Variability in Stomatal Adaptation to Drought among Grapevine Cultivars: Genotype-Dependent Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Stomatal Density in Developing Leaves
2.3. RNA Isolation and RT-qPCR Analysis of Target Genes Involved in Stomatal Development
2.4. Statistical Analysis
3. Results
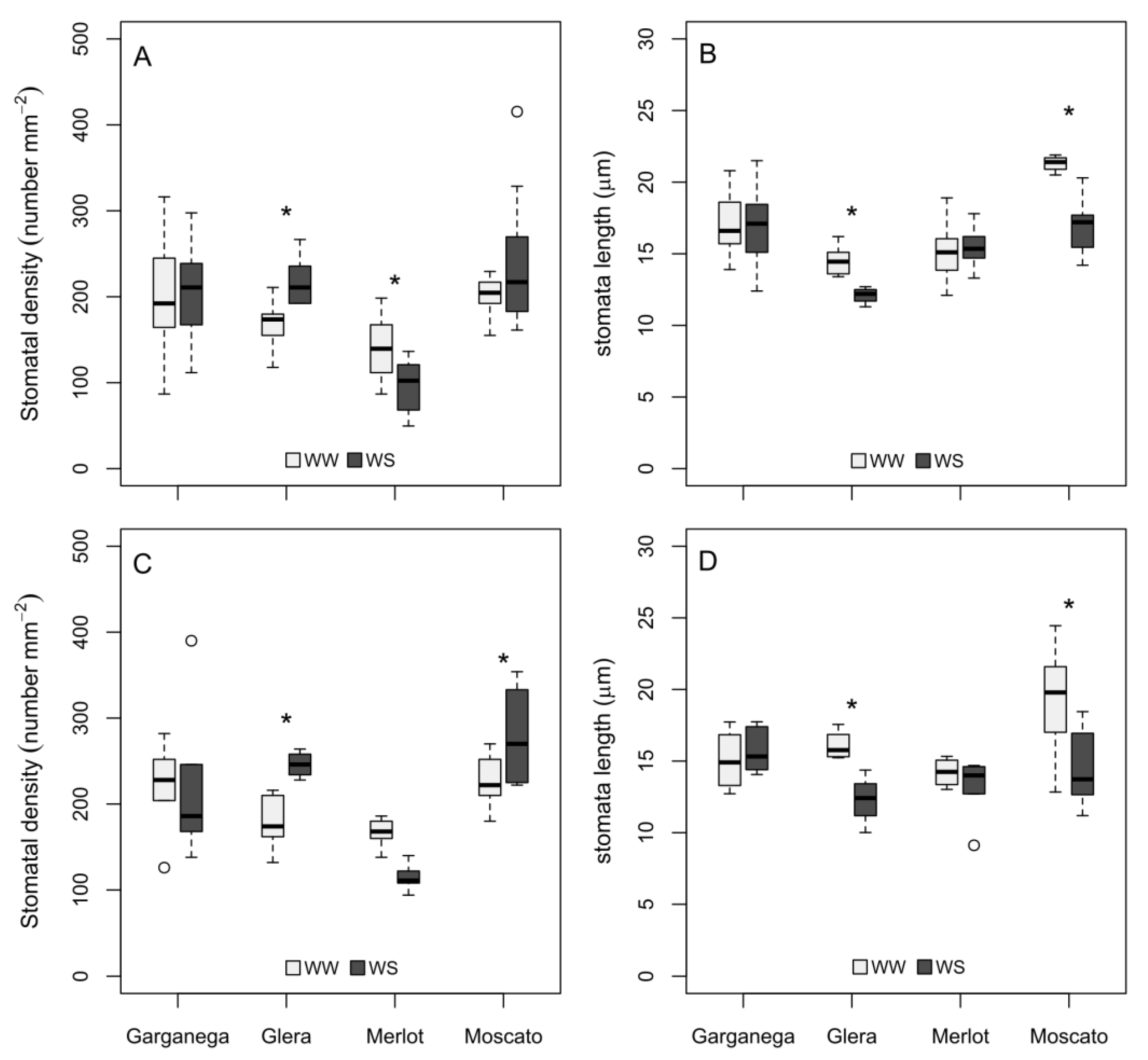
3.1. Stomatal Density and Size in Developing Leaves
3.2. Expression of Target Genes Involved in Water Stress Sensing and Stomagenesis in Developing Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spinoni, J.; Naumann, G.; Vogt, J.; Barbosa, P. Meteorological Droughts in Europe: Events and Impacts: Past Trends and Future Projection; European Union: Luxembourg, 2016. [Google Scholar]
- Düring, H. Stomatal Adaptation of Leaves to Drought. Vitis 1990, 29, 366–370. [Google Scholar]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1998, 42, 16. [Google Scholar] [CrossRef]
- Galmés, J.; Ochogavía, J.M.; Gago, J.; Roldán, E.J.; Cifre, J.; Conesa, M.À. Leaf Responses to Drought Stress in Mediterranean Accessions of Solanum Lycopersicum: Anatomical Adaptations in Relation to Gas Exchange Parameters. Plant Cell Environ. 2013, 36, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Bota, J.; Tomás, M.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among Grapevine Cultivars in Their Stomatal Behavior and Water Use Efficiency under Progressive Water Stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Tortosa, I.; Escalona, J.M.; Bota, J.; Tomás, M.; Hernández, E.; Escudero, E.G.; Medrano, H. Exploring the Genetic Variability in Water Use Efficiency: Evaluation of Inter and Intra Cultivar Genetic Diversity in Grapevines. Plant Sci. 2016, 251, 35–43. [Google Scholar] [CrossRef]
- Levin, A.D.; Williams, L.E.; Matthews, M.A. A Continuum of Stomatal Responses to Water Deficits among 17 Wine Grape Cultivars (Vitis vinifera). Funct. Plant Biol. 2019, 47, 11–25. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Yang, H.M.; Wang, G.X. Leaf Stomatal Densities and Distribution in Triticum Aestivum under Drought and CO2 Enrichment. Chin. J. Plant Ecol. 2001, 25, 312. [Google Scholar]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative Impacts of Water Stress on the Leaf Anatomy of a Drought-Resistant and a Drought-Sensitive Olive Cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Laajimi, N.O.; Boussadia, O.; Skhiri, F.H.; Teixeira da Silva, J.A.; Rezgui, S.; Hellali, R. Anatomical Adaptations in Vegetative Structures of Apricot Tree (Prunus armeniaca L.) Cv. “Amor El Euch” Grown under Water Stress. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 46–51. [Google Scholar]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of Stress on the Leaf Anatomy of Sugarcane Cultivars with Different Drought Tolerance (Saccharum officinarum, Poaceae). Rev. Biol. Trop. 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Hardie, W.J.; Smith, J.P. Stomatal Density of Grapevine Leaves (Vitis vinifera L.) Responds to Soil Temperature and Atmospheric Carbon Dioxide. Aust. J. Grape Wine Res. 2011, 17, 147–152. [Google Scholar] [CrossRef]
- Gokbayrak, Z.; Dardeniz, A.; Bal, M. Stomatal Density Adaptation of Grapevine to Windy Conditions. Trakia J. Sci. 2008, 6, 41–60. [Google Scholar]
- Palliotti, A.; Silvestroni, O.; Petoumenou, D.; Vignaroli, S.; Berrios, J.G. Evaluation of Low-Energy Demand Adaptive Mechanisms in Sangiovese Grapevine during Drought. OENO One 2008, 42, 41–47. [Google Scholar] [CrossRef]
- Theodorou, N.; Koundouras, S.; Zioziou, E.; Nikolaou, N. Responses of Leaf Stomatal Density and Anatomy to Water Deficit in Four Winegrape Cultivars (Vitis vinifera L.). In Proceedings of the 3rd Internetional Ampelos Symposyum, Santorini, Greece, 30–31 May 2013. [Google Scholar]
- Montoro, A.; López-Urrea, R.; Fereres, E. Role of Stomata Density in the Water Use of Grapevines. Acta Hortic. 2016, 1115, 41–47. [Google Scholar] [CrossRef]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of environmental factors light, CO2, temperature, and relative humidity on stomatal opening and development: A review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Herrmann, A.; Torii, K.U. Shouting out loud: Signaling modules in the regulation of stomatal development. Plant Physiol. 2021, 185, 765–780. [Google Scholar] [CrossRef]
- Jewaria, P.K.; Hara, T.; Tanaka, H.; Kondo, T.; Betsuyaku, S.; Sawa, S.; Sakagami, Y.; Aimoto, S.; Kakimoto, T. Differential Effects of the Peptides Stomagen, EPF1 and EPF2 on Activation of MAP Kinase MPK6 and the SPCH Protein Level. Plant Cell Physiol. 2013, 54, 1253–1262. [Google Scholar] [CrossRef]
- Zoulias, N.; Harrison, E.L.; Casson, S.A.; Gray, J.E. Molecular Control of Stomatal Development. Biochem. Eng. J. 2018, 475, 441–454. [Google Scholar] [CrossRef]
- Clemens, M.; Faralli, M.; Lagreze, J.; Bontempo, L.; Piazza, S.; Varotto, C.; Malnoy, M.; Oechel, W.; Rizzoli, A.; Dalla Costa, L. VvEPFL9-1 Knock-Out via CRISPR/Cas9 Reduces Stomatal Density in Grapevine. Front. Plant Sci. 2022, 13, 878001. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the brakes on: Abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014, 202, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Balestrini, R.; Vitali, M.; Pagliarani, C.; Perrone, I.; Schubert, A.; Lovisolo, C. Gene Expression in Vessel-Associated Cells upon Xylem Embolism Repair in Vitis vinifera L. Petioles. Planta 2014, 239, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Pagliarani, C.; Moine, A.; Chitarra, W.; Nerva, L.; Catoni, M.; Tavazza, R.; Matić, S.; Vallino, M.; Secchi, F.; Noris, E. The C4 protein of tomato yellow leaf curl Sardinia virus primes drought tolerance in tomato through morphological adjustments. Hortic. Res. 2022, 9, uhac164. [Google Scholar] [CrossRef] [PubMed]
- Scienza, A. Atlante Geologico Dei Vini d’Italia: Vitigno, Suolo e Fattori Climatici; Giunti: Firenze, Italy, 2015; ISBN 9788809883222. [Google Scholar]
- Gaiotti, F.; Nerva, L.; Fila, G.; Lovat, L.; Belfiore, N.; Chitarra, W. Comparative effects of drought stress on leaf gas exchange, foliar ABA and leaf orientation in four grapevine cultivars grown in Northern Italy. Physiol. Plant. 2023, 175, e14063. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera) Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.P. Vine Water Status Is a Key Factor in Grape Ripening and Vintage Quality for Red Bordeaux Wine. How Can It Be Assessed for Vineyard Management Purposes. J. Int. Sci. Vigne Vin. 2009, 43, 121–134. [Google Scholar]
- Miras-Avalos, J.M.; Araujo, E.S. Optimization of Vineyard Water Management: Challenges, Strategies, and Perspectives. Water 2021, 13, 746. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Nerva, L.; Giudice, G.; Quiroga, G.; Belfiore, N.; Lovat, L.; Perria, R.; Volpe, M.G.; Moffa, L.; Sandrini, M.; Gaiotti, F.; et al. Mycorrhizal Symbiosis Balances Rootstock-Mediated Growth-Defence Tradeoffs. Biol. Fertil. Soils 2021, 58, 17–34. [Google Scholar] [CrossRef]
- Chitarra, W.; Cuozzo, D.; Ferrandino, A.; Secchi, F.; Palmano, S.; Perrone, I.; Boccacci, P.; Pagliarani, C.; Gribaudo, I.; Mannini, F.; et al. Dissecting Interplays between Vitis vinifera L. and Grapevine Virus B (GVB) under Field Conditions. Mol. Plant Pathol. 2018, 19, 2651–2666. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in Maximum Stomatal Conductance Constrained by Negative Correlation between Stomatal Size and Density: An Analysis Using Eucalyptus Globulus. Plant. Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Bosabalidis, A.M.; Kofidis, G. Comparative Effects of Drought Stress on Leaf Anatomy of Two Olive Cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy Stomata, Photosynthesis and Plant Water Use Efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Dong, Y.; Zhao, Y.; Geng, A.; Xia, X.; Yin, W. PdEPF1 Regulates Water-Use Efficiency and Drought Tolerance by Modulating Stomatal Density in Poplar. Plant Biotechnol. J. 2016, 14, 849–860. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with Reduced Stomatal Density Conserves Water and Has Improved Drought Tolerance under Future Climate Conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.J.; Sack, F.D. Variable Timing of Developmental Progression in the Stomatal Pathway in Arabidopsis Cotyledons. New Phytol. 2002, 153, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dow, G.J.; Bergmann, D.C. Patterning and Processes: How Stomatal Development Defines Physiological Potential. Curr. Opin. Plant Biol. 2014, 21, 67–74. [Google Scholar] [CrossRef]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and Salinity Stress in Grapevines: Early and Late Changes in Transcript and Metabolite Profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerva, L.; Chitarra, W.; Fila, G.; Lovat, L.; Gaiotti, F. Variability in Stomatal Adaptation to Drought among Grapevine Cultivars: Genotype-Dependent Responses. Agriculture 2023, 13, 2186. https://doi.org/10.3390/agriculture13122186
Nerva L, Chitarra W, Fila G, Lovat L, Gaiotti F. Variability in Stomatal Adaptation to Drought among Grapevine Cultivars: Genotype-Dependent Responses. Agriculture. 2023; 13(12):2186. https://doi.org/10.3390/agriculture13122186
Chicago/Turabian StyleNerva, Luca, Walter Chitarra, Gianni Fila, Lorenzo Lovat, and Federica Gaiotti. 2023. "Variability in Stomatal Adaptation to Drought among Grapevine Cultivars: Genotype-Dependent Responses" Agriculture 13, no. 12: 2186. https://doi.org/10.3390/agriculture13122186





