Is Grazing Good for Wet Meadows? Vegetation Changes Caused by White-Backed Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Animal Management
2.3. Field Study
2.4. Statistical Analyses
3. Results
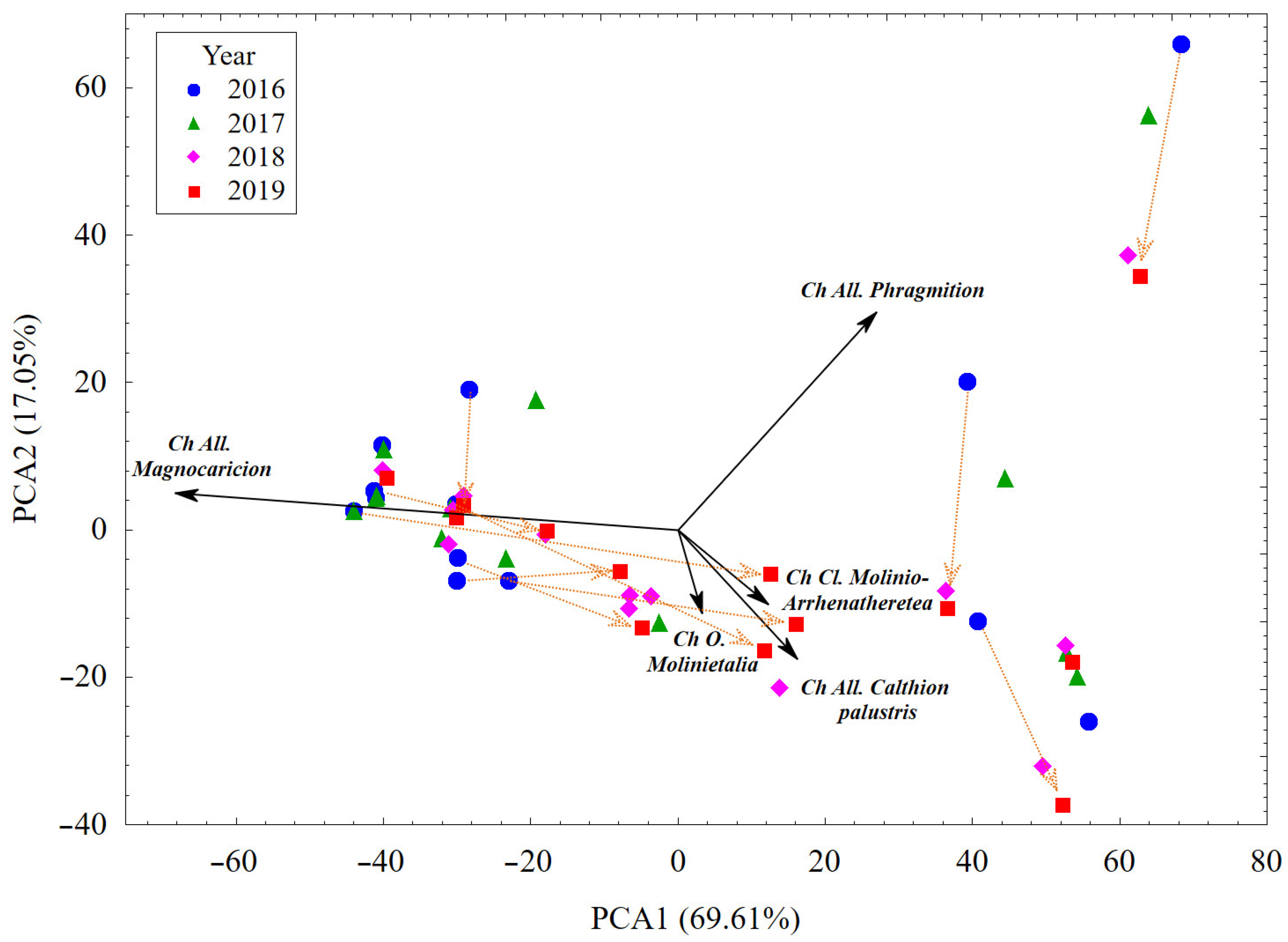
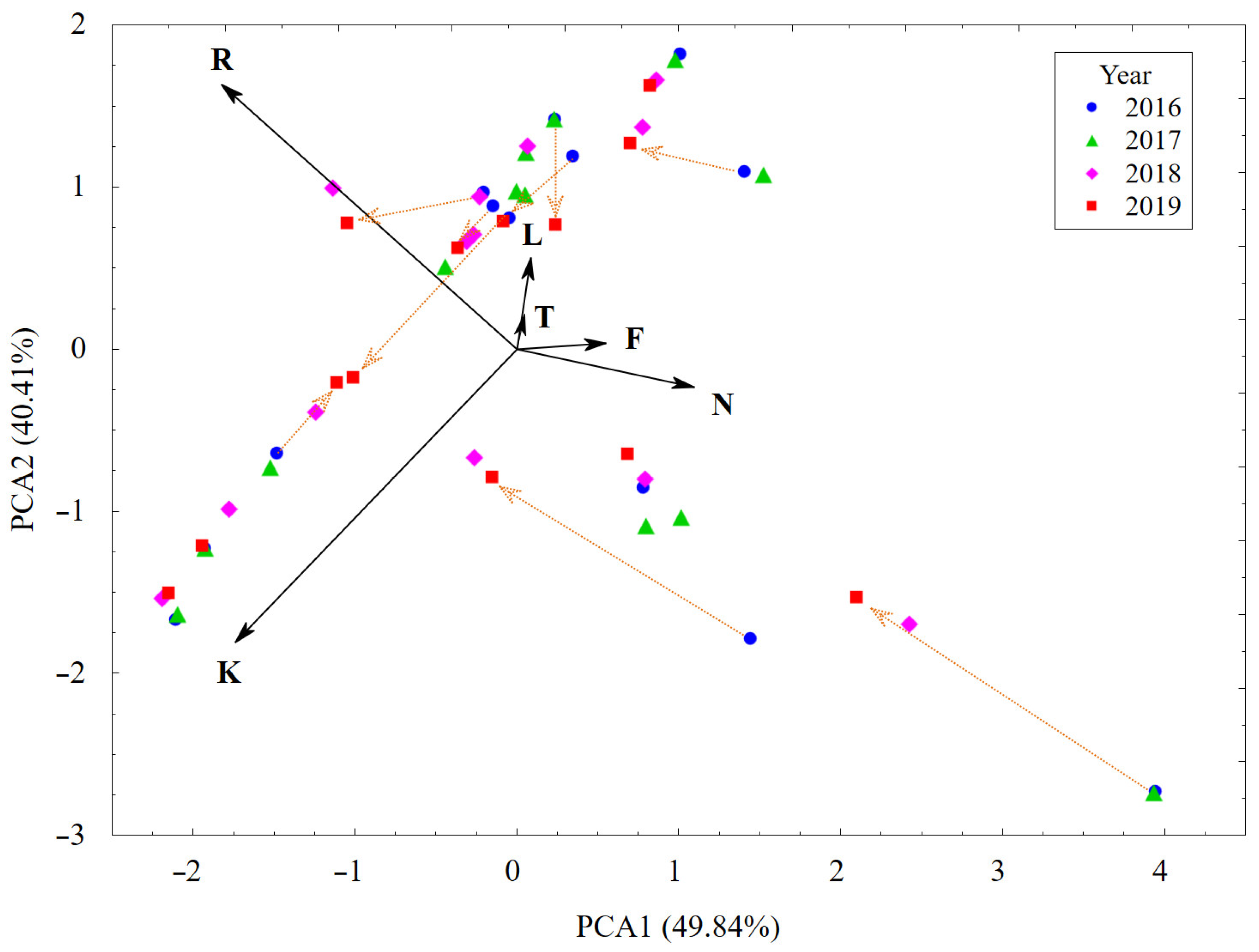
3.1. Plant Cover and Their Changes vs. Habitat Conditions
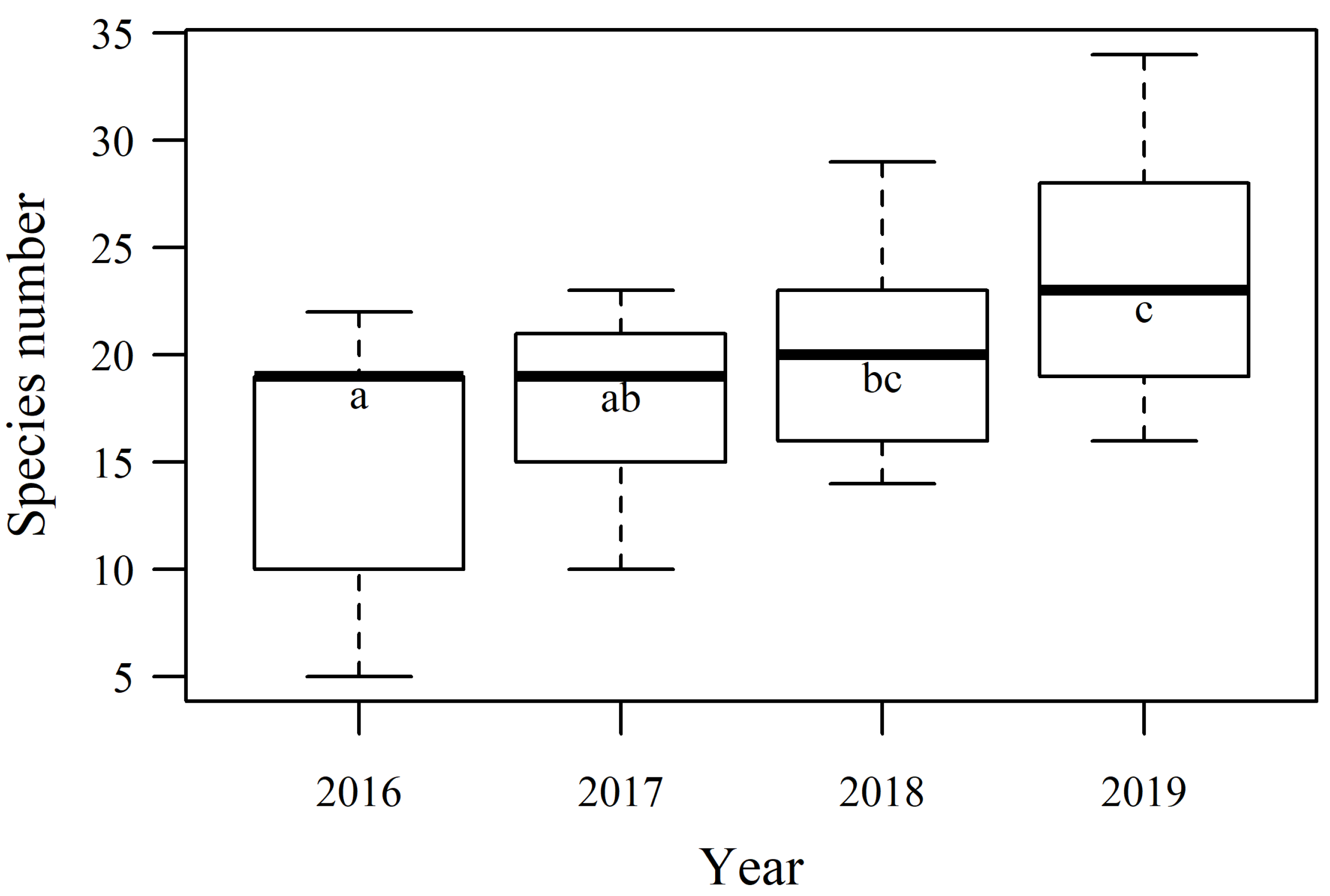
3.2. Biodiversity Dynamics
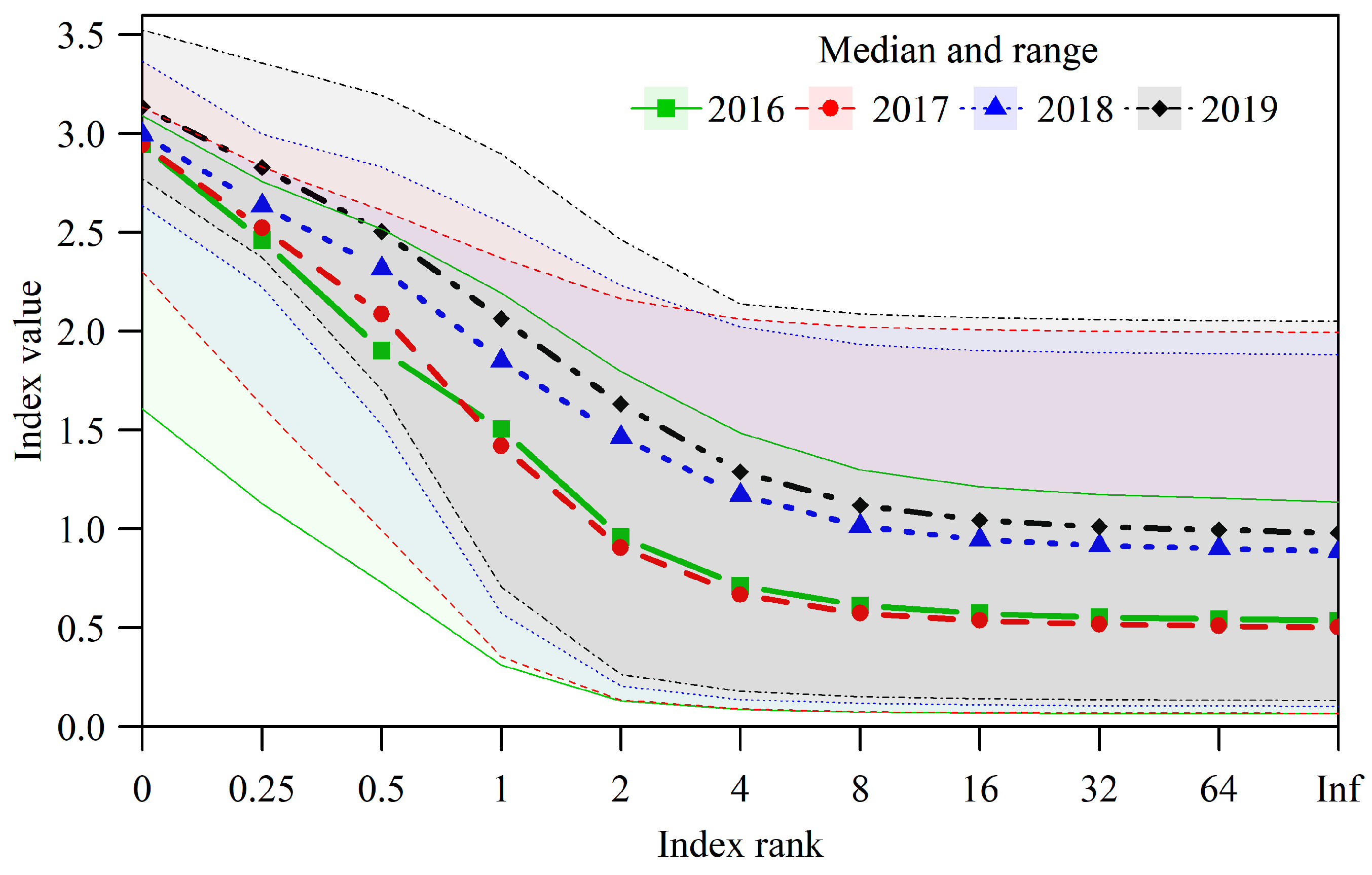
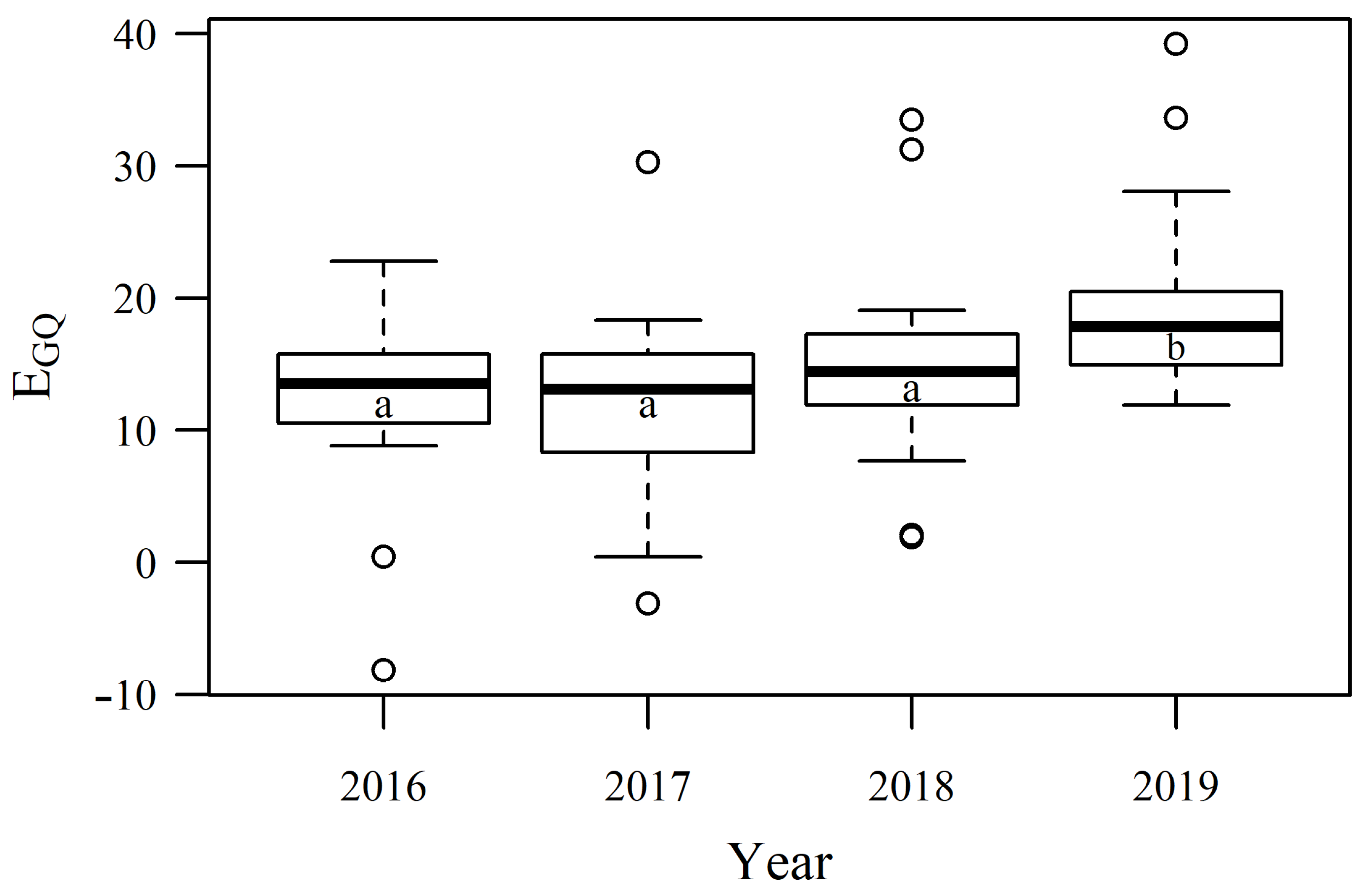
3.3. Dynamics of Pastures Forage Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen-Diaz, B.; Jackson, R.D.; Bartolome, J.W.; Tate, K.W.; Oates, L.G. Long-term grazing study in spring-fed wetlands reveals management tradeoffs. Calif. Agric. 2004, 583, 144–148. [Google Scholar]
- Kulik, M.; Bochniak, A.; Baryła, R.; Warda, M.; Wyłupek, T. Changes of the Phragmitetea Class Vegetation in the Bystra Valley, Eastern Poland. Rocz. Ochr. Sr. 2018, 20, 1049–1065. [Google Scholar]
- Oles, K.M.; Weixelman, D.A.; Lile, D.F.; Tate, K.W.; Snell, L.K.; Roche, L.M. Riparian Meadow Response to Modern Conservation Grazing Management. Environ. Manag. 2017, 60, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Marty, J.T. Effects of cattle grazing on diversity in ephemeral wetlands. Conserv. Biol. 2005, 195, 1626–1632. [Google Scholar]
- Vera, P.; Belda, E.J.; Kvist, L.; Encabo, S.I.; Monros, J.S. Habitat preference of endangered Eastern Iberian Reed Buntings Emberiza schoeniclus witherbyi. Bird Study 2011, 58, 238–247. [Google Scholar] [CrossRef]
- Wyłupek, T.; Klikocka, H.; Kulik, M.; Lipińska, H.; Gawryluk, A. Floristic diversity and use value of phytocoenoses in a section of the Wieprz river valley in Roztocze National Park for feeding the konik polski horse. Agron. Sci. 2018, 744, 123–134. [Google Scholar] [CrossRef]
- Chabuz, W.; Kulik, M.; Sawicka-Zugaj, W.; Żółkiewski, P.; Warda, M.; Pluta, M.; Lipiec, A.; Bochniak, A.; Zdulski, J. Impact of the type of use of permanent grasslands areas in mountainous regions on the floristic diversity of habitats and animal welfare. Glob. Ecol. Conserv. 2019, 19, e00629. [Google Scholar] [CrossRef]
- Kulik, M.; Warda, M.; Gawryluk, A.; Bochniak, A.; Patkowski, K.; Lipiec, A.; Gruszecki, T.M.; Pluta, M.; Bielińska, E.; Futa, B. Grazing of native livestock breeds as a method of grassland protection in Roztocze National Park, Eastern Poland. J. Ecol. Eng. 2020, 213, 61–69. [Google Scholar]
- Rysiak, A.; Chabuz, W.; Sawicka-Zugaj, W.; Zdulski, J.; Grzywaczewski, G.; Kulik, M. Comparative impacts of grazing and mowing on the floristics of grasslands in the buffer zone of Polesie National Park; eastern Poland. Glob. Ecol. Conserv. 2021, 27, e01612. [Google Scholar] [CrossRef]
- Belsky, A.J.; Matzke, A.; Uselman, S. Survey of livestock influences on stream and riparian ecosystems in the western United States. J. Soil Water Conserv. 1999, 54, 419–431. [Google Scholar]
- Kulik, M. Changes of biodiversity and species composition of Molinia meadow depending on use method. Pol. J. Environ. Stud. 2014, 233, 773–782. [Google Scholar]
- Myers, L.; Kane, J. The impact of summer cattle grazing on surface water quality in high elevation mountain meadows. Water Qual. Expo. Health 2011, 31, 51–62. [Google Scholar] [CrossRef]
- Long, J.W.; Pope, K. Wet meadows. In Science Synthesis to Support Socioecological Resilience in the Sierra Nevada and Southern Cascade Range; Long, J.W., Quinn-Davidson, L., Skinner, C.N., Eds.; USDA Forest Service, U.S. Department of Agriculture, Pacific Southwest Research Station: Albany, CA, USA, 2014; Chapter 6.3; pp. 341–372. [Google Scholar]
- Burton, E.C.; Gray, M.J.; Schmutzer, A.C.; Miller, D.L. Differential responses of postmetamorphic amphibians to cattle grazing in wetlands. J. Wildl. Manag. 2009, 732, 269–277. [Google Scholar] [CrossRef]
- Kaszewski, B.M. Climatic Conditions in Lublin Region; Wydawnictwo UMCS: Lublin, Poland, 2008; p. 76. [Google Scholar]
- Skowera, B.; Puła, J. Extreme pluviothermic conditions in the spring period in the area of Poland in 1971–2000. Acta Agrophys. 2004, 31, 171–177. [Google Scholar]
- Chmist-Sikorska, J.; Kępińska-Kasprzak, M.; Struzik, P. Agricultural drought assessment on the base of Hydro-thermal Coefficient of Selyaninov in Poland. Ital. J. Agrometeorol. 2022, 1, 3–12. [Google Scholar] [CrossRef]
- Eurostat. Glossary, Livestock Unit LSU. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Glossary,Livestock_unit_LSU (accessed on 15 June 2020).
- Braun-Blanquet, J. Plant Sociology. The Study of Plant Communities, 3rd ed.; Springer Publishing: New York, NY, USA, 1964; p. 631. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland—A Checklist; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; p. 442. [Google Scholar]
- Matuszkiewicz, W. Polish Plant Communities Guidebook; Polish Scientific Publishers: Warsaw, Poland, 2008; p. 536. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe; hierarchical floristic classification system of vascular plant; bryophyte; lichen; and algal communities. App. Veg. Sci. 2016, 19 (Suppl. 1), 3–264. [Google Scholar] [CrossRef]
- Novák, J. Evaluation of grassland quality. Ekol. Bratisl. 2004, 232, 127–143. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NY, USA, 2010; p. 255. [Google Scholar]
- Kindt, R.; Van Damme, P.; Simons, A.J. Tree diversity in western Kenya, using profiles to characterise richness and evenness. Biodivers. Conserv. 2006, 154, 1253–1270. [Google Scholar] [CrossRef]
- Avila, M.R.; Nabinger, C.; Schneider-Canny, R.; Fedrigo, J.K. Botanical composition of a natural rangeland over seeded with annual ryegrass under N fertilization. Sci. Agropecu. 2019, 102, 303–305. [Google Scholar] [CrossRef]
- Samuel, D.; Derero, A.; Kebebew, Z.; Hadgu, K.M. Tree species diversity and spatial distribution patterns on agricultural landscapes in sub-humid Oromia; Ethiopia. Agrofor. Syst. 2019, 933, 1015–1029. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 338–372. [Google Scholar]
- Ellenberg, H.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa, 2nd ed.; Verlag Erich Goltze KG: Göttingen, Germany, 1991; p. 197. [Google Scholar]
- Czarnecka, B.; Chabudziński, Ł. Assessment of flora diversity in a minor river valley using ecological indicator values, Geographical Information Systems and Digital Elevation Models. Cent. Eur. J. Biol. 2014, 9, 220–231. [Google Scholar] [CrossRef]
- R Core Team. R, A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 January 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package Vegan: Community Ecology Package. R Package Version 4.2.2. 2019. Available online: http://CRAN.Rproject.org/package=vegan (accessed on 1 January 2022).
- Acreman, M.; Holden, J. How wetlands affect floods. Wetlands 2013, 33, 773–786. [Google Scholar] [CrossRef]
- Hatfield, R.G.; LeBuhn, G. Patch and landscape factors shape community assemblage of bumble bees, Bombus spp. Hymenoptera, Apidae, in montane meadows. Biol. Conserv. 2007, 139, 150–158. [Google Scholar] [CrossRef]
- Norton, J.B.; Jungst, L.J.; Norton, U.; Olsen, H.R.; Tate, K.W.; Horwath, W.R. Soil carbon and nitrogen storage in upper montane riparian meadows. Ecosystems 2011, 14, 1217–1231. [Google Scholar] [CrossRef]
- Roche, L.M.; Latimer, A.M.; Eastburn, D.J.; Tate, K.W. Cattle grazing and conservation of a meadow-dependent amphibian species in the Sierra Nevada. PLoS ONE 2012, 7, e35734. [Google Scholar] [CrossRef] [PubMed]
- Davy, J.S.; Roche, L.M.; Robertson, A.V.; Nay, D.E.; Tate, K.W. Introducing cattle grazing to a noxious weed-dominated rangeland shifts plant communities. Calif. Agric. 2015, 69, 230–236. [Google Scholar] [CrossRef]
- Matejkova, I.; Van Diggelen, R.; Prach, K. An attempt to restore a central European species-rich mountain grassland through grazing. Appl. Veg. Sci. 2003, 6, 161–168. [Google Scholar] [CrossRef]
- Swanson, S.; Wyman, S.; Evans, C. Practical Grazing Management to Maintain or Restore Riparian Functions and Values on Rangelands. J. Rangel. Appl. 2015, 2, 1–28. [Google Scholar]
- Kiss, T.; Lévai, P.; Ferencz, Á.; Szentes, S.; Hufnagel, L.; Nagy, A.; Balogh, Á.; Pintér, O.; Saláta, D.; Házi, J.; et al. Change of composition and diversity of species and grassland management between different grazing intensity in pannonian dry and wet grasslands. App. Ecol. Environ. Res. 2011, 93, 197–230. [Google Scholar] [CrossRef]
- Kampmann, D.; Herzog, F.; Jeanneret, P.; Konold, W.; Peter, M.; Walter, T.; Wildi, O.; Lüscher, A. Mountain grassland bio diversity, Impact of site conditions versus management type. J. Nat. Conserv. 2007, 161, 12–25. [Google Scholar]
- Virágh, K.; Bartha, S. The effect of current dynamical state of a loess steppe community on its responses to disturbances. Tiscia 1996, 30, 3–13. [Google Scholar]
- Luoto, M.; Pykälä, J.; Kuussaari, M. Decline of landscape-scale habitat and species diversity after the end of cattle grazing. J. Nat. Conserv. 2003, 11, 171–178. [Google Scholar] [CrossRef]
- Pykälä, J. Plant species responses to cattle grazing in mesic semi-natural grassland. Agric. Ecosyst. Environ. 2005, 108, 109–117. [Google Scholar] [CrossRef]
- Házi, J.; Bartha, S.; Szentes, S.; Wichmann, B.; Penksza, K. Seminatural grassland management by mowing of Calamagrostis epigejos in Hungary. Plant Biosyst. 2011, 1453, 699–707. [Google Scholar] [CrossRef]
- Bullock, J.M.; Pakeman, R.J. Grazing of lowland heath in England, Management methods and their effects on healthland vegetation. Biol. Conserv. 1997, 79, 1–13. [Google Scholar] [CrossRef]
- Jutila, H. Effect of grazing on the vegetation of shore meadows along the Bothnian Sea, Finland. Plant Ecol. 1999, 140, 77–88. [Google Scholar] [CrossRef]
- Baker, B.W.; Peinetti, H.R.; Coughenour, M.B. Resilience of willow stems after release from intense elk browsing. Rangel. Ecol. Manag. 2005, 586, 575–581. [Google Scholar] [CrossRef]
- Stringham, T.K.; Krueger, W.C.; Thomas, D.R. Application of non-equilibrium ecology to rangeland riparian zones. J. Range Manag. 2001, 543, 210–217. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes and Ecosystem Properties, 2nd ed.; Wiley: Chichester, UK, 2001; p. 417. [Google Scholar]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 175, 293–302. [Google Scholar] [CrossRef]
- Tölgyesi, C.; Bátori, Z.; Erdős, L.; Gallé, R.; Körmöczi, L. Plant diversity patterns of a Hungarian steppe-wetland mosaic in relation to grazing regime and land use history. Tuexenia 2013, 35, 399–416. [Google Scholar]
- BiolFlor, Version 1.1. Search and information system on vascular plants in Germany. Available online: http://www.ufz.de/biolflor/ (accessed on 1 January 2022).
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klimes, L.; Klimesová, J.; et al. The LEDA Traitbase, A database of life-history traits of Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Kulik, M.; Warda, M.; Bochniak, A.; Stamirowska-Krzaczek, E.; Turos, P.; Dąbrowska-Zielińska, K.; Bzowska-Bakalarz, M.; Bieganowski, A.; Trendak, M. The species diversity of grasslands in the Middle Wieprz Valley PLH060005 depending on meadow type and mowing frequency. Rocz. Ochr. Sr. 2019, 21, 543–555. [Google Scholar]
- Jastrzębska, M.; Jastrzębski, W.P.; Hołdyński, C.; Kostrzewska, M.K. Weed species diversity in organic and integrated farming systems. Acta Agrobot. 2013, 66, 113–124. [Google Scholar] [CrossRef]
- Bakker, J.P. Nature Management by Grazing and Cutting: On the Ecological Significance of Grazing and Cutting Regimes Applied to Restore Former Species-Rich Grassland Communities in the Netherlands; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; p. 400. [Google Scholar]
- Nösberger, J.; Messerli, M.; Carlen, C. Biodiversity in grassland. Ann. Zootech. 1998, 47, 383–393. [Google Scholar] [CrossRef]
- Parsons, A.J.; Rowarth, J.; Thornley, J.; Newton, P. Primary production of grasslands, herbage accumulation and use, and impacts of climate change. In Grassland Productivity and Ecosystem Services; Lemaire, G., Hodgson, J., Chabbi, A., Eds.; CAB International: Wallingford, UK, 2011; pp. 3–18. [Google Scholar]
- Soussana, J.F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 1901, 9–17. [Google Scholar] [CrossRef]
- Bedoin, F.; Kristensen, T. Sustainability of grassland-based beef production—Case studies of Danish suckler farms. Livest. Sci. 2013, 158, 189–198. [Google Scholar] [CrossRef]
- Tate, K.; Roche, L.; Lile, D.; George, H. Forage and Cattle Response to Sierra Meadow Restoration; University of California Cooperative Extensionl: Davis, CA, USA, 2011; Available online: https://s3.amazonaws.com/american-rivers-website/wp-content/uploads/2016/06/21173418/5-Forage-Model.pdf (accessed on 1 January 2022).
- Ludewig, K.; Donath, T.W.; Zelle, B.; Eckstein, R.L.; Mosner, E.; Otte, A.; Jensen, K. Effects of Reduced Summer Precipitation on Productivity and Forage Quality of Floodplain Meadows at the Elbe and the Rhine River. PLoS ONE 2015, 10, e0124140. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Y.; Xu, J.; Wu, Z. Effects of climatic factors on the net primary productivity in the source region of Yangtze River, China. Sci. Rep. 2021, 11, 1376. [Google Scholar] [CrossRef]
- Teuber, L.M.; Hölzel, N.; Fraser, L.H. Livestock grazing in intermountain depressional wetlands—Effects on plant strategies, soil characteristics and biomass. Agric. Ecosyst. Environ. 2013, 175, 21–28. [Google Scholar] [CrossRef]
| Average Air Temperature (°C) | ||||
|---|---|---|---|---|
| Month | ||||
| 2016 | 2017 | 2018 | 2019 | |
| Jan | −3.9 | −5.7 | −0.2 | −3.5 |
| Feb | 3.3 | −1.6 | −4.3 | 2.1 |
| Mar | 3.5 | 5.8 | −1 | 5 |
| Apr | 9.5 | 7.7 | 13.1 | 9.4 |
| May | 14.1 | 13.5 | 16.4 | 12.7 |
| June | 18.3 | 17.9 | 18 | 20.9 |
| July | 19.3 | 18.3 | 19.8 | 18.4 |
| Aug | 18.2 | 19.7 | 20.1 | 19.6 |
| Sept | 15.1 | 13.7 | 15.7 | 14.3 |
| Oct | 6.8 | 9.2 | 9.7 | 10.8 |
| Nov | 2.6 | 3.8 | 4.5 | 6.3 |
| Dec | −0.2 | 1.8 | 0.3 | 2.6 |
| Year average | 8.9 | 8.8 | 9.5 | 9.9 |
| Total rainfall and/or snowmelt (mm) | ||||
| Month | ||||
| 2016 | 2017 | 2018 | 2019 | |
| Jan | 27.17 | 4.82 | 12.69 | 22.86 |
| Feb | 26.4 | 20.05 | 25.91 | 12.95 |
| Mar | 40.64 | 32.76 | 24.12 | 27.94 |
| Apr | 79.75 | 31.25 | 35.57 | 45.72 |
| May | 84.58 | 58.99 | 49.79 | 109.72 |
| June | 46.99 | 50.29 | 51.58 | 23.62 |
| July | 85.86 | 87.12 | 111.99 | 53.33 |
| Aug | 45.71 | 25.15 | 31.23 | 79.49 |
| Sept | 13.71 | 123.45 | 19.05 | 19.56 |
| Oct | 92.46 | 88.14 | 28.97 | 26.4 |
| Nov | 53.58 | 28.71 | 9.13 | 51.56 |
| Dec | 35.3 | 36.33 | 39.11 | 52.32 |
| Sum | 632.15 | 587.06 | 439.14 | 525.47 |
| K Index Values | K Index Classes by [16] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Year | ||||||||
| 2016 | 2017 | 2018 | 2019 | 2016 | 2017 | 2018 | 2019 | ||
| Month | Month | ||||||||
| Jan | Jan | ||||||||
| Feb | Feb | ||||||||
| Mar | Mar | ||||||||
| Apr | 2.8 | 1.35 | 0.91 | 1.62 | Apr | vw | o | d | qw |
| May | 2 | 1.46 | 1.01 | 2.88 | May | qw | o | qd | vw |
| June | 0.86 | 0.94 | 0.96 | 0.38 | June | d | d | d | exd |
| July | 1.48 | 1.59 | 1.89 | 0.97 | July | o | o | qw | d |
| Aug | 0.84 | 0.43 | 0.52 | 1.35 | Aug | d | vd | vd | o |
| Sept | 0.3 | 3 | 0.4 | 0.46 | Sept | exd | vw | exd | vd |
| Oct | 4.53 | 3.19 | 1 | 0.81 | Oct | exw | exw | d | d |
| Nov | Nov | ||||||||
| Dec | Dec | ||||||||
| Year | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|
| Livestock (pcs.) | 15 | 43 | 38 | 28 |
| LSU | 6.0 | 21.7 | 54.3 | 28.0 |
| Stocking rate LSU ha−1 | 0.12 | 0.45 | 1.12 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulik, M.; Bochniak, A.; Chabuz, W.; Żółkiewski, P.; Rysiak, A. Is Grazing Good for Wet Meadows? Vegetation Changes Caused by White-Backed Cattle. Agriculture 2023, 13, 261. https://doi.org/10.3390/agriculture13020261
Kulik M, Bochniak A, Chabuz W, Żółkiewski P, Rysiak A. Is Grazing Good for Wet Meadows? Vegetation Changes Caused by White-Backed Cattle. Agriculture. 2023; 13(2):261. https://doi.org/10.3390/agriculture13020261
Chicago/Turabian StyleKulik, Mariusz, Andrzej Bochniak, Witold Chabuz, Paweł Żółkiewski, and Anna Rysiak. 2023. "Is Grazing Good for Wet Meadows? Vegetation Changes Caused by White-Backed Cattle" Agriculture 13, no. 2: 261. https://doi.org/10.3390/agriculture13020261
APA StyleKulik, M., Bochniak, A., Chabuz, W., Żółkiewski, P., & Rysiak, A. (2023). Is Grazing Good for Wet Meadows? Vegetation Changes Caused by White-Backed Cattle. Agriculture, 13(2), 261. https://doi.org/10.3390/agriculture13020261








