±0.11) ab |
| Seed treatment | |||||
| Ssp | 200.00 (±3.85) bc | 7.25 (±0.14) a | 0.63 (±0.004) a | 0.82 (±0.001) a | 6.17 (±0.08) a |
| SH2O | 191.72 (±3.08) c | 7.08 (±0.19) a | 0.60 (±0.004) c | 0.81 (±0.001) a | 5.66 (±0.09) b |
| Ssp + Xcc | 213.81 (±4.43) a | 6.41 (±0.18) b | 0.63 (±0.002) ab | 0.82 (±0.003) a | 5.03 (±0.09) c |
| SXcc | 203.48 (±5.11) ab | 6.33 (±0.34) b | 0.62 (±0.002) b | 0.81 (±0.002) a | 5.03 (±0.09) c |
| Treatment | APX (μmol min−1 g−1 FM) | GPX (μmol/tetraguaiacol min−1 g−1) | TAC (mML−1) | Phenols (mg GAE g−1 FM) |
|---|---|---|---|---|
| Substrate treatment | ||||
| Sp | 0.49 (±0.01) a | 9.66 (±0.47) b | 2.12 (± 0.06) a | 29.79 (±0.17) d |
| C-H2O | 0.50 (±0.04) a | 10.98 (±0.03) a | 1.70 (±0.15) a | 40.96 (±0.55) a |
| Sp + Xcc | 0.44 (±0.01) a | 9.68 (±0.09) b | 2.53 (± 0.26) a | 36.49 (±0.23) b |
| Xcc | 0.52 (±0.03) a | 8.32 (±0.25) c | 2.17 (± 0.17) a | 31.57 (±0.11) c |
| Seed treatment | ||||
| Ssp | 0.39 (±0.01) b | 4.86 (±0.01) c | 2.11 (± 0.13) a | 29.00 (±1.01) a |
| SH2O | 0.41 (±0.03) ab | 9.28 (±0.09) a | 2.34 (± 0.15) a | 29.21 (±0.08) a |
| Ssp + Xcc | 0.49 (±0.03) a | 9.51 (±0.05) a | 2.65 ( ±0.13) a | 29.41 (±0.34) a |
| SXcc | 0.29 (±0.03) c | 7.33 (±0.09) b | 2.07 (±0.10) b | 27.20 (±0.52) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, S.; Ragasova, L.N.; Tekielska, D.; Fidurski, M.; Sekara, A.; Pokluda, R. Serendipita indica as a Plant Growth Promoter and Biocontrol Agent against Black Rot Disease in Cabbage Grown in a Phytotron. Agriculture 2023, 13, 2048. https://doi.org/10.3390/agriculture13112048
Saleem S, Ragasova LN, Tekielska D, Fidurski M, Sekara A, Pokluda R. Serendipita indica as a Plant Growth Promoter and Biocontrol Agent against Black Rot Disease in Cabbage Grown in a Phytotron. Agriculture. 2023; 13(11):2048. https://doi.org/10.3390/agriculture13112048
Chicago/Turabian StyleSaleem, Sana, Lucia Nedorost Ragasova, Dorota Tekielska, Maciej Fidurski, Agnieszka Sekara, and Robert Pokluda. 2023. "Serendipita indica as a Plant Growth Promoter and Biocontrol Agent against Black Rot Disease in Cabbage Grown in a Phytotron" Agriculture 13, no. 11: 2048. https://doi.org/10.3390/agriculture13112048
APA StyleSaleem, S., Ragasova, L. N., Tekielska, D., Fidurski, M., Sekara, A., & Pokluda, R. (2023). Serendipita indica as a Plant Growth Promoter and Biocontrol Agent against Black Rot Disease in Cabbage Grown in a Phytotron. Agriculture, 13(11), 2048. https://doi.org/10.3390/agriculture13112048






