Effects of Silicon Alone and Combined with Organic Matter and Trichoderma harzianum on Sorghum Yield, Ions Accumulation and Soil Properties under Saline Irrigation
Abstract
:1. Introduction
2. Materials and Methods
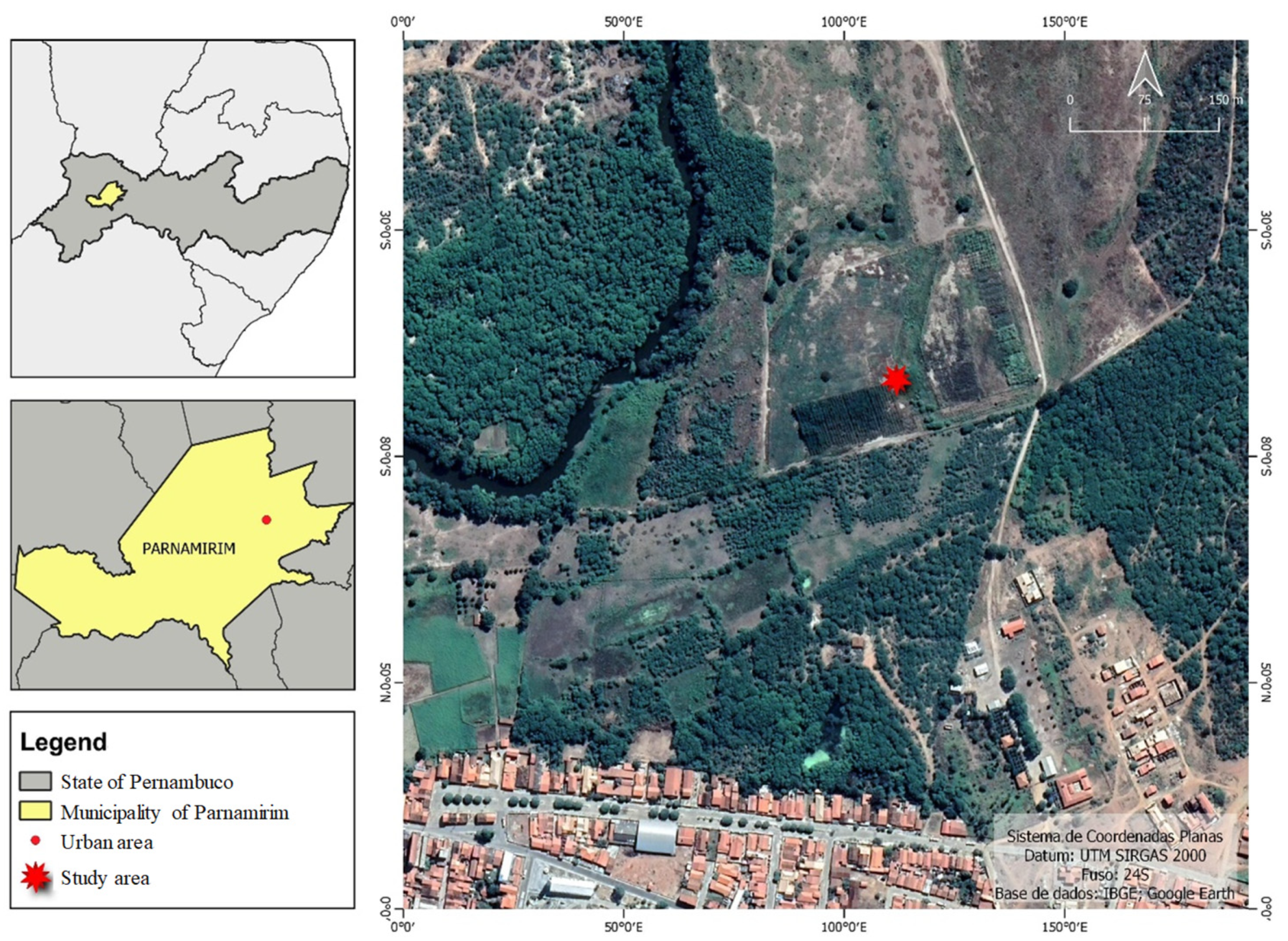
2.1. Study Area
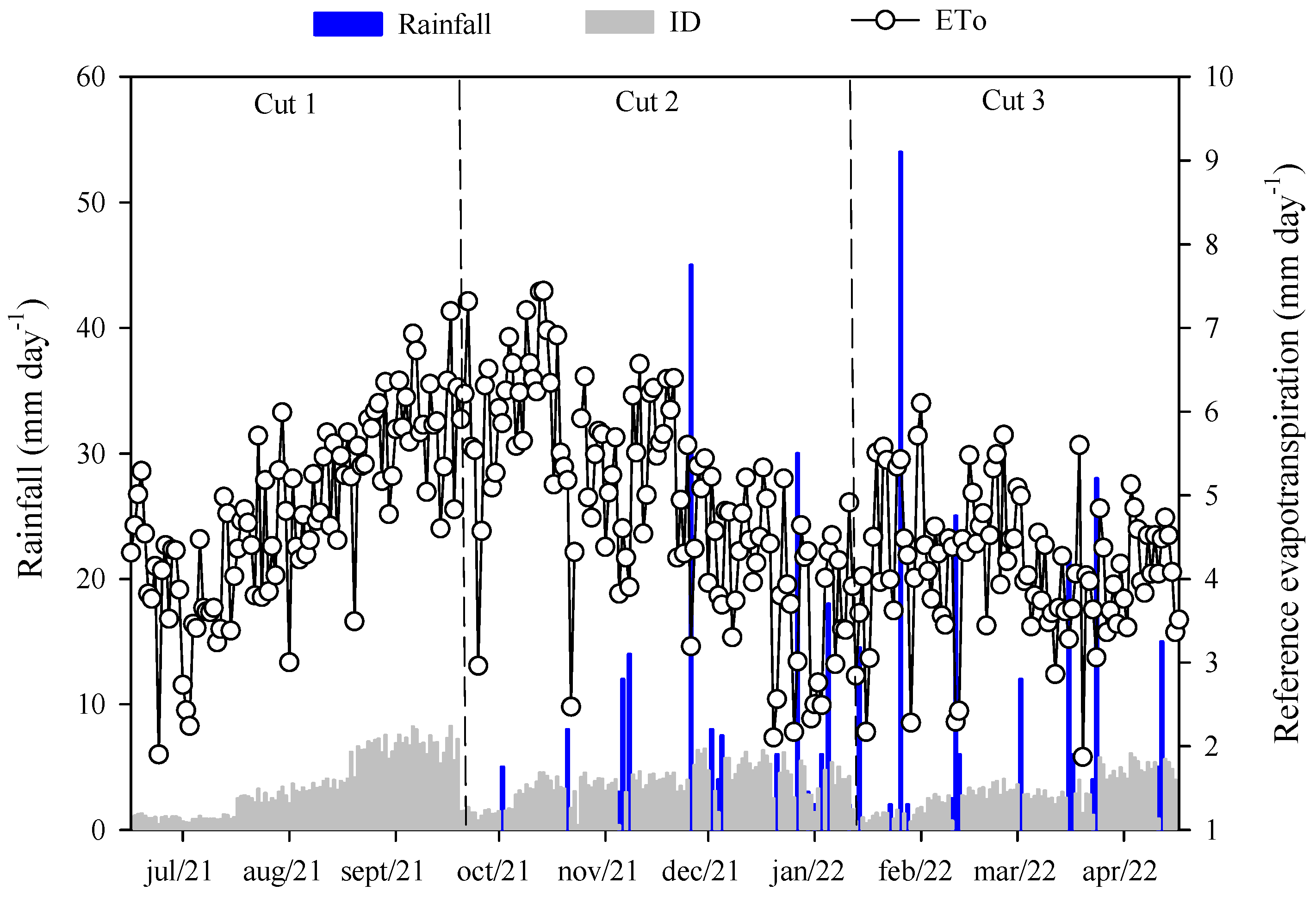
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Analysis
2.4.1. Soluble Complex
2.4.2. Exchangeable Complex
2.5. Content of Sodium, Potassium, Calcium, Magnesium, Chloride, and Phosphorus in the Plant
2.6. Sorghum Productivity and Water Use Efficiency (WUE)
2.7. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
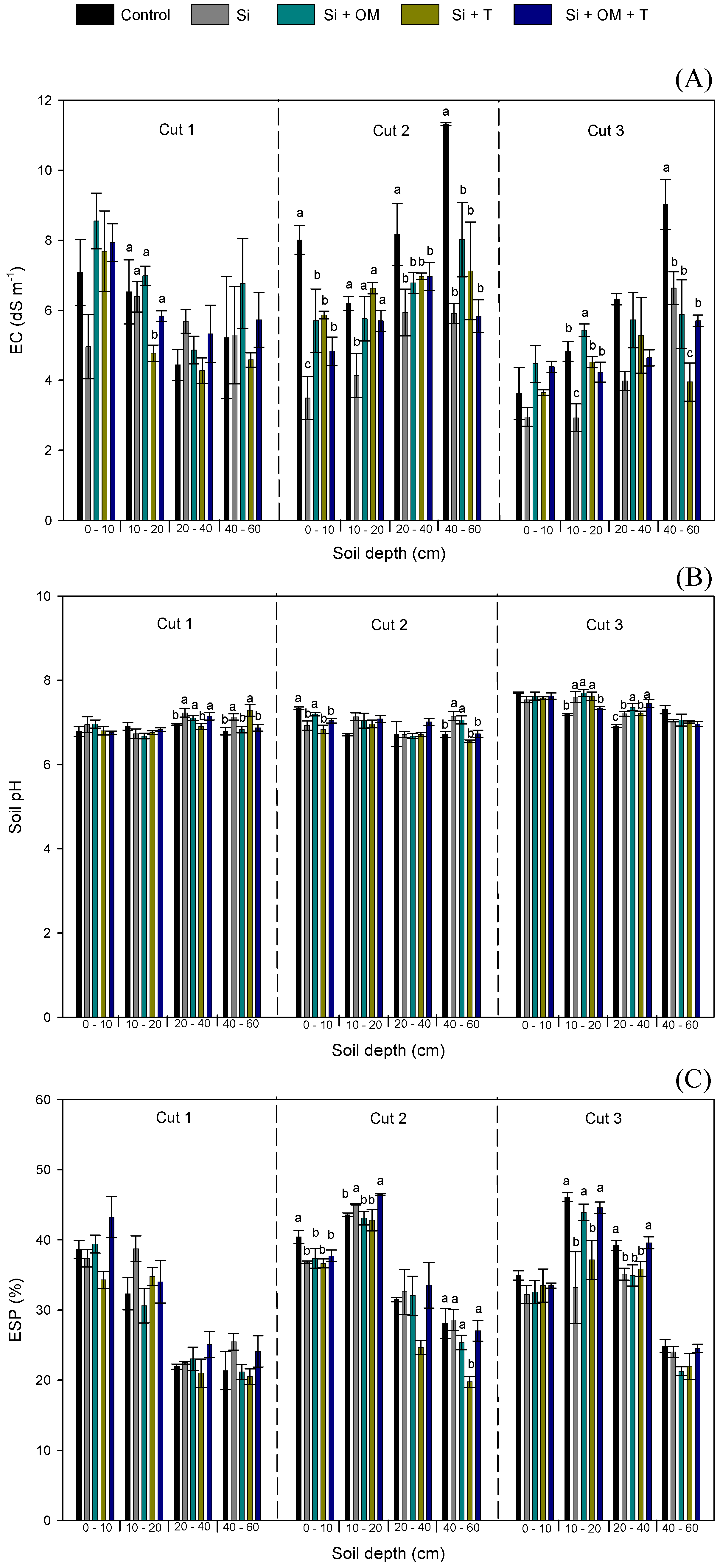
3.1.1. Salinity Parameters
3.1.2. Soluble Complex
3.1.3. Sorptive Complex
3.2. Ions Content at Plant Tissue
3.3. Sorghum Yield and Water Use Efficiency
4. Discussion
4.1. Salinity Parameters
4.2. Soluble and Sorptive Complex
4.3. Ions Content at Plant Tissue Level
4.4. Sorghum Yield and Water Use Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Bouri, S. Use of HYDRUS-1D–GIS tool for evaluating effects of climate changes on soil salinization and irrigation management. Arch. Agron. Soil Sci. 2020, 66, 193–207. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chu, L.; Lu, H.; Qi, W.; Chen, X.; Liu, J.; Kuang, S.; Tang, B.; Wong, V. Towards sustainable agriculture for the salt-affected soil. L. Degrad. Dev. 2019, 30, 574–579. [Google Scholar] [CrossRef]
- Lima, A.O.; Lima-Filho, F.P.; Dias, N.S.; Chipana-Rivera, R.; Ferreira Neto, M.; Souza, A.M.; Rego, P.R.A.; Fernandes, C.S. Variation of the water table and salinity in alluvial aquifers of underground dams in the semi-arid region of Rio Grande do Norte, Brazil. Biosci. J. 2019, 35, 477–484. [Google Scholar] [CrossRef]
- Fernandes, J.G.; Freire, M.B.G.S.; Cunha, J.C.; Galvíncio, J.D.; Correia, M.M.; Santos, P.R. Qualidade físico-química das águas utilizadas no Perímetro Irrigado Cachoeira II, Serra Talhada, Pernambuco. Rev. Bras. Ciênc. Agrár.–Braz. J. Agric. Sci. 2009, 4, 27–34. [Google Scholar] [CrossRef]
- Lessa, C.I.N.; Lacerda, C.F.; Cajazeiras, C.C.D.A.; Neves, A.L.R.; Lopes, F.B.; Silva, A.O.; Souza, H.C.; Gheyi, H.R.; Nogueira, R.S.; Lima, S.C.R.V.; et al. Potential of Brackish Groundwater for Different Biosaline Agriculture Systems in the Brazilian Semi-Arid Region. Agriculture 2023, 13, 550. [Google Scholar] [CrossRef]
- Pessoa, L.G.M.; Freire, M.B.G.D.S.; Wilcox, B.P.; Green, C.H.M.; Araújo, R.J.T.; Filho, J.C.D.A. Spectral reflectance characteristics of soils in northeastern Brazil as influenced by salinity levels. Environ. Monit. Assess. 2016, 188, 616. [Google Scholar] [CrossRef]
- Pessoa, L.G.M.; Freire, M.B.G.S.; Green, C.H.M.; Miranda, M.F.A.; Filho, J.C.A.; Pessoa, W.R.L.S. Assessment of soil salinity status under different land-use conditions in the semiarid region of Northeastern Brazil. Ecol. Indic. 2022, 141, 109139. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Salah, E.G.I. Interactive impacts of soil salinity and jasmonic acid and humic acid on growth parameters, forage yield and photosynthesis parameters of sorghum plants. S. Afr. J. Bot. 2022, 146, 293–303. [Google Scholar] [CrossRef]
- Saadat, S.; Homaee, M. Modeling sorghum response to irrigation water salinity at early growth stage. Agric. Water Manag. 2015, 152, 119–124. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Song, R.; Shao, H.; Song, F.; Xu, H.; Lu, Y. Silicon improves maize photosynthesis in saline-alkaline soils. Sci. World J. 2015, 2015, 245072. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglansmicrocarpa L. Seedlings. Plants 2022, 11, 2381. [Google Scholar] [CrossRef]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Perez, S.C.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Pessoa, L.G.M.; Silva, L.F.D.S.; Freire, M.B.G.D.S.; Ferreira-Silva, S.L.; Green, C.H.M.; Melo, H.F.; Fernandes, J.G.; Freire, F.J. Effectiveness of soil conditioners to enhance salt extraction ability of Salicornia ramosissima in saline-sodic soil for different soil moisture contents. Int. J. Phytoremediat. 2022, 24, 447–455. [Google Scholar] [CrossRef]
- Silva, M.M.A.; Pessoa, L.G.M.; Simplício, J.B.; Souza, W.L.D.S.; Freire, M.B.G.D.S.; Souza, E.S.; Santos, E.S.; Miranda, M.F.A.; Junior, C.C.P. Soil conditioners as candidates to mitigate salt/water stress effects on sorghum growth and soil properties. Aust. J. Crop Sci. 2021, 15, 98–106. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Junior, G.D.S.S.; Gratao, P.L.; Felisberto, G.; Viciedo, D.O.; Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Guspta, O.P.; Devana, B.N.; Tripathi, D.K.; et al. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef]
- Abdolzadeh, A.; Zarooshan, M.; Sadeghipour, H.R.; Mehrabanjoubani, P. Effect of silicon and nano silicon application on wheat (C3) and sorghum (C4) under salinity stress. J. Plant Prod. Res. 2022, 29, 173–190. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Kumari, R.; Bhatnagar, S.; Mehla, N.; Vashistha, A. Potential of organic amendments (AM fungi, PGPR, vermicompost and seaweeds) in combating salt stress: A Review. Plant Stress 2022, 6, 100111. [Google Scholar] [CrossRef]
- Miranda, M.F.A.; Freire, M.B.G.S.; Almeida, B.G.; Freire, F.J.; Pessoa, L.G.M.; Freire, A.G. Phytodesalination and chemical and organic conditioners to recover the chemical properties of saline-sodic soil. Soil Sci. Soc. Am. J. 2021, 85, 132–145. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.; Liu, Z.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 11152. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Huqail, A.A.A.; Egamberdieva, D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Interact. 2014, 9, 857–868. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yang, T.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef]
- Cruz, J.L.; Coelho, E.F.; Coelho Filho, M.A.; Santos, A.A. Salinity reduces nutrients absorption and efficiency of their utilization in cassava plants. Ciência Rural 2018, 48, 1–12. [Google Scholar] [CrossRef]
- Hoffmann, J.; Berni, R.; Hausman, J.F.; Guerriero, G. A review on the beneficial role of silicon against salinity in non-accumulator crops: Tomato as a model. Biomolecules 2020, 10, 1284. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.F.; Rodrigues, E.S.C.; Silva, W.G.; Galvão, S.R.S. Classificação da precipitação pluviométrica anual para o município de Parnamirim—PE utilizando Índice de Anomalia de Chuva (IAC). Rev. Semiárido Visu 2019, 7, 275–284. [Google Scholar] [CrossRef]
- Embrapa. Sistema Brasileiro de Classificação de Solos; Centro Nac.: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration: Guidelines for computing crop requirements. Irrig. Drain. Pap. 1998, 56, 300. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Soil Sci. Soc. Am. J. 1954, 18, 348. [Google Scholar] [CrossRef]
- Freitas, G.A.; Sousa, C.R.; Capone, A.; Afférri, F.S.; Silva, R.R. Adubação orgânica no sulco de plantio e sua influência no desenvolvimento do sorgo. J. Biotechnol. Biodivers. 2012, 3, 61–67. [Google Scholar] [CrossRef]
- USSL. Diagnosis and Improvement of Saline and Alkali Soils; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorous in Soils by Extraction with Sodium Bicarbonate; US Department: Washington, DC, USA, 1954. [Google Scholar]
- Silva, M.M.A.; Santos, H.R.B.; Silva, E.N.; Neto, J.B.; Hermínio, P.J.; Ramalho, T.L.; Nunes, V.G.; Simões, A.N.; Souza, E.S.; Ferreira-Silva, S.L. Higher control of Na+ and Cl− transport to the shoot along with K+/Na+ selectivity is determinant for differential salt resistance in grapevine rootstocks. J. Plant Growth Regul. 2023, 42, 5713–5726. [Google Scholar] [CrossRef]
- Embrapa. Manual de Análises Químicas de Solos. Plantas e Fertilizantes; Embrapa Informação Tecnológica: Rio de Janeiro, Brasil, 2009. [Google Scholar]
- Queiroz, G.C.M.; Medeiros, J.F.; Silva, R.R.; Morais, F.M.S.; Sousa, L.V.; Souza, M.V.P.; Santos, E.N.; Ferreira, F.N.; Silva, J.M.C.; Clemente, M.I.B.; et al. Growth, solute accumulation, and ion distribution in sweet sorghum under salt and drought stresses in a Brazilian Potiguar semiarid área. Agriculture 2023, 13, 803. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef]
- Véras, M.L.M.; Alves, L.S.; Silva, T.I.; Silva, I.N.; Costa, A.S.D.N.; Melo, E.N.; Cordovil, H.P.L.; Dantas, A.P.J.; Souza, N.A.; Dias, T.J. Silicon as mitigator of salt stress in mango tree seedlings. Aust. J. Crop Sci. 2021, 15, 1146–1150. [Google Scholar] [CrossRef]
- Rizwan, A.; Zia-ur-Rehman, M.; Rizwan, M.; Usman, M.; Anayatullah, S.; Alharby, H.F.; Bamagoos, A.A.; Alharbi, B.M.; Ali, S. Effects of silicon nanoparticles and conventional Si amendments on growth and nutrient accumulation by maize (Zea mays L.) grown in saline-sodic soil. Environ. Res. 2023, 227, 115740. [Google Scholar] [CrossRef] [PubMed]
- Black, A.S.; Campbell, A.S. Ionic strength of soil solution and its effect on charge properties of some New Zealand soils. J. Soil Sci. 1982, 33, 249–262. [Google Scholar] [CrossRef]
- Ramírez-Jaramillo, G.; Lozano-Contreras, M.G.; Ramírez-Silva, J.H. Agroclimatic conditions for growing Sorghum bicolor L. Moench, under irrigation conditions in Mexico. OALib 2020, 7, 100813. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yao, R.; Wang, X.; Xie, W. Short-term effects of biochar and gypsum on soil hydraulic properties and sodicity in a saline-alkali soil. Pedosphere 2020, 30, 694–702. [Google Scholar] [CrossRef]
- Miles, N.; Manson, A.D.; Rhodes, R.; van Antwerpen, R.; Weigel, A. Extractable Silicon in Soils of the South African Sugar Industry and Relationships with Crop Uptake. Commun. Soil Sci. Plant Anal. 2014, 45, 2949–2958. [Google Scholar] [CrossRef]
- Yanai, J.; Taniguchi, H.; Nakao, A. Evaluation of available silicon content and its determining factors of agricultural soils in Japan. Soil Sci. Plant Nutr. 2016, 62, 511–518. [Google Scholar] [CrossRef]
- Camargo, M.S.; Pereira, H.S.; Korndörfer, G.H.; Queiroz, A.A.; Reis, C.B. Soil reaction and absorption of silicon by rice. Sci. Agric. 2007, 64, 176–180. [Google Scholar] [CrossRef]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.B.G.S.; Ruiz, H.A.; Ribeiro, M.R.; Ferreira, P.A.; Alvarez, V.H.; Freire, F.J. Estimativa do risco de sodificação de solos de Pernambuco pelo uso de águas salinas. Rev. Bras. Eng. Agríc. Ambient. 2003, 7, 227–232. [Google Scholar] [CrossRef]
- Minhas, P.S.; Qadir, M.; Yadav, R.K. Groundwater irrigation induced soil sodification and response options. Agric. Water Manag. 2019, 215, 74–85. [Google Scholar] [CrossRef]
- Pessoa, L.G.M.; Freire, M.B.G.S.; Filho, J.C.A.; Santos, P.R.; Miranda, M.F.A.; Freire, F.J. Characterization and classification of halomorphic soils in the semiarid region of northeastern Brazil. J. Agric. Sci. 2019, 11, 405. [Google Scholar] [CrossRef]
- Silva, M.O.; Freire, M.B.G.S.; Mendes, A.M.S.; Freire, F.J.; Duda, G.P.; Sousa, C.E.S. Risco de salinização em quatro solos do Rio Grande do Norte sob irrigação com águas salinas. Rev. Bras. Ciênc. Agrár.–Braz. J. Agric. Sci. 2007, 2, 8–14. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Yang, H.; Fan, G.; Zhou, D. Short-term land use conversions influence the profile distribution of soil salinity and sodicity in northeastern China. Ecol. Indic. 2018, 88, 79–87. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L. Vermiculite and humic acid improve the quality of green waste compost as a growth medium for Centaurea cyanus L. Environ. Technol. Innov. 2021, 24, 101945. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. The effectiveness of composted green waste amended with vermiculite and humic acid powders as an alternative cultivation substrate for cornflower cultivation. Commun. Soil Sci. Plant Anal. 2021, 52, 2945–2957. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.J. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef]
- Kusumawati, R.; Pangestu, H.E.; Basmal, J. Effect of Trichoderma addition on sargassum organic fertilizer. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012059. [Google Scholar] [CrossRef]
- Pratiwi, V.; Oktarina, H.; Sriwati, R. The potential of Trichoderma spp. and Pseudomonas auregenosa as patchouli waste decomposer. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012019. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-induced acidification is an early trigger for changes in arabidopsis root growth and determines fungal phytostimulation. Front. Plant Sci. 2017, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.A.; Inoue, T.T.; Esper Neto, M.; Muniz, A.S. Princípios de fertilidade do solo, adubação e nutrição mineral. In Hortaliças-Fruto; EDUEM: Maringá, Brazil, 2018; pp. 113–162. [Google Scholar] [CrossRef]
- Carneiro, C.E.A.; Fioretto, R.A.; Fonseca, I.C.B.; Carneiro, G.E.S. Calcário, potássio, fosfato e silício na produtividade do solo. Acta Sci. Agron. 2006, 28, 465–470. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 258, 29–39. [Google Scholar] [CrossRef]
- Hunt, J.F.; Ohno, T.; He, Z.; Honeycutt, C.W.; Dail, D.B. Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic matter. Biol. Fertil. Soils 2007, 44, 277–288. [Google Scholar] [CrossRef]
- Yan, X.; Wang, D.; Zhang, H.; Zhang, G.; Wei, Z. Organic amendments affect phosphorus sorption characteristics in a paddy soil. Agric. Ecosyst. Environ. 2013, 175, 47–53. [Google Scholar] [CrossRef]
- Bi, Q.-F.; Li, K.J.; Zheng, B.X.; Liu, X.P.; Li, H.Z.; Jin, B.J.; Ding, K.; Yang, X.; Lin, X.Y.; Zhu, Y.G. Partial replacement of inorganic phosphorus (P) by organic manure reshapes phosphate mobilizing bacterial community and promotes P bioavailability in a paddy soil. Sci. Total Environ. 2020, 703, 134977. [Google Scholar] [CrossRef]
- Qaswar, M.; Chai, R.; Ahmed, W.; Jing, H.; Han, T.; Liu, K.; Zhang, H. Partial substitution of chemical fertilizers with organic amendments increased rice yield by changing phosphorus fractions and improving phosphatase activities in fluvo-aquic soil. J. Soils Sediments 2020, 20, 1285–1296. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Long-term organic substitution management affects soil phosphorus speciation and reduces leaching in greenhouse vegetable production. J. Clean. Prod. 2021, 327, 129464. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Ding, L.-J.; Su, J.-Q.; Sun, G.-X.; Wu, J.-S.; Wei, W.-X. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biotechnol. 2018, 102, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ling, X.; Iqbal, B.; Zhou, Z.; Meng, Y. Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch. Agron. Soil Sci. 2021, 69, 18–31. [Google Scholar] [CrossRef]
- El-Baki, G.A.; Mostafa, D. The potentiality of Trichoderma harzianum in alleviation the adverse effects of salinity in faba bean plants. Acta Biol. Hung. 2014, 65, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Higgs, D.; Murillo-Amador, B.; Aydemir, S.; Girgin, A.R. Silicon improves salinity tolerance in wheat plants. Environ. Exp. Bot. 2008, 62, 10–16. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Sousa Junior, G.S.; Felisberto, G. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol. Biochem. 2019, 142, 224–233. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Sousa Junior, G.S.; Viciedo, D.O.; Díaz, Y.P.; Calzada, K.P.; Gratão, P.L. Silicon alleviates sodium toxicity in sorghum and sunflower plants by enhancing ionic homeostasis in roots and shoots and increasing dry matter accumulation. Silicon 2021, 13, 475–486. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Li, J.; Tanaka, K.; Oka, M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol. Plant. 2013, 35, 3099–3107. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Flowers, T.J.; Gong, H. Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 2013, 170, 847–853. [Google Scholar] [CrossRef]
- Abbas, T.; Balal, R.M.; Shahid, M.A.; Pervez, M.A.; Ayyub, C.M.; Aqueel, M.A.; Javaid, M.M. Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiol. Plant. 2015, 37, 6. [Google Scholar] [CrossRef]
- Soylemezoglu, G.; Demir, K.; Inal, A.; Gunes, A. Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci. Hortic. 2009, 123, 240–246. [Google Scholar] [CrossRef]
- Karimi, G.; Pourakbar, L.; Moghaddam, S.S.; Danesh, Y.R.; Djordjevi’c, J.P. Effectiveness of fungal bacterial biofertilizers on agrobiochemical attributes of quinoa (Chenopodium quinoa willd.) under salinity stress. Int. J. Environ. Sci. Technol. 2022, 19, 11989–12002. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.D.C.; Mello, S.C.M.; Lobo Júnior, M.; Silva, M.C. Controle de Fusarium oxysporum f.sp. phaseoli in vitro e em sementes, e promoção do crescimento inicial do feijoeiro comum por Trichoderma harzianum. Trop. Plant Pathol. 2011, 36, 28–34. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Wiethan, M.M.S.; Vey, R.T.; Oliveira, J.C.P.; Köpp, M.M.; Silva, A.C.F. Trichoderma na promoção do desenvolvimento de plantas de Paspalum regnellii Mez. Rev. Ciênc. Agrár. 2019, 42, 131–140. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A.; et al. Effect of colonization of Trichoderma harzianum on growth development and CBD content of hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef]
- Instituto Agronômico de Pernambuco (IPA). Sorgo Sudão: Sudão 4202—Cultivar Tolerante a Salinidade e Com Aptidão Para Feno. 2007. Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/487334 (accessed on 15 October 2023).
- Aishwarya, S.; Viswanath, H.S.; Singh, A.; Singh, R. Biosolubilization of different nutrients by Trichoderma spp. and their mechanisms involved: A review. Int. J. Adv. Agric. Sci. Technol. 2020, 7, 34–39. [Google Scholar]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamram, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Sousa, R.A.; Lacerda, C.F.; Neves, A.L.R.; Costa, R.N.T.; Hernandez, F.F.F.; Sousa, C.H.C. Crescimento do sorgo em função da irrigação com água salobra e aplicação de compostos orgânicos. Rev. Bras. Agric. Irrig. 2018, 12, 2315–2326. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Klepper, K.; Ahmad, R.; Blair, G. Impact of feedlot manure and nitrogen additions on forage yields, nutrient balance and soil nitrate, phosphate and salinity. Commun. Soil Sci. Plant Anal. 2020, 51, 2658–2669. [Google Scholar] [CrossRef]
- Yang, R.; Sun, Z.; Liu, X.; Long, X.; Gao, L.; Shen, Y. Biomass composite with exogenous organic acid addition supports the growth of sweet sorghum (Sorghum bicolor ‘Dochna’) by reducing salinity and increasing nutrient levels in coastal saline–alkaline soil. Front. Plant Sci. 2023, 14, 1163195. [Google Scholar] [CrossRef]
- Cunha, E.E.; Lima, J.M.P. Caracterização de genótipos e estimativa de parâmetros genéticos de características produtivas de sorgo forrageiro. Rev. Bras. Zootec. 2010, 39, 701–706. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Pires, A.J.V.; Viana, A.E.S.; Matsumoto, S.N.; Carvalho, G.G.P.; Ribeiro, L.S.O. Produtividade, composição química e características agronômicas de diferentes forrageiras. Rev. Bras. Zootec. 2010, 39, 2604–2610. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Comassetto, D.S.; Dornelles, R.R.; Rosa, F.Q.; Oaigen, R.P.; Castagnara, D.D.; Valle, T.A.; Azevedo, E.B. Produção, composição bromatológica e fenológica de forrageiras estivais na Região Sul do Brasil. Agrarian 2020, 13, 82–92. [Google Scholar] [CrossRef]
- Alayafi, A.H.; Al-Solaimani, S.G.M.; El-Wahed, M.H.A.; Alghabari, F.M.; El Sabagh, A. Silicon supplementation enhances productivity, water use efficiency and salinity tolerance in maize. Front. Plant Sci. 2022, 13, 953451. [Google Scholar] [CrossRef]
- Khan, W.; Aziz, T.; Hussain, I.; Ramzani, P.M.A.; Reichenauer, T.G. Silicon: A beneficial nutrient for maize crop to enhance photochemical efficiency of photosystem II under salt stress. Arch. Agron. Soil Sci. 2017, 63, 599–611. [Google Scholar] [CrossRef]
- Wang, X.; Nie, J.; Wang, P.; Zhao, J.; Yang, Y.; Wang, S.; Zang, H. Does the replacement of chemical fertilizer nitrogen by manure benefit water use efficiency of winter wheat—Summer maize systems? Agric. Water Manag. 2021, 243, 106428. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Zhang, X.; Zhang, S.; Chen, Y. Organic manure input improves soil water and nutrients use for sustainable maize (Zea mays. L) productivity on the Loess Plateau. PLoS ONE 2020, 15, e0238042. [Google Scholar] [CrossRef] [PubMed]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.S.; Paula, A.M.; Ferrari, L.H.; Silva, J.; Pinheiro, J.B.; Cajamarca, S.M.N.; Busato, J.G. Trichoderma-inriched vermicompost extracts reduces nematode biotic stress in tomato and bell pepper crops. Agronomy 2021, 11, 1655. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Tubana, B.S.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Sousa, T.P.; Chaibub, A.A.; Silva, G.B.; Filippi, M.C.C. Trichoderma asperellum modulates defense genes and potentiates gas exchanges in upland rice plants. Physiol. Mol. Plant Pathol. 2020, 112, 101561. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.P.; Olmedo-Monfil, V.; Lara-Banda, M.; Zúñiga-Romo, E.R.; Aréchiga-Carvajal, E.T. Promotion of plant growth in arid zones by selected Trichoderma spp. strains with adaptation plasticity to alkaline pH. Biology 2022, 11, 1206. [Google Scholar] [CrossRef]
- Ahmed, B.A.O.; Inoue, M.; Moritani, S. Effect of saline water irrigation and manure application on the available water content, soil salinity, and growth of wheat. Agric. Water Manag. 2010, 97, 165–170. [Google Scholar] [CrossRef]
| Soil Property | Soil Layer (cm) | |||
|---|---|---|---|---|
| 0–10 | 10–20 | 20–40 | 40–60 | |
| Chemical property | ||||
| pH | 6.05 | 6.09 | 6.16 | 6.32 |
| OM (g kg−1) | 25.08 | 19.35 | 18.00 | 11.75 |
| P (mg dm−3) | 28.40 | 19.73 | 14.83 | 9.75 |
| K+ (cmolc dm−3) | 0.51 | 0.34 | 0.24 | 0.14 |
| Na+ (cmolc dm−3) | 0.30 | 0.37 | 0.52 | 0.82 |
| Ca2+ (cmolc dm−3) | 9.06 | 8.54 | 8.79 | 9.98 |
| Mg2+ (cmolc dm−3) | 5.29 | 5.40 | 5.21 | 5.53 |
| H + Al (cmolc dm−3) | 0.96 | 0.89 | 0.76 | 0.60 |
| SB (cmolc dm−3) | 15.14 | 14.65 | 14.93 | 16.48 |
| CEC (cmolc dm−3) | 16.10 | 15.54 | 15.69 | 17.08 |
| ESP (%) | 1.86 | 2.38 | 3.31 | 4.80 |
| Saturation extract | ||||
| EC (dS m−1) | 3.56 | 6.21 | 7.22 | 5.77 |
| Ca2+ (mmolc L−1) | 31.88 | 58.88 | 60.45 | 43.85 |
| Mg2+ (mmolc L−1) | 12.03 | 22.78 | 25.15 | 18.00 |
| K+ (mmolc L−1) | 9.63 | 5.78 | 5.08 | 2.13 |
| Na+ (mmolc L−1) | 5.58 | 9.40 | 12.18 | 13.15 |
| SAR (mmolc L−1) | 1.20 | 1.50 | 1.83 | 2.23 |
| Soil texture | ||||
| Sand (%) | 14.20 | 10.30 | 11.30 | 10.92 |
| Silt (%) | 72.12 | 75.62 | 71.62 | 75.48 |
| Clay (%) | 13.68 | 14.08 | 17.08 | 13.60 |
| pH | EC | Ca2+ | Mg2+ | K+ | Na+ | Cl− | HCO3− | SO42− | B | Cu | Fe | Mn | Zn | SAR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | ----------------- mmolc L−1 ----------------- | --------- mg L−1 --------- | mmolc L−0.5 | |||||||||||
| 7.23 | 3.12 | 9.29 | 9.23 | 0.12 | 11.73 | 26.38 | 5.70 | 0.76 | 0.11 | 0.02 | 0.02 | 0.03 | 0.20 | 3.85 |
| D | C | N | P | K+ | Na+ | Ca2+ | Mg2+ | pH | EC |
|---|---|---|---|---|---|---|---|---|---|
| g cm−3 | -------------------------------- g kg−1 --------------------------------- | dS m−1 | |||||||
| 0.8158 | 119.70 | 17.90 | 8.70 | 4.50 | 1.10 | 27.70 | 10.20 | 7.87 | 1.87 |
| Soil Layer (cm) | Cut 1 | Cut 2 | Cut 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Si + OM | Si + T | Si + T + OM | C | Si | Si + OM | Si + T | Si + T + OM | C | Si | Si + OM | Si + T | Si + T + OM | |
| K+ (mmolc L−1) | |||||||||||||||
| 0–10 | 0.79 c | 0.51 d | 1.50 a | 0.97 b | 1.53 a | 0.67 b | 0.67 b | 0.76 b | 0.92 a | 0.94 a | 0.36 c | 0.44 c | 0.40 c | 0.56 a | 0.49 b |
| 10–20 | 0.71 b | 0.64 b | 0.80 b | 0.65 b | 1.13 a | 0.31 b | 0.22 b | 0.34 b | 0.47 a | 0.31 b | 0.38 a | 0.24 b | 0.31 a | 0.32 a | 0.31 a |
| 20–40 | 0.42 b | 0.45 b | 0.39 b | 0.49 b | 0.60 a | 0.35 a | 0.24 b | 0.29 b | 0.36 a | 0.25 b | 0.34 | 0.29 | 0.36 | 0.30 | 0.23 |
| 40–60 | 0.39 | 0.47 | 0.52 | 0.36 | 0.36 | 0.30 b | 0.28 b | 0.34 b | 0.64 a | 0.33 b | 0.40 a | 0.43 a | 0.33 b | 0.30 b | 0.29 b |
| Na+ (mmolc L−1) | |||||||||||||||
| 0–10 | 124.37 a | 84.69 b | 144.26 a | 90.22 b | 119.69 a | 133.57 a | 72.07 c | 107.30 b | 90.58 c | 83.41 c | 60.27 c | 48.98 d | 74.95 b | 86.12 a | 76.61 b |
| 10–20 | 77.42 b | 111.44 a | 95.03 b | 87.48 b | 90.82 b | 122.82 a | 98.4 b | 116.85 a | 94.76 b | 114.46 a | 103.56 a | 68.97 b | 80.24 b | 71.91 b | 113.73 a |
| 20–40 | 43.62 | 50.41 | 56.41 | 42.21 | 67.62 | 81.03 a | 80.43 a | 62.52 b | 70.28 a | 55.95 b | 93.08 a | 74.95 b | 102.57 a | 63.46 b | 88.76 a |
| 40–60 | 46.34 c | 75.67 b | 57.83 c | 52.01 c | 99.85 a | 121.33 a | 63.12 b | 72.07 b | 61.32 b | 63.71 b | 51.22 b | 82.59 a | 52.51 b | 43.57 b | 57.58 b |
| Cl− (mmolc L−1) | |||||||||||||||
| 0–10 | 64.58 a | 29.58 b | 88.75 a | 44.58 b | 73.75 a | 72.08 a | 20.00 b | 41.25 b | 44.58 b | 31.67 b | 13.33 c | 13.75 c | 27.92 a | 22.08 b | 32.08 a |
| 10–20 | 68.75 a | 56.25 b | 59.58 b | 44.58 c | 45.42 c | 53.75 a | 26.25 b | 45.42 a | 52.08 a | 52.92 a | 34.58 | 20.63 | 25.63 | 39.58 | 27.50 |
| 20–40 | 41.25 | 42.50 | 30.42 | 36.25 | 42.08 | 79.38 a | 37.92 c | 57.92 b | 59.58 b | 58.75 b | 50.42 a | 27.08 b | 56.25 a | 40.42 b | 29.17 b |
| 40–60 | 42.08 | 42.92 | 37.92 | 31.25 | 43.75 | 98.33 a | 47.08 b | 52.08 b | 58.75 b | 46.25 b | 84.17 a | 44.17 b | 50.42 b | 27.08 c | 44.58 b |
| Ca2+ (mmolc L−1) | |||||||||||||||
| 0–10 | 23.36 b | 18.61 b | 39.50 a | 26.31 b | 43.30 a | 29.20 a | 13.19 b | 33.12 a | 25.09 a | 25.44 a | 10.54 b | 10.43 b | 16.93 a | 14.94 a | 18.77 a |
| 10–20 | 35.19 b | 29.75 b | 46.04 a | 23.14 b | 30.81 b | 24.50 b | 14.06 c | 32.72 a | 29.80 a | 23.98 b | 17.38 a | 11.11 b | 20.63 a | 19.18 a | 20.21 a |
| 20–40 | 31.42 | 30.75 | 33.69 | 30.43 | 35.71 | 38.26 a | 23.79 b | 38.18 a | 42.91 a | 40.45 a | 26.81 a | 16.56 c | 31.28 a | 32.68 a | 22.85 b |
| 40–60 | 34.28 | 38.26 | 42.30 | 32.26 | 39.28 | 60.73 a | 30.96 b | 37.45 b | 63.01 a | 42.03 b | 38.00 a | 28.54 a | 43.56 a | 19.64 b | 32.24 a |
| Mg2+ (mmolc L−1) | |||||||||||||||
| 0–10 | 12.07 b | 9.46 b | 31.87 a | 22.99 a | 24.11 a | 15.58 | 7.08 | 12.56 | 14.15 | 10.48 | 5.10 c | 4.11 c | 9.05 a | 7.46 b | 9.55 a |
| 10–20 | 19.34 | 16.95 | 20.27 | 16.48 | 17.06 | 13.77 b | 6.91 b | 11.08 b | 21.23 a | 13.60 b | 9.76 | 4.28 | 5.65 | 5.92 | 5.65 |
| 20–40 | 17.61 | 20.41 | 16.90 | 15.22 | 18.68 | 28.80 a | 14.32 b | 22.55 a | 16.95 b | 16.84 b | 13.28 a | 8.28 b | 16.95 a | 7.41 b | 12.29 a |
| 40–60 | 33.60 a | 28.36 a | 10.94 b | 15.99 b | 20.38 b | 23.26 | 18.82 | 15.63 | 16.62 | 20.52 | 31.43 a | 14.43 b | 24.91 a | 9.66 b | 16.79 b |
| Soil Layer (cm) | Cut 1 | Cut 2 | Cut 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Si + OM | Si + T | Si + T + OM | C | Si | Si + OM | Si + T | Si + T + OM | C | Si | Si + OM | Si + T | Si + T + OM | |
| K+ (cmolc kg−1) | |||||||||||||||
| 0–10 | 1.14 | 1.54 | 1.35 | 1.11 | 1.38 | 0.79 c | 1.23 a | 1.01 b | 1.25 a | 1.03 b | 0.58 b | 0.96 a | 0.65 b | 0.74 b | 0.63 b |
| 10–20 | 0.59 c | 0.60 c | 0.85 b | 0.69 c | 1.07 a | 0.45 b | 0.35 b | 0.54 b | 0.77 a | 0.69 a | 0.42 | 0.29 | 0.41 | 0.43 | 0.45 |
| 20–40 | 0.35 a | 0.26 b | 0.28 b | 0.24 b | 0.33 a | 0.31 | 0.26 | 0.26 | 0.37 | 0.35 | 0.22 | 0.24 | 0.21 | 0.21 | 0.21 |
| 40–60 | 0.21 | 0.22 | 0.19 | 0.21 | 0.26 | 0.33 a | 0.23 b | 0.26 b | 0.37 a | 0.23 b | 0.21 | 0.22 | 0.14 | 0.21 | 0.24 |
| Na+ (cmolc kg−1) | |||||||||||||||
| 0–10 | 6.23 | 6.02 | 6.34 | 5.52 | 6.96 | 6.51 a | 5.93 b | 6.02 b | 5.90 b | 6.07 b | 5.63 | 5.19 | 5.25 | 5.39 | 5.39 |
| 10–20 | 5.02 | 6.02 | 4.76 | 5.41 | 5.29 | 6.86 | 7.00 | 6.70 | 6.66 | 7.22 | 7.16 a | 5.16 b | 6.83 a | 5.77 b | 6.93 a |
| 20–40 | 3.44 | 3.53 | 3.62 | 3.29 | 3.94 | 4.94 | 5.12 | 5.03 | 3.87 | 5.26 | 6.15 a | 5.51 b | 5.48 b | 5.63 b | 6.21 a |
| 40–60 | 3.65 | 4.35 | 3.62 | 3.50 | 4.12 | 4.80 a | 4.88 a | 4.33 a | 3.38 b | 4.62 a | 4.25 | 4.10 | 3.64 | 3.75 | 4.19 |
| Ca2+ (cmolc kg−1) | |||||||||||||||
| 0–10 | 14.79 b | 15.31 b | 18.67 a | 14.19 b | 16.12 b | 18.12 b | 16.52 b | 18.23 b | 19.45 b | 22.79 a | 18.21 b | 17.88 b | 18.72 a | 16.78 c | 19.30 a |
| 10–20 | 15.28 | 15.41 | 15.84 | 15.13 | 16.05 | 17.41 | 16.74 | 16.18 | 16.75 | 17.09 | 16.38 | 16.04 | 16.59 | 16.56 | 16.08 |
| 20–40 | 16.75 b | 16.47 b | 18.67 a | 18.00 a | 19.20 a | 18.07 | 18.61 | 18.29 | 18.31 | 19.47 | 16.38 | 16.81 | 16.49 | 16.49 | 17.16 |
| 40–60 | 20.52 a | 19.70 a | 17.08 b | 17.96 b | 19.68 a | 19.32 | 18.34 | 20.12 | 20.04 | 19.45 | 18.02 | 17.25 | 17.21 | 17.52 | 17.44 |
| Mg2+ (cmolc kg−1) | |||||||||||||||
| 0–10 | 9.85 b | 9.02 c | 11.04 a | 9.90 b | 10.93 a | 9.06 b | 8.29 c | 8.73 b | 8.73 b | 10.49 a | 13.34 a | 12.49 b | 12.92 b | 12.57 b | 13.32 a |
| 10–20 | 10.20 | 10.34 | 10.95 | 10.31 | 10.86 | 8.73 a | 8.84 a | 8.91 a | 8.27 b | 9.17 a | 12.90 a | 13.21 a | 12.95 a | 11.70 b | 13.19 a |
| 20–40 | 12.00 a | 9.87 b | 11.96 a | 9.17 b | 9.39 b | 9.15 | 9.30 | 9.61 | 9.46 | 9.90 | 11.85 b | 12.16 b | 13.30 a | 11.78 b | 12.81 a |
| 40–60 | 13.25 a | 13.03 a | 11.56 b | 12.42 a | 11.28 b | 11.81 | 11.02 | 10.53 | 9.81 | 11.04 | 13.98 | 14.64 | 12.75 | 12.55 | 13.69 |
| P (mg kg−1) | |||||||||||||||
| 0–10 | 45.72 b | 56.19 b | 123.17 a | 43.10 b | 113.66 a | 38.14 c | 44.79 c | 97.20 b | 40.63 c | 148.32 a | 42.16 b | 32.29 b | 81.07 a | 38.60 b | 70.40 a |
| 10–20 | 35.52 c | 42.11 c | 52.67 b | 38.41 c | 69.87 a | 23.87 | 34.42 | 37.21 | 39.06 | 47.86 | 26.94 c | 35.57 b | 41.96 a | 28.16 c | 44.73 a |
| 20–40 | 24.43 | 25.81 | 26.77 | 27.74 | 24.84 | 24.63 | 23.03 | 25.21 | 28.08 | 31.62 | 30.97 a | 23.09 b | 34.92 a | 23.87 b | 20.92 b |
| 40–60 | 23.34 | 30.13 | 24.87 | 21.58 | 28.85 | 17.10 c | 26.42 b | 21.18 c | 32.56 a | 26.42 b | 30.18 | 21.06 | 21.62 | 27.53 | 26.77 |
| Treatment | Na+ (g) | K+ (g) | Cl− (g) | P (mg) | Ca2+ (g) | Mg2+ (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut 1 | Cut 2 | Cut 3 | Cut 1 | Cut 2 | Cut 3 | Cut 1 | Cut 2 | Cut 3 | Cut 1 | Cut 2 | Cut 3 | Cut 1 | Cut 2 | Cut 3 | Cut 1 | Cut 2 | Cut 3 | |
| Leaves | ||||||||||||||||||
| Control | 0.05 | 0.15 a | 0.06 | 0.78 b | 0.67 | 0.36 | 0.41 | 0.32 | 0.31 a | 33.84 | 43.73 a | 27.09 c | 0.20 b | 0.22 | 0.16 b | 0.13 b | 0.10 c | 0.09 |
| Si | 0.07 | 0.06 b | 0.04 | 0.90 b | 0.48 | 0.30 | 0.44 | 0.24 | 0.24 b | 44.47 | 32.49 b | 30.44 b | 0.26 a | 0.24 | 0.21 a | 0.18 a | 0.12 c | 0.10 |
| Si + OM | 0.06 | 0.08 b | 0.05 | 0.85 b | 0.57 | 0.41 | 0.38 | 0.30 | 0.22 b | 41.27 | 48.72 a | 23.93 c | 0.21 b | 0.24 | 0.22 a | 0.17 a | 0.11 c | 0.10 |
| Si + T | 0.06 | 0.10 b | 0.07 | 0.84 b | 0.64 | 0.38 | 0.36 | 0.37 | 0.23 b | 46.79 | 43.16 a | 25.05 c | 0.27 a | 0.26 | 0.23 a | 0.18 a | 0.13 b | 0.11 |
| Si + OM + T | 0.06 | 0.10 b | 0.05 | 1.06 a | 0.63 | 0.39 | 0.52 | 0.34 | 0.30 a | 47.92 | 45.66 a | 34.01 a | 0.28 a | 0.29 | 0.25 a | 0.20 a | 0.15 a | 0.13 |
| Stems | ||||||||||||||||||
| Control | 0.16 b | 0.22 b | 0.07 | 2.65 b | 2.11 | 0.86 b | 2.07 a | 1.14 | 0.82 b | 23.75 | 41.42 | 24.33 b | 0.21 a | 0.22 b | 0.21 b | 0.22 a | 0.23 a | 0.21 |
| Si | 0.11 b | 0.19 b | 0.14 | 2.72 b | 2.19 | 1.68 a | 1.47 b | 1.32 | 1.06 b | 16.94 | 35.99 | 53.85 a | 0.15 b | 0.18 b | 0.20 b | 0.15 b | 0.17 b | 0.23 |
| Si + OM | 0.21 b | 0.30 b | 0.17 | 3.75 a | 2.93 | 1.82 a | 2.07 a | 1.19 | 1.32 a | 31.03 | 56.92 | 41.75 b | 0.22 a | 0.20 b | 0.28 a | 0.25 a | 0.20 b | 0.26 |
| Si + T | 0.32 a | 0.80 a | 0.15 | 4.16 a | 2.35 | 1.92 a | 2.21 a | 1.73 | 1.27 a | 19.99 | 56.99 | 64.87 a | 0.21 a | 0.30 a | 0.26 a | 0.20 a | 0.25 a | 0.27 |
| Si + OM + T | 0.17 b | 0.39 b | 0.12 | 3.22 b | 2.82 | 1.79 a | 2.14 a | 1.46 | 1.30 a | 24.33 | 60.54 | 67.31 a | 0.23 a | 0.29 a | 0.27 a | 0.23 a | 0.29 a | 0.28 |
| Panicles | ||||||||||||||||||
| Control | 0.02 | 0.02 | 0.01 | 0.15 b | 0.14 | 0.05 c | 0.05 | 0.02 | 0.03 | 6.52 c | 14.36 | 5.31 | 0.01 c | 0.02 c | 0.03 c | 0.03 c | 0.02 b | 0.02 c |
| Si | 0.03 | 0.03 | 0.01 | 0.19 b | 0.13 | 0.04 c | 0.10 | 0.04 | 0.03 | 12.01 b | 11.26 | 3.16 | 0.02 b | 0.04 b | 0.06 b | 0.05 b | 0.04 b | 0.04 b |
| Si + OM | 0.05 | 0.02 | 0.02 | 0.42 a | 0.14 | 0.10 a | 0.11 | 0.04 | 0.06 | 18.60 a | 13.05 | 9.85 | 0.04 a | 0.06 a | 0.09 a | 0.09 a | 0.06 a | 0.07 a |
| Si + T | 0.05 | 0.03 | 0.01 | 0.27 b | 0.13 | 0.04 c | 0.11 | 0.02 | 0.04 | 20.38 a | 15.21 | 532 | 0.03 b | 0.06 a | 0.06 b | 0.06 a | 0.06 a | 0.06 a |
| Si + OM + T | 0.06 | 0.02 | 0.01 | 0.35 a | 0.14 | 0.07 b | 0.10 | 0.03 | 0.07 | 20.02 a | 12.72 | 12.24 | 0.03 a | 0.06 a | 0.07 b | 0.07 a | 0.06 a | 0.07 a |
| Plant shoot | ||||||||||||||||||
| Control | 0.23 | 0.38 b | 0.14 | 3.58 b | 2.93 | 1.28 b | 2.54 | 1.49 | 1.16 b | 64.11 b | 99.50 b | 56.73 c | 0.42 c | 0.46 b | 0.40 b | 0.37 c | 0.35 b | 0.31 b |
| Si | 0.21 | 0.28 b | 0.18 | 3.82 b | 2.79 | 2.03 a | 2.02 | 1.60 | 1.33 b | 73.41 b | 79.74 b | 87.45 b | 0.44 c | 0.47 b | 0.46 b | 0.38 c | 0.33 b | 0.38 b |
| Si + OM | 0.32 | 0.40 b | 0.24 | 5.02 a | 3.64 | 2.33 a | 2.57 | 1.53 | 1.61 a | 90.91 a | 118.69 a | 75.53 c | 0.48 b | 0.49 b | 0.59 a | 0.51 a | 0.38 b | 0.43 a |
| Si + T | 0.43 | 0.93 a | 0.23 | 5.26 a | 3.12 | 2.35 a | 2.67 | 2.12 | 1.54 a | 87.16 a | 115.36 a | 95.25 b | 0.51 a | 0.62 a | 0.56 a | 0.44 b | 0.44 a | 0.43 a |
| Si + OM + T | 0.30 | 0.51 b | 0.19 | 4.63 a | 3.60 | 2.25 a | 2.76 | 1.83 | 1.66 a | 92.27 a | 118.93 a | 113.55 a | 0.55 a | 0.64 a | 0.59 a | 0.50 a | 0.49 a | 0.47 a |
| Saline Attenuator | DMY (t ha−1) | FMY (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut 1 | Cut 2 | Cut 3 | Total Yield | Cut 1 | Cut 2 | Cut 3 | Total Yield | |
| Control | 16.99 b | 17.59 b | 10.21 c | 44.80 b | 52.33 b | 52.28 c | 41.76 b | 146.37 b |
| Si | 18.09 b | 19.34 b | 11.71 c | 49.14 b | 55.42 b | 49.25 c | 41.58 b | 146.24 b |
| Si + OM | 22.09 a | 21.06 b | 18.88 a | 62.03 a | 62.58 a | 58.78 b | 61.07 a | 182.43 a |
| Si + T | 19.96 b | 27.36 a | 15.21 b | 62.53 a | 58.61 b | 67.14 a | 53.70 a | 179.45 a |
| Si + OM + T | 23.69 a | 20.56 b | 14.80 b | 59.05 a | 66.20 a | 51.22 c | 52.20 a | 169.62 a |
| Saline Attenuator | WUE (g DM L−1 H2O) | |||
|---|---|---|---|---|
| Cut 1 | Cut 2 | Cut 3 | Total WUE | |
| Control | 4.89 b | 3.55 b | 2.55 c | 10.98 b |
| Si | 5.20 b | 3.90 b | 2.92 c | 12.02 b |
| Si + OM | 6.35 a | 4.25 b | 4.71 a | 15.30 a |
| Si + T | 5.74 b | 5.52 a | 3.79 b | 15.05 a |
| Si + OM + T | 6.81 a | 4.14 b | 3.69 b | 14.65 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.O.N.d.; Pessoa, L.G.M.; Silva, E.M.d.; Silva, L.R.d.; Freire, M.B.G.d.S.; Souza, E.S.d.; Ferreira-Silva, S.L.; França, J.G.E.d.; Silva, T.G.F.d.; Alencar, E.L.d.N. Effects of Silicon Alone and Combined with Organic Matter and Trichoderma harzianum on Sorghum Yield, Ions Accumulation and Soil Properties under Saline Irrigation. Agriculture 2023, 13, 2146. https://doi.org/10.3390/agriculture13112146
Silva JONd, Pessoa LGM, Silva EMd, Silva LRd, Freire MBGdS, Souza ESd, Ferreira-Silva SL, França JGEd, Silva TGFd, Alencar ELdN. Effects of Silicon Alone and Combined with Organic Matter and Trichoderma harzianum on Sorghum Yield, Ions Accumulation and Soil Properties under Saline Irrigation. Agriculture. 2023; 13(11):2146. https://doi.org/10.3390/agriculture13112146
Chicago/Turabian StyleSilva, José Orlando Nunes da, Luiz Guilherme Medeiros Pessoa, Emanuelle Maria da Silva, Leonardo Raimundo da Silva, Maria Betânia Galvão dos Santos Freire, Eduardo Soares de Souza, Sérgio Luiz Ferreira-Silva, José Geraldo Eugênio de França, Thieres George Freire da Silva, and Eurico Lustosa do Nascimento Alencar. 2023. "Effects of Silicon Alone and Combined with Organic Matter and Trichoderma harzianum on Sorghum Yield, Ions Accumulation and Soil Properties under Saline Irrigation" Agriculture 13, no. 11: 2146. https://doi.org/10.3390/agriculture13112146





