The Breeding of Waxy Sorghum Using Traditional Three-Line Method and Marker-Assisted Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Breeding Materials
2.1.1. Liangnuo No.1 F4 Inbreeding Line
2.1.2. Taiwanese Native Waxy Sorghum SB6
2.1.3. BTx623
2.1.4. F5 Waxy Lines from the TCR170617/SB6
2.1.5. Crop Management
2.2. DNA Extraction and Primer Sequence
2.2.1. DNA Extraction and PCR Conditions
2.2.2. Primer Sequence for Foreground Selection
2.3. Yield Trials
2.3.1. Preliminary Yield Trials
2.3.2. Advanced Yield Trials
2.3.3. Statistical Analysis
3. Results
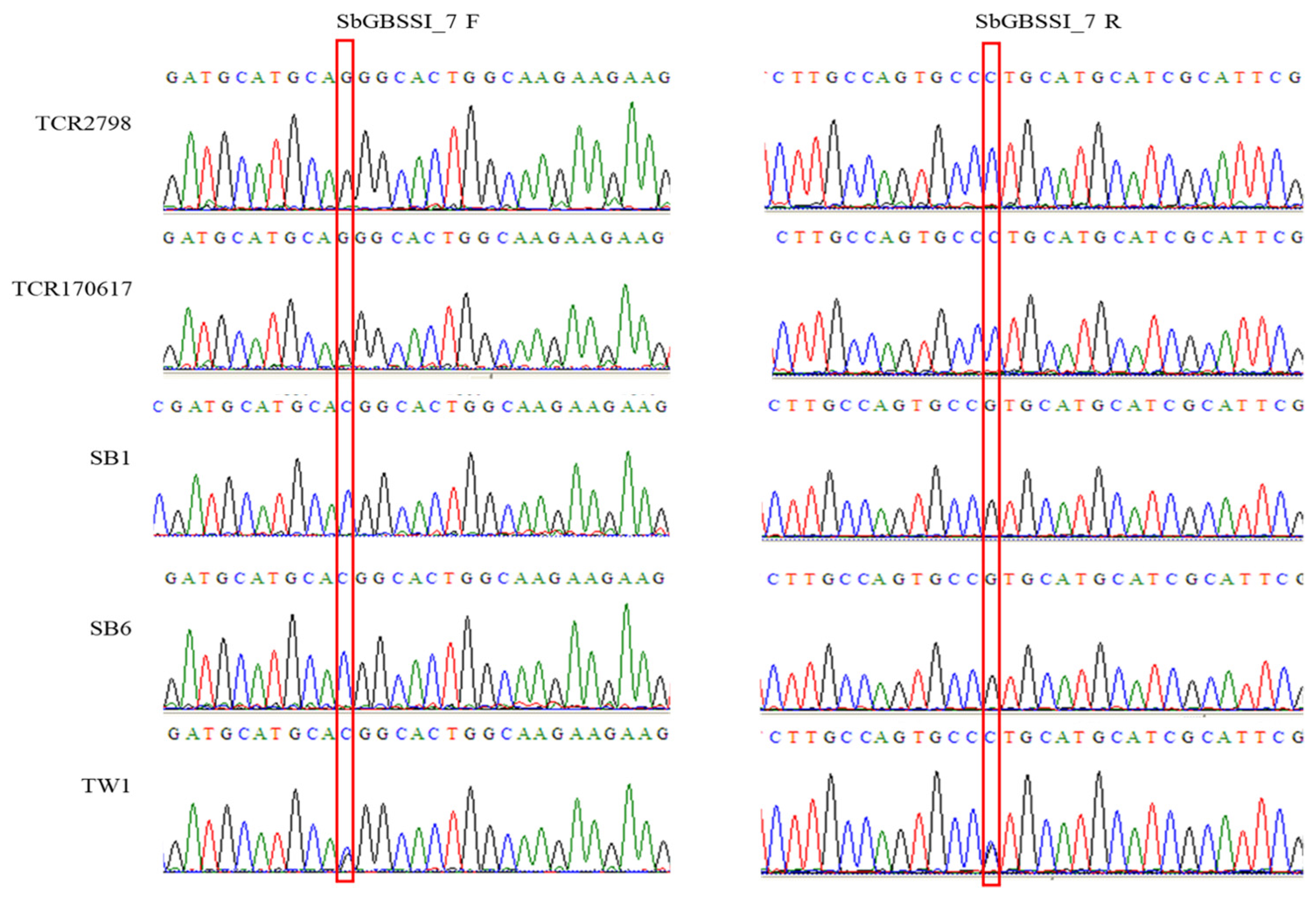
3.1. The Waxy Alleles wxa and wxc
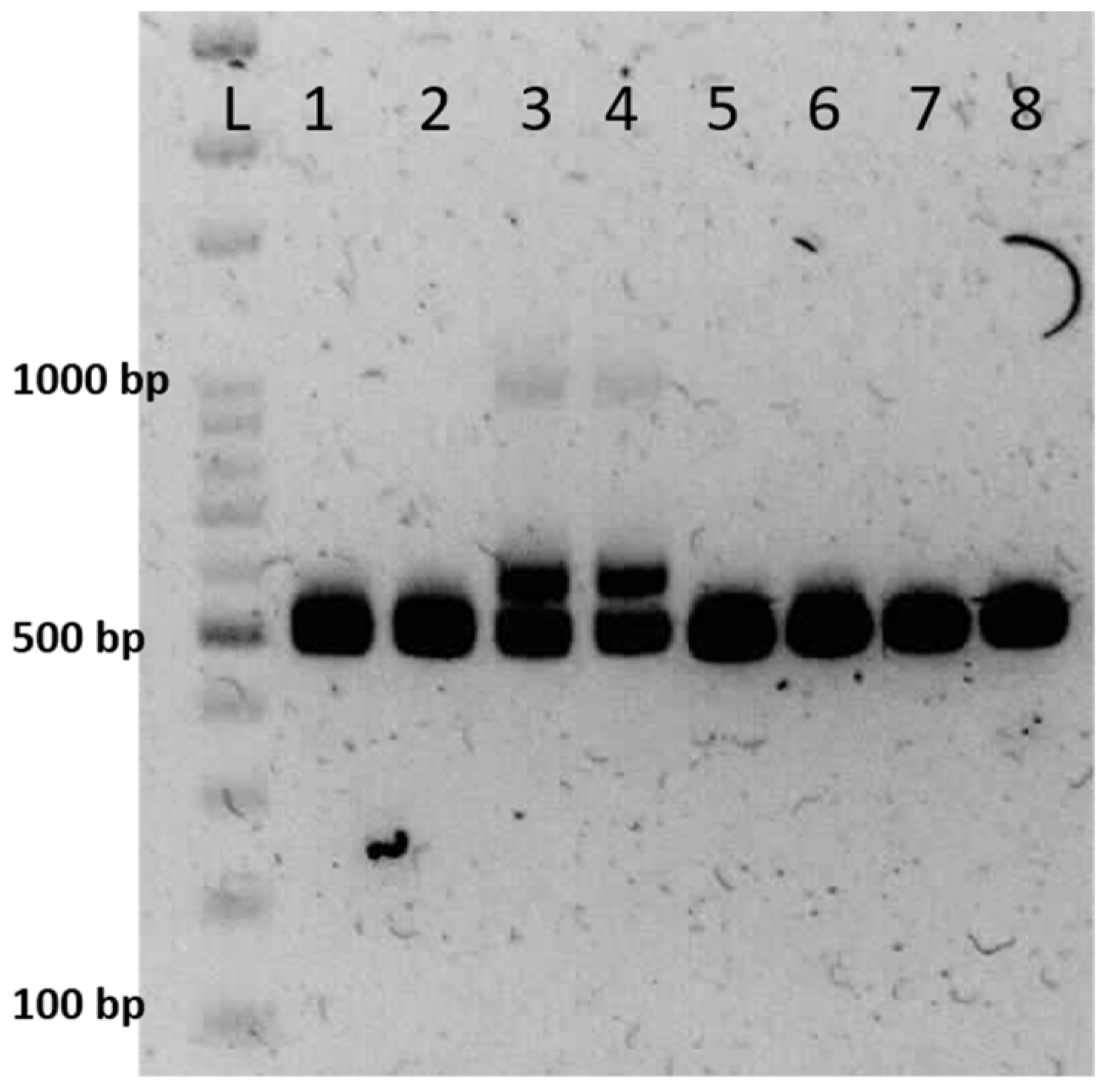
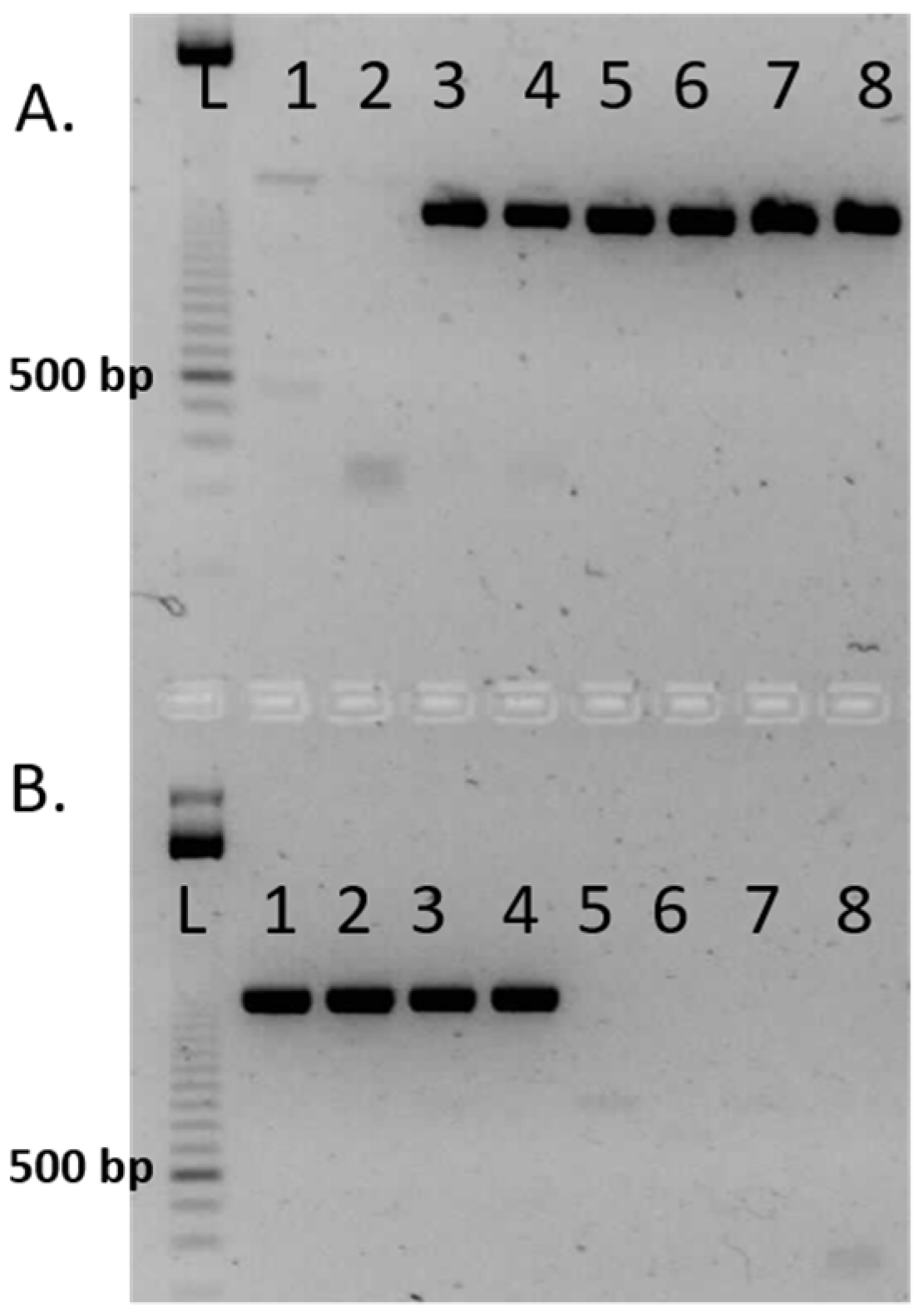
3.2. The Selection of A-Line
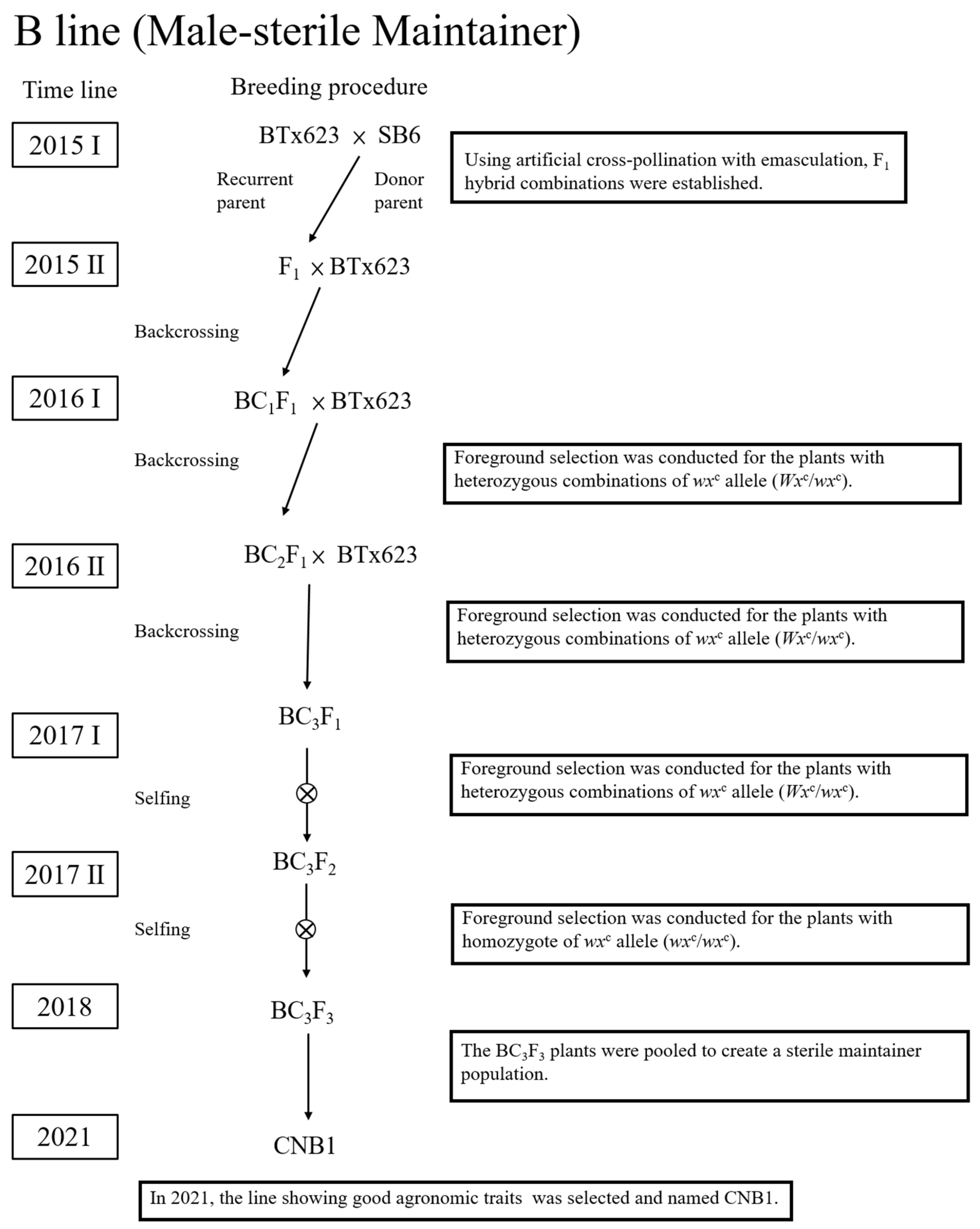
3.3. The Selection of B-Line
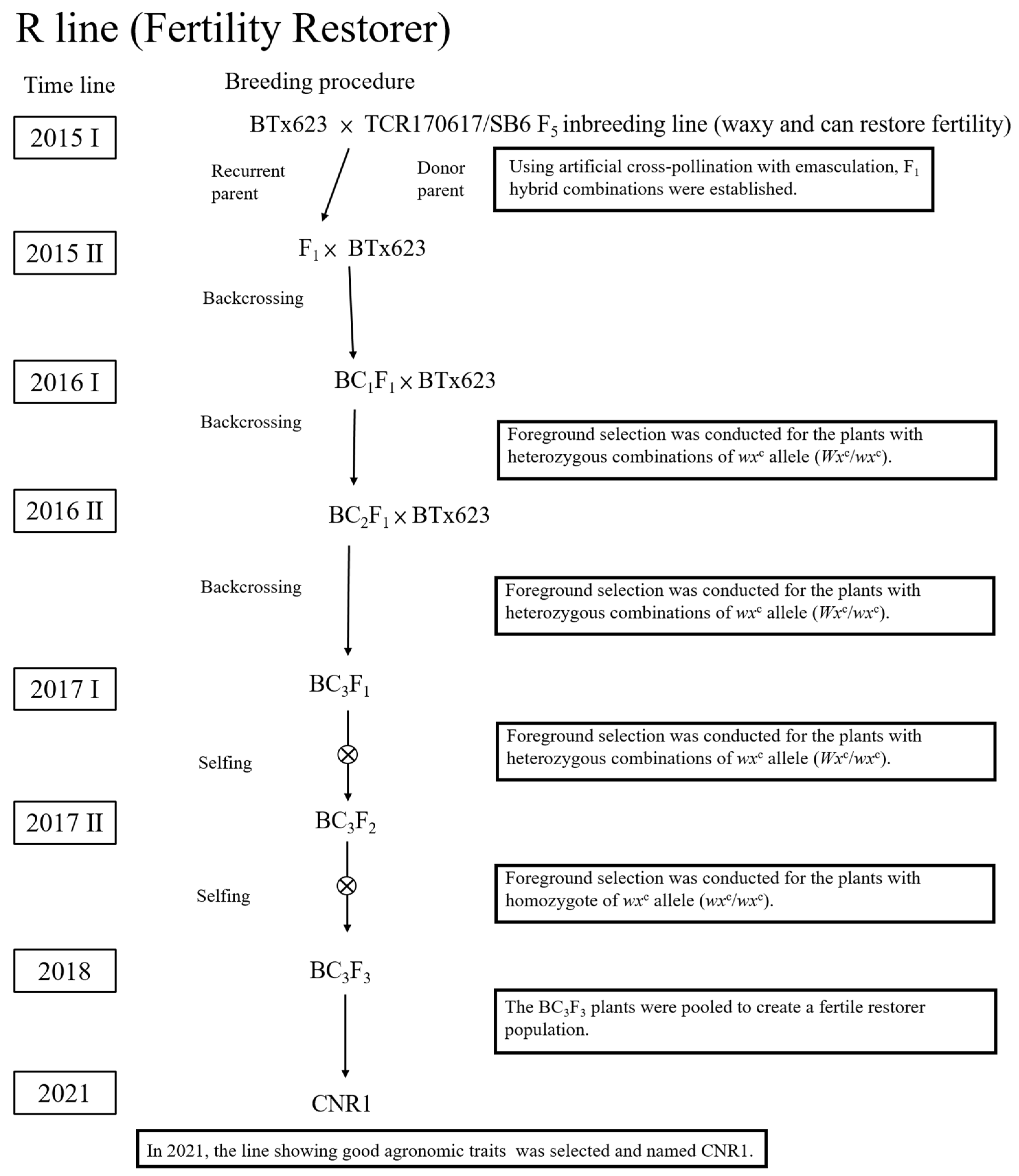
3.4. The Selection of R-Line
3.5. Agronomy Traits Analysis
3.5.1. Preliminary Trials
3.5.2. Advanced Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananda, G.K.; Myrans, H.; Norton, S.L.; Gleadow, R.; Furtado, A.; Henry, R.J. Wild sorghum as a promising resource for crop improvement. Front. Plant Sci. 2020, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/countryprofiles/en/ (accessed on 22 September 2022).
- Doggett, H. Sorghum, 2nd ed.; Longman: London, UK; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Dicko, M.H.; Gruppen, H.; Traoré, A.S.; Voragen, A.G.; Van Berkel, W.J. Sorghum grain as human food in Africa: Relevance of content of starch and amylase activities. Afr. J. Biotechnol. 2006, 5, 384–395. [Google Scholar]
- Rooney, W.L. Sorghum. In Cellulosic Energy Cropping Systems; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 109–129. [Google Scholar]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo-Lingani, H.; Owusu-Kwarteng, J.; Glover, R.; Diawara, B.; Jakobsen, M.; Jespersen, L. Sustainable production of African traditional beers with focus on dolo, a West African sorghum-based alcoholic beverage. Front. Sustain. Food Syst. 2021, 5, 672410. [Google Scholar] [CrossRef]
- Almodares, A.; Hadi, M.R.; Ahmadpour, H. Sorghum stem yield and soluble carbohydrates under different salinity levels. Afr. J. Biotechnol. 2008, 7, 4051–4055. [Google Scholar]
- Almodares, A.; Hadi, M.R. Production of bioethanol from sweet sorghum: A review. Afr. J. Agric. Res. 2009, 4, 772–780. [Google Scholar]
- McIntyre, C.L.; Drenth, J.; Gonzalez, N.; Henzell, R.G.; Jordan, D.R. Molecular characterization of the waxy locus in sorghum. Genome 2008, 51, 524–533. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Graybosch, R.A.; Funnell, D.L. Occurrence of the waxy alleles wxa and wxb in waxy sorghum plant introductions and their effect on starch thermal properties. Crop Sci. 2007, 47, 1927–1933. [Google Scholar] [CrossRef]
- Sattler, S.E.; Singh, J.; Haas, E.J.; Guo, L.; Sarath, G.; Pedersen, J.F. Two distinct waxy alleles impact the granule-bound starch synthase in sorghum. Mol. Breed. 2009, 24, 349–359. [Google Scholar] [CrossRef][Green Version]
- Kawahigashi, H.; Oshima, M.; Nishikawa, T.; Okuizumi, H.; Kasuga, S.; Yonemaru, J.I. A novel waxy allele in sorghum landraces in East Asia. Plant Breed. 2013, 132, 305–310. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, G.; Li, Y.; Fan, J.; Ding, G.; Zhao, J.; Wang, W. Identification of two novel waxy alleles and development of their molecular markers in sorghum. Genome 2013, 56, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, M.; Morino, K.; Nakajima, M.; Okagaki, R.J.; Wessler, S.R.; Izawa, T.; Shimamoto, K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 1998, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Vrinten, P.; Nakamura, T.; Yamamori, M. Molecular characterization of waxy mutations in wheat. Mol. Genet. Genom. 1999, 261, 463–471. [Google Scholar] [CrossRef]
- Saito, M.; Konda, M.; Vrinten, P.; Nakamura, K.; Nakamura, T. Molecular comparison of waxy null alleles in common wheat and identification of a unique null allele. Theor. Appl. Genet. 2004, 108, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Kiribuchi-Otobe, C.; Hirano, H.; Suzuki, Y.; Fujita, M. Detection of single nucleotide polymorphism (SNP) controlling the waxy character in wheat by using a derived cleaved amplified polymorphic sequence (dCAPS) marker. Theor. Appl. Genet. 2003, 107, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Domon, E.; Fuijita, M.; Ishikawa, N. The insertion/deletion polymorphisms in the waxy gene of barley genetic resources from East Asia. Theor. Appl. Genet. 2002, 104, 132–138. [Google Scholar] [CrossRef]
- Fukunaga, K.; Kawase, M.; Kato, K. Structural variation in the Waxy gene and differentiation in foxtail millet [Setaria italica (L.) P. Beauv.]: Implications for multiple origins of the waxy phenotype. Mol. Genet. Genom. 2002, 268, 214–222. [Google Scholar] [CrossRef]
- Varagona, M.J.; Purugganan, M.; Wessler, S.R. Alternative splicing induced by insertion of retrotransposons into the maize waxy gene. Plant Cell 1992, 4, 811–820. [Google Scholar]
- Marillonnet, S.; Wessler, S.R. Retrotransposon insertion into the maize waxy gene results in tissue-specific RNA processing. Plant Cell 1997, 9, 967–978. [Google Scholar] [CrossRef][Green Version]
- Yang, R.; Piao, Z.; Wan, C.; Lee, G.; Ruan, X.; Bai, J. Breeding for three-line japonica hybrid rice combinations with high resistant starch content using molecular marker-assisted selection. Breed. Sci. 2020, 70, 409–414. [Google Scholar] [CrossRef]
- Cao, L.Y.; Zhan, X.D. Chinese experiences in breeding three-line, two-line and superhybrid rice. In Rice: Germplasm, Genetics and Improvement; InTech: Rijeka, Croatia, 2014; pp. 279–308. [Google Scholar]
- Wu, Y.P.; Chang, Y.C.; Kuo, H.I.; Lin, B.N.; Wang, S.M.; Tseng, Y.C. The development of two high-yield and high-quality functional rice cultivars using marker-assisted selection and conventional breeding methods. Int. J. Mol. Sci. 2022, 23, 4678. [Google Scholar] [CrossRef] [PubMed]
- Boyles, R.E.; Brenton, Z.W.; Kresovich, S. Genetic and genomic resources of sorghum to connect genotype with phenotype in contrasting environments. Plant J. 2019, 97, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Baloch, F.S.; Altaf, M.T.; Liaqat, W.; Bedir, M.; Nadeem, M.A.; Cömertpay, G.; Sun, H.J. Recent advancements in the breeding of sorghum crop: Current status and future strategies for marker-assisted breeding. Front. Genet. 2023, 14, 1150616. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Li, Z.; Leng, C.; Lu, C.; Luo, H.; Liu, Y.; Jing, H.C. Sorghum breeding in the genomic era: Opportunities and challenges. Theor. Appl. Genet. 2021, 134, 1899–1924. [Google Scholar] [CrossRef] [PubMed]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Maize and sorghum as raw materials for brewing, a review. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Zhao, G.; Soe Htet, M.N.; Jia, Z.; Yan, Z.; Feng, B. Liquor flavour is associated with the physicochemical property and microbial diversity of fermented grains in waxy and non-waxy sorghum (Sorghum bicolor) during fermentation. Front. Microbiol. 2021, 12, 618458. [Google Scholar] [CrossRef]




| Line | Heading Days | Days to Harvesting | Height (cm) | Panicle Length (cm) | Panicle Weight (g) | Number of Seeds per Panicle | Seed Weight per Panicle (g) | Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|---|
| CNA1 | 60 a | 111 a | 148.5 ± 1.0 a | 31.1 ± 1.5 a | 69.61 ± 3.0 b | 1872 ± 38.6 c | 51.70 ± 0.2 b | 2913 ± 9.3 b |
| CNB1 | 56 b | 106 b | 151.9 ± 2.7 b | 31.2 ± 0.4 a | 96.12 ± 5.0 a | 3335 ± 11.0 a | 78.70 ± 2.8 a | 4434 ± 154.9 a |
| CNR1 | 53 c | 97 c | 132.5 ± 0.4 c | 30.9 ± 0.2 a | 91.95 ± 2.6 a | 2951 ± 95.2 b | 74.68 ± 2.1 a | 4310 ± 118.3 a |
| Line | Heading Days | Days to Harvesting | Height (cm) | Panicle Length (cm) | Panicle Weight (g) | Number of Seeds per Panicle | Seed Weight per Panicle (g) | Thousand-Seed Weight (g) |
|---|---|---|---|---|---|---|---|---|
| CNA1 | 59.75 ± 2.4 a | 109.00 ± 2.0 a | 110.36 ± 1.4 c | 31.95 ± 1.4 a | 28.54 ± 6.7 c | 398.4 ± 341 c | 8.58 ± 7.6 c | 20.82 ± 1.5 b |
| CNB1 | 60.50 ± 2.3 a | 107.00 ± 2.4 a | 115.68 ± 4.6 a | 29.85 ± 1.4 c | 109.22 ± 33.4 a | 3327.1 ± 1013 a | 83.86 ± 26 a | 25.15 ± 1.6 a |
| CNR1 | 56.75 ± 0.4 a | 104.75 ± 2.9 a | 102.45 ± 3.7 d | 30.74 ± 1.4 bc | 106.96 ± 27.4 a | 3137.3 ± 708 a | 85.46 ± 22.9 a | 27.08 ± 1.6 a |
| CNH1 | 59.00 ± 2.5 a | 106.00 ± 1.0 a | 112.71 ± 2.3 b | 30.90 ± 0.86 b | 88.40 ± 22.3 b | 2687.8 ± 545 b | 71.46 ± 19.6 b | 26.41 ± 1.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-P.; Chang, Y.-C.; Kuo, S.-C.; Liao, D.-J.; Shen, T.-Y.; Kuo, H.-I.; Wang, S.-W.; Tseng, Y.-C. The Breeding of Waxy Sorghum Using Traditional Three-Line Method and Marker-Assisted Selection. Agriculture 2023, 13, 2054. https://doi.org/10.3390/agriculture13112054
Wu Y-P, Chang Y-C, Kuo S-C, Liao D-J, Shen T-Y, Kuo H-I, Wang S-W, Tseng Y-C. The Breeding of Waxy Sorghum Using Traditional Three-Line Method and Marker-Assisted Selection. Agriculture. 2023; 13(11):2054. https://doi.org/10.3390/agriculture13112054
Chicago/Turabian StyleWu, Yong-Pei, Yu-Chi Chang, Su-Chen Kuo, Dah-Jing Liao, Ting-Yu Shen, Hsin-I Kuo, Sheng-Wen Wang, and Yu-Chien Tseng. 2023. "The Breeding of Waxy Sorghum Using Traditional Three-Line Method and Marker-Assisted Selection" Agriculture 13, no. 11: 2054. https://doi.org/10.3390/agriculture13112054
APA StyleWu, Y.-P., Chang, Y.-C., Kuo, S.-C., Liao, D.-J., Shen, T.-Y., Kuo, H.-I., Wang, S.-W., & Tseng, Y.-C. (2023). The Breeding of Waxy Sorghum Using Traditional Three-Line Method and Marker-Assisted Selection. Agriculture, 13(11), 2054. https://doi.org/10.3390/agriculture13112054






