Directing the Apple Rhizobiome toward Resiliency Post-Fumigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Collection and Preparation of Soil
2.3. ARD Bioassay
2.4. Evaluation of Composted Materials
2.5. Soil Amendments
2.6. Rootstock Genotype
2.7. Assessment of Rootstock Growth
2.8. 16S/ITS Sequencing for Assessment of Microbial Community Composition
2.9. Amendment Impacts on Soil Health Properties
2.10. (Artificial) Re-Infestation Experiment
2.11. Quantification of P. ultimum in Soil
3. Results
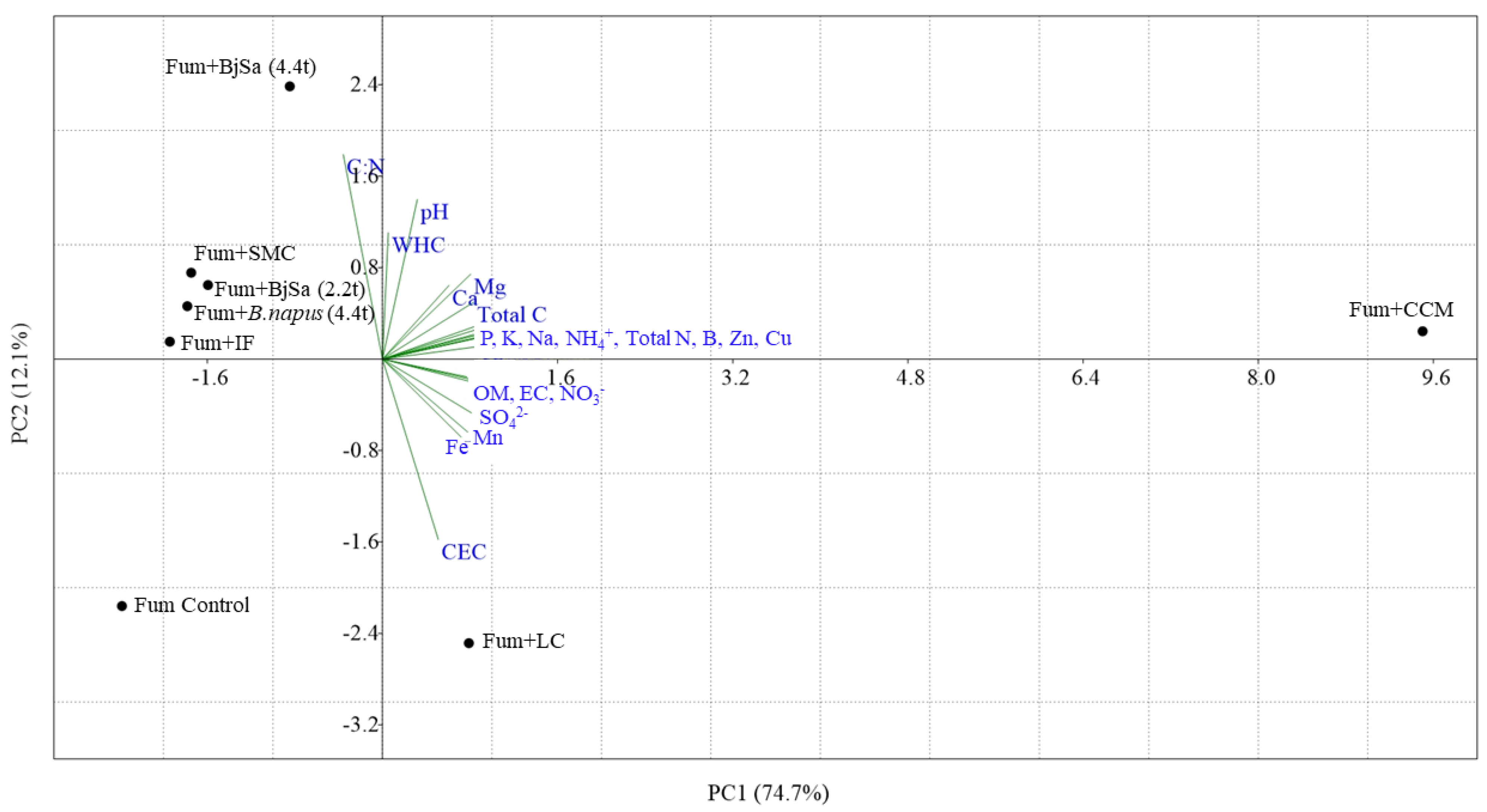
3.1. Amendment Impacts on Soil Health Properties
3.2. ARD Bioassay
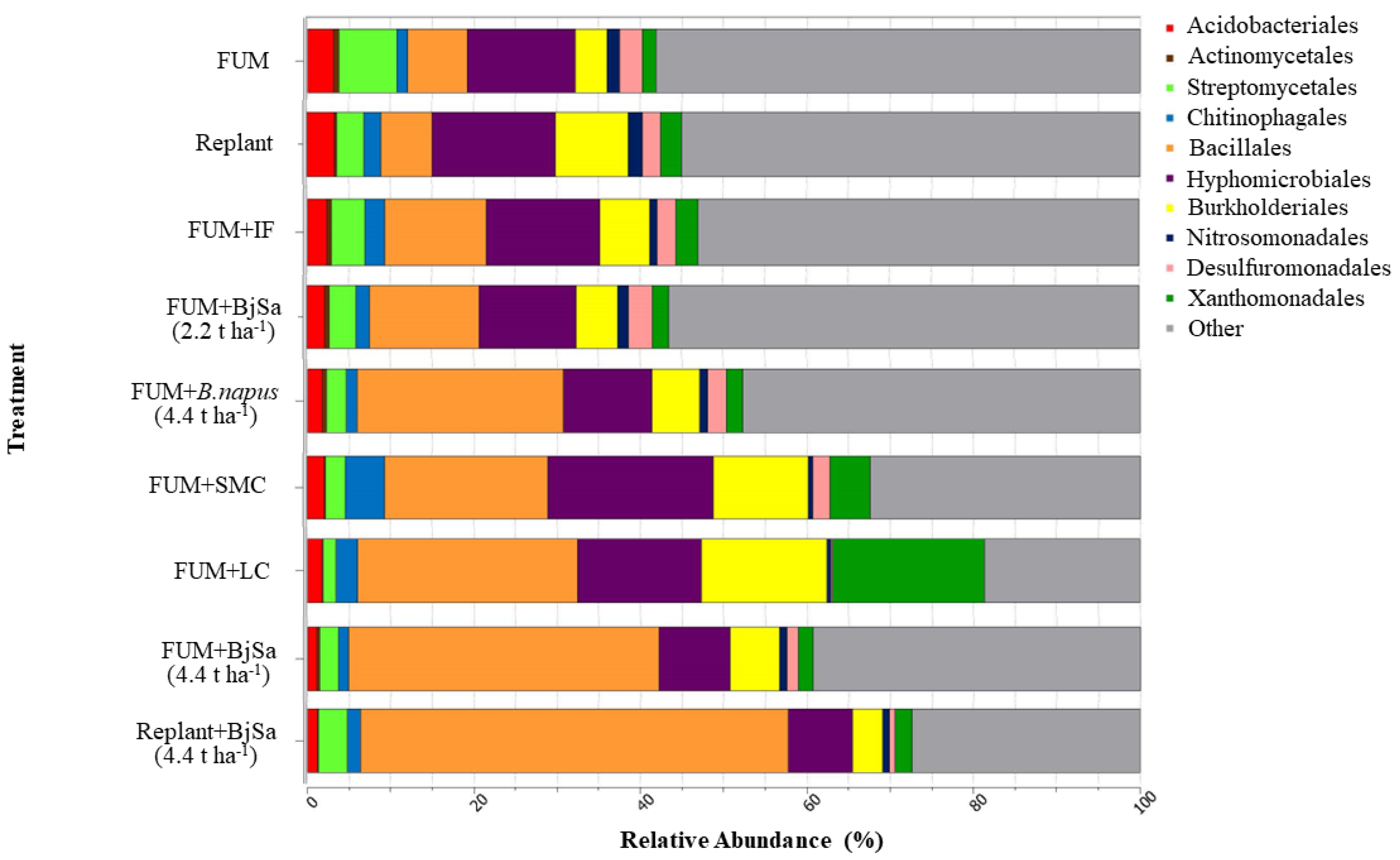
3.3. Amendment-Based Changes to Rhizosphere Microbial Community Composition (Four Weeks Post-Planting)
3.4. In-Depth Assessment of Microbial Community Sequence Data
3.5. Bacterial and Fungal Rhizosphere Community Trajectory at Harvest (8 Weeks Post-Planting)
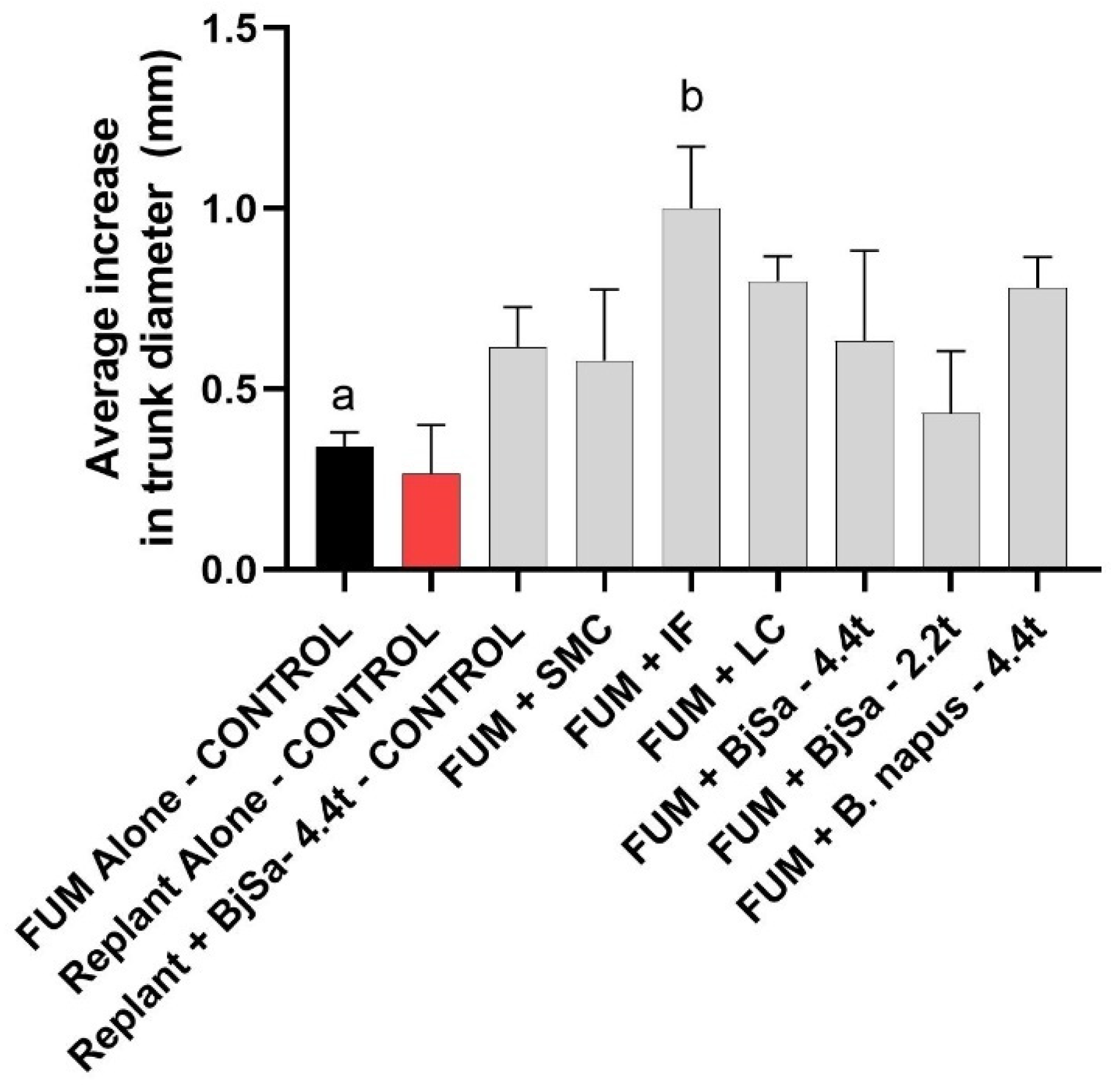
3.6. Impacts on Plant Fitness
3.7. (Artificial) Re-Infestation Experiment
3.8. Assessment of Postharvest Pathogens in Bulk Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzola, M.; Hewavitharana, S.S.; Strauss, S.L. Brassica seed meal soil amendments transform the rhizosphere microbiome and improve apple production through resistance to pathogen reinfestation. J. Phytopathol. 2015, 105, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Mazzola, M. Field evaluation of reduced rate Brassicaceae seed meal amendment and rootstock genotype on the microbiome and control of apple replant disease. J. Phytopathol. 2019, 109, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Luzzati, J.; Katan, J. Alternatives for soil fumigation in combating apple replant disease. Acta Hortic. 1998, 477, 107–114. [Google Scholar] [CrossRef]
- Braun, G.; Fuller, K.D.; McRae, K.; Fillmore, S.A.E. Response of ‘Honeycrisp®’ apple trees to combinations of pre-plant fumigation, deep ripping, and hog manure compost incorporation in a soil with replant disease. HortScience 2010, 45, 1702–1707. [Google Scholar] [CrossRef]
- Spiegel, Y.; Chet, I.; Cohn, E. Use of chitin for controlling plant plant-parasitic nematodes. Plant Soil 1987, 98, 337–345. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Juárez, M.F.-D.; Insam, H.; Schweizer, S.; Naef, A.; Topp, A.-R.; Kelderer, M.; Rühmer, T.; Baab, G.; Henfrey, J.; et al. Performance evaluation of locally available composts to reduce replant disease in apple orchards of central Europe. Renew. Agric. Food Syst. 2019, 34, 543–557. [Google Scholar] [CrossRef]
- Watson, T.T.; Nelson, L.M.; Neilsen, D.; Neilsen, G.H.; Forge, T.A. Soil amendments influence Pratylenchus penetrans populations, beneficial rhizosphere microorganisms, and growth of newly planted sweet cherry. Appl. Soil Ecol. 2017, 118, 212–220. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Pierson, E.A.; Jo, Y.-K. Host-mediated microbiome engineering (HMME) of drought tolerance in the wheat rhizosphere. PLoS ONE 2019, 14, e0225933. [Google Scholar] [CrossRef]
- Friesen, M.L.; Porter, S.S.; Stark, S.C.; von Wettberg, E.J.; Sachs, J.L.; Martinez-Romero, E. Microbially mediated plant functional traits. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 23–46. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- El-Ghaouth, A.; Smilanick, J.L.; Brown, G.E.; Ippolito, A.; Wisniewski, M.; Wilson, C.L. Application of Candida saitoana and glycolchitosan for the control of postharvest diseases of apple and citrus fruit under semi-commercial conditions. Plant Dis. 2000, 84, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. J. Phytopathol. 1998, 88, 930–938. [Google Scholar] [CrossRef]
- Dupont, T.; Granatstein, D. Compost Use for Tree Fruit. WSU Extension Factsheet, FS337E. 2020. Available online: http://treefruit.wsu.edu/publications/compost-use-for-tree-fruit/ (accessed on 17 May 2021).
- Van Horn, C.; Somera, T.S.; Mazzola, M. Comparative analysis of the rhizosphere and endophytic microbiomes across apple rootstock genotypes in replant orchard soils. Phytobiomes J. 2021, 5, 231–243. [Google Scholar] [CrossRef]
- Mazzola, M.; Gu, Y.H. Impact of wheat cultivation on microbial communities from replant soils and apple growth in greenhouse trials. J. Phytopathol. 2000, 90, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Somera, T.S.; Freilich, S.; Mazzola, M. Comprehensive analysis of the apple rhizobiome as influenced by different Brassica seed meals and rootstocks in the same soil/plant system. Appl. Soil Ecol. 2020, 157, 103766. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. ASD 2021, 41, 5. [Google Scholar] [CrossRef]
- Brown, J.; Davis, J.B.; Brown, D.A.; Seip, L.; Gosselin, T.; Wysocki, D. Registration of ‘Athena’ winter rapeseed. Crop Sci. 2004, 45, 800–801. [Google Scholar] [CrossRef]
- Brown, J.; Davis, J.B.; Brown, D.A.; Seip, L.; Gosselin, T. Registration of ‘Pacific Gold’ oriental condiment mustard. Crop Sci. 2004, 44, 2271–2272. [Google Scholar] [CrossRef]
- Brown, J.; Davis, J.B.; Erickson, D.A.; Brown, A.P.; Seip, L. Registration of ‘IdaGold’ mustard. Crop Sci. 1997, 38, 541. [Google Scholar]
- BC Tree Fruit Production Guide. BCFGA. Available online: https://www.bctfpg.ca/horticulture/fruit-tree-nutrition/ (accessed on 17 May 2021).
- Reig, G.; Lordan, J.; Miranda Sazo, M.; Hoying, S.; Fargione, M.; Reginato, G.; Donahue, D.J.; Francescatto, P.; Fazio, G.; Robinson, T. Long-term performance of ‘Gala’, Fuji’ and ‘Honeycrisp’ apple trees grafted on Geneva® rootstocks and trained to four production systems under New York State climatic conditions. Sci. Hort. 2019, 244, 277–293. [Google Scholar] [CrossRef]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr. Opin. Microbiol. 2018, 44, 41–49. [Google Scholar] [CrossRef]
- Mazzola, M.; Brown, J.; Zhao, X.; Fazio, G. Interaction of brassicaceous seed meal and apple rootstock on recovery of Pythium spp. and Pratylenchus penetrans from roots grown in replant soils. Plant Dis. 2009, 93, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. J. Phytopathol. 2001, 91, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, D.M.N.; Weerakoon, N.; Reardon, C. Long-term suppression of Pythium abappressorium induced by Brassica juncea seed meal amendment is biologically mediated. Soil Biol. Biochem. 2012, 51, 44–52. [Google Scholar] [CrossRef]
- Tambong, J.T.; Lévesque, C.A.; Nyoni, M.; Mazzola, M.; Wessels, J.P.B.; McLeod, A.; Van der Heyden, H.; Wallon, T.; Carisse, O.; Huang, D.; et al. Identification and quantification of pathogenic Pythium spp. from soils in eastern Washington using real-time polymerase chain reaction. Phytopathology 2006, 96, 637–647. [Google Scholar]
- Somera, T.S.; Mazzola, M. Toward a holistic view of orchard ecosystem dynamics: A comprehensive review of the multiple factors governing development or suppression of apple replant disease. Front. Microbiol. 2022, 13, 949404. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Kumar, M.; Kumari, P.; Reddy, C.; Jha, B. Solvent tolerant marine bacterium Bacillus aquimaris secreting organic solvent stable alkaline cellulase. Chemosphere 2011, 83, 706–712. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. An alkali-halotolerant cellulase from Bacillus flexus isolated from green seaweed Ulva lactuca. Carbohydr. Polym. 2011, 83, 891–897. [Google Scholar] [CrossRef]
- Puspasari, F.; Nurachman, Z.; Noer, A.S.; Radjasa, O.K.; van der Maarel, M.J.E.C.; Natalia, D. Characteristics of raw starch degrading α-amylase from Bacillus aquimaris MKSC 6.2 associated with soft coral Sinularia sp. Starch-Stärke 2011, 63, 461–467. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Role of bacteria in the management of plant parasitic nematodes: A review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- Jang, Y.-L.; Kim, S.G.; Kim, Y.H. Biocontrol efficacies of Bacillus species against Cylindrocarpon destructans causing ginseng root rot. Plant Pathol. J. 2011, 27, 333–341. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Q.; Liu, L.; Liu, H.; Wang, Y.; Peng, D.; Ruan, L.; Raymond, B.; Sun, M. Comparative genomics of Bacillus thuringiensis reveals a path to specialized exploitation of multiple invertebrate hosts. MBio 2017, 8, e00822-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Fu, X.; Li, Y.; Wang, Q. Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol. Technol. 2016, 115, 113–121. [Google Scholar] [CrossRef]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Zhang, F.; Zheng, D.; Chang, Y.; Xu, L.; Huang, L. Biocontrol activity of Bacillus velezensis D4 against apple Valsa canker. Biol. Control 2021, 163, 104760. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Z.; He, Y. Isolation, purification, and identification of antifungal protein produced by Bacillus subtilis SL-44 and anti-fungal resistance in apple. ESPR 2023, 30, 62080–62093. [Google Scholar] [CrossRef]
- Utkhede, R.S.; Smith, E.M. Promotion of apple tree growth and fruit production by the EBW-4 strain of Bacillus subtilis in apple replant disease soil. Can. J. Microbiol. 1992, 38, 1270–1273. [Google Scholar] [CrossRef]
- Mehta, P.; Chauhan, A.; Mahajan, R.; Mahajan, P.K.; Shirkot, C.K. Strain of Bacillus circulans isolated from apple rhizosphere showing plant growth promoting potential. Curr. Sci. 2010, 98, 538–542. [Google Scholar]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Brown, J. Efficacy of brassicaceous seed meal formulations for the control of apple replant disease in conventional and organic production systems. Plant Dis. 2010, 94, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, C.; Fang, D.; He, X.; Wei, M.; Zhuo, C.; Jin, J.; Shen, B.; Li, R.; Ling, N.; et al. Manipulating the soil microbiomes during a community recovery process with plant beneficial species for the suppression of Fusarium wilt of watermelon. AMB Express 2021, 11, 87. [Google Scholar] [CrossRef]
- Cohen, M.F.; Mazzola, M. Resident bacteria, nitric oxide emission and particle size modulate the effect of Brassica napus seed meal on disease incited by Rhizoctonia solani and Pythium spp. Plant Soil 2006, 286, 75–86. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
- Khalil, M.I.; Youssef, S.A.; Tartoura, K.A.; Eldesoky, A.A. Comparative evaluation of physiological and biochemical alteration in tomato plants infected by Alternaria alternata in response to Trichoderma viride and Chaetomium globosum application. Physiol. Mol. Plant Pathol. 2021, 115, 101671. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Anke, H.; Stadler, M.; Mayer, A.; Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nemato, phagous fungi and Ascomycetes. Can. J. Bot. 1995, 73, 932–939. [Google Scholar] [CrossRef]
- Kuvarina, A.E.; Gavryushina, I.A.; Kulko, A.B.; Ivanov, I.A.; Rogozhin, E.A.; Georgieva, M.L.; Sadykova, V.S. The Emericellipsins A–E from an alkalophilic fungus Emericellopsis alkalina show potent activity against multidrug-resistant pathogenic fungi. J. Fungi 2021, 7, 153. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Production of chitinase from thermophilic Humicola grisea and its application in production of bioactive chitooligosaccharides. Int. J. Biol. Macromol. 2017, 104, 1641–1647. [Google Scholar] [CrossRef]
- Sneh, B.; Humble, S.J.; Lockwood, J.L. Parasitism of oospores of Phytophthora megasperma var. sojae, P. cactorum, Pythium sp., and Aphanomyces euteiches in soil by Oomycetes, Chytridiomycetes, Hyphomycetes, Actinomycetes, and Bacteria. Phytopathology 1977, 67, 622–628. [Google Scholar] [CrossRef]
- Boonsang, N.; Dethoup, T.; Singburaudom, N.; Gomes, N.G.M.; Kijjoa, A. In vitro antifungal activity screening of crude extracts of soil fungi against plant pathogenic fungi. J. Biopest. 2014, 7, 156. [Google Scholar]
- Mestre, M.C.; Fontenla, S.; Bruzone, M.C.; Fernández, N.V.; Dames, J. Detection of plant growth enhancing features in psychrotolerant yeasts from Patagonia (Argentina). J. Basic Microbiol. 2016, 56, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.J.R.; Stewart, A. Glasshouse screening for biological control agents of Phytophthora cactorum on apple (Malus domestica). N. Z. J. Crop Hortic. Sci. 2001, 29, 159–169. [Google Scholar] [CrossRef]
- Lira, V.L.; Santos, D.V.; Barbosa, R.N.; Costa, A.F.; Maia, L.C.; Moura, R.M. Biocontrol potential of fungal filtrates on the reniform nematode (Rotylenchulus reniformis) in coriander and cowpea. Nematropica 2020, 50, 86–95. [Google Scholar]
- Eroshin, V.K.; Dedyukhina, E.G. Effect of lipids from Mortierella hygrophila on plant resistance to phytopathogens. World J. Microbiol. Biotechnol. 2002, 18, 165–167. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Chooi, Y.H.; Cacho, R.; Tang, Y. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem. Biol. 2010, 17, 483–494. [Google Scholar] [CrossRef]
- Vaartaja, O.; Agnihotri, V.P. Interaction of nutrients and four antifungal antibiotics in their effects on Pythium species in vitro and in soil. Plant Soil 1969, 30, 49–61. [Google Scholar] [CrossRef]
- Davis, R.F.; Backman, P.A.; Rodriguez-Kabana, R.; Kokalis-Burelle, N. Biological control of apple fruit diseases by Chaetomium globosum formulations containing cellulose. Biol. Control 1992, 2, 118–123. [Google Scholar] [CrossRef]
- Di Pietro, A.; Gut-Rella, M.; Pachlatko, J.P.; Schwinn, F.J. Role of antibiotics produced by Chaetomium globosum in biocontrol of Pythium ultimum, a causal agent of damping-off. Phytopathology 1992, 82, 131–135. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Vannacci, G. Preharvest application of beneficial fungi as a strategy to prevent postharvest mycotoxin contamination: A review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Berka, R.M.; Grigoriev, I.V.; Otillar, R.; Salamov, A.; Grimwood, J.; Reid, I.; Ishmael, N.; John, T.; Darmond, C.; Moisan, M.C.; et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 2011, 29, 922–927. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. AEM 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]



| Management Strategy | Annual Cost (USD) |
|---|---|
| Conventional soil fumigation | 700–1700 |
| Weed control (labor + materials; 2 applications per year) | 200–1012 |
| Fertilizer (labor + materials) | 60α |
| ab Brassica seed meal (4.4 t ha−1) | 1380–3482 |
| ab Brassica seed meal (2.2 t ha−1) | 700–1700 |
| a Shiitake mushroom compost (2% v:v) * | 940 |
| Insect (mealworm) frass (3.2 t ha−1) | 920 |
| a Composted chicken manure (1.2 t ha−1) | 50 |
| a Chitin-based amendment (20–40 L ha−1) | 20–40 |
| Treatment | Application Rate | Notes |
|---|---|---|
| Fumigated replant soil | NA | Disease-conducive control |
| Unfumigated replant soil | NA | Disease-conducive control |
| Unfumigated replant soil + BjSa SM | 4.4 ton ha−1 | Disease-suppressive control |
| FUM + BjSa SM | 4.4 ton ha−1 | |
| FUM + BjSa SM | 2.2 ton ha−1 | |
| FUM + B. napus SM | 4.4 ton ha−1 | |
| FUM + SMC | 2% v/v | Rate estimated based on cost |
| FUM + IF | 3.2 ton ha−1 | Rate recommended by mfr. |
| FUM + CCM | 1.7 ton ha−1 | Rate estimated based on EC |
| FUM + LC | 20 L ha−1 | Rate recommended by mfr. |
| Treatment | pH | EC § | CEC † | Na # | Ca # | Mg # | K # | WHC (in/ft) | OM (%) | Total N (%) | Total C (%) | C:N | NO3 ‡ | NH4 ‡ | SO42 ‡ | P ‡ | K ‡ | B ‡ | Zn ‡ | Mn ‡ | Cu ‡ | Fe ‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fumigated replant soil * | 7.20 | 0.29 | 8.80 | 1.00 | 84.10 | 22.50 | 16.80 | 1.51 | 1.70 | 0.09 | 0.69 | 7.60 | 14.50 | 3.60 | 17 | 24 | 578 | 0.23 | 6.20 | 2.70 | 0.90 | 17 |
| Fumigated replant soil | 6.00 | 0.30 | 9.10 | 1.30 | 56.70 | 16.20 | 11.10 | 1.28 | 1.40 | 0.08 | 0.65 | 8.70 | 7.90 | 1.10 | 28 | 16 | 393 | 0.17 | 6.00 | 1.70 | 0.60 | 24 |
| Unfumigated replant soil | 6.20 | 0.68 | 11.20 | 1.10 | 57.70 | 16.10 | 14.80 | 1.78 | 1.60 | 0.11 | 0.96 | 9.00 | 23.10 | 1.60 | 64 | 41 | 645 | 0.30 | 10.50 | 1.70 | 2.60 | 27 |
| Unfumigated replant soil + BjSa SM (4.4 t ha−1) | 5.90 | 0.59 | 10.80 | 1.20 | 64.00 | 18.10 | 17.40 | 1.75 | 1.60 | 0.11 | 0.99 | 9.30 | 44.90 | 2.80 | 80 | 41 | 737 | 0.35 | 10.30 | 4.50 | 3.00 | 37 |
| FUM + BjSa SM (2.2 t ha−1) | 7.00 | 0.45 | 8.00 | 2.70 | 72.40 | 21.80 | 16.90 | 1.40 | 1.40 | 0.10 | 0.89 | 9.30 | 34.80 | 13.50 | 38 | 40 | 531 | 0.24 | 7.10 | 2.20 | 1.10 | 14 |
| FUM + BjSa SM (4.4 t ha−1) | 6.60 | 0.74 | 7.80 | 4.90 | 77.70 | 25.80 | 23.10 | 1.95 | 1.50 | 0.11 | 1.12 | 10.20 | 69.50 | 14.30 | 55 | 40 | 706 | 0.32 | 7.00 | 3.70 | 1.40 | 17 |
| FUM + B.napus SM (4.4 t ha−1) | 6.60 | 0.53 | 8.20 | 1.50 | 71.00 | 21.40 | 14.90 | 1.38 | 1.30 | 0.08 | 0.79 | 10.10 | 79.70 | 1.40 | 32 | 21 | 477 | 0.25 | 6.60 | 2.60 | 0.70 | 27 |
| FUM + SMC | 7.50 | 0.36 | 8.50 | 1.20 | 71.50 | 20.90 | 14.10 | 1.41 | 1.90 | 0.09 | 0.89 | 9.70 | 2.50 | 4.50 | 16 | 23 | 471 | 0.15 | 5.90 | 1.80 | 0.50 | 13 |
| FUM + IF | 7.10 | 0.05 | 8.70 | 1.20 | 66.10 | 20.80 | 14.40 | 1.50 | 1.60 | 0.08 | 0.78 | 9.30 | 16.00 | 1.50 | 23 | 40 | 489 | 0.17 | 6.90 | 1.20 | 0.60 | 13 |
| FUM + CCM | 7.80 | 3.29 | 9.50 | 29.50 | 84.20 | 39.50 | 87.70 | 1.46 | 2.60 | 0.23 | 1.96 | 8.70 | 277.00 | 266.80 | 271 | 157 | 3262 | 3.35 | 25.10 | 29.60 | 15.00 | 43 |
| FUM + LC | 5.20 | 1.56 | 9.30 | 2.70 | 79.80 | 20.90 | 13.10 | 1.57 | 1.90 | 0.12 | 0.97 | 8.30 | 147.50 | 8.50 | 168 | 44 | 474 | 0.15 | 7.80 | 21.50 | 1.00 | 28 |
| Postharvest Fungal Pathogen | Unfumigated | Unfumigated + 4.4 t BjSa SM | Fumigated | FUM + IF | FUM +SMC | FUM+LC | FUM + 4.4 t BjSa SM |
|---|---|---|---|---|---|---|---|
| Alternaria alternata | 0.17 * | 0.02 † | 0.05 † | 1.25 | 0.03 † | 0.01 *,† | 0.04 † |
| Aspergillus parasiticus | 0 | 0 | 0.01 | 0.01 | 0.01 † | 0 | 0 |
| Aspergillus niger | 0.01 | 2.14 | 0.01 | 0 | 0.01 | 0.01 | 0.01 |
| Cladosporium cladosporioides | 0.04 | 0.06 | 0.02 | 0.02 | 0 *,† | 0.01 | 0.09 |
| Glomerella cingulata | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucor circinelloides | 0 | 0 | 0 | 0 | 0 | 0.07 | 0 |
| Penicillium expansum | 0 | 0 | 0 | 0.17† | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somera, T.; Mazzola, M.; Cook, C. Directing the Apple Rhizobiome toward Resiliency Post-Fumigation. Agriculture 2023, 13, 2104. https://doi.org/10.3390/agriculture13112104
Somera T, Mazzola M, Cook C. Directing the Apple Rhizobiome toward Resiliency Post-Fumigation. Agriculture. 2023; 13(11):2104. https://doi.org/10.3390/agriculture13112104
Chicago/Turabian StyleSomera, Tracey, Mark Mazzola, and Chris Cook. 2023. "Directing the Apple Rhizobiome toward Resiliency Post-Fumigation" Agriculture 13, no. 11: 2104. https://doi.org/10.3390/agriculture13112104
APA StyleSomera, T., Mazzola, M., & Cook, C. (2023). Directing the Apple Rhizobiome toward Resiliency Post-Fumigation. Agriculture, 13(11), 2104. https://doi.org/10.3390/agriculture13112104








