Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Growth Experiments
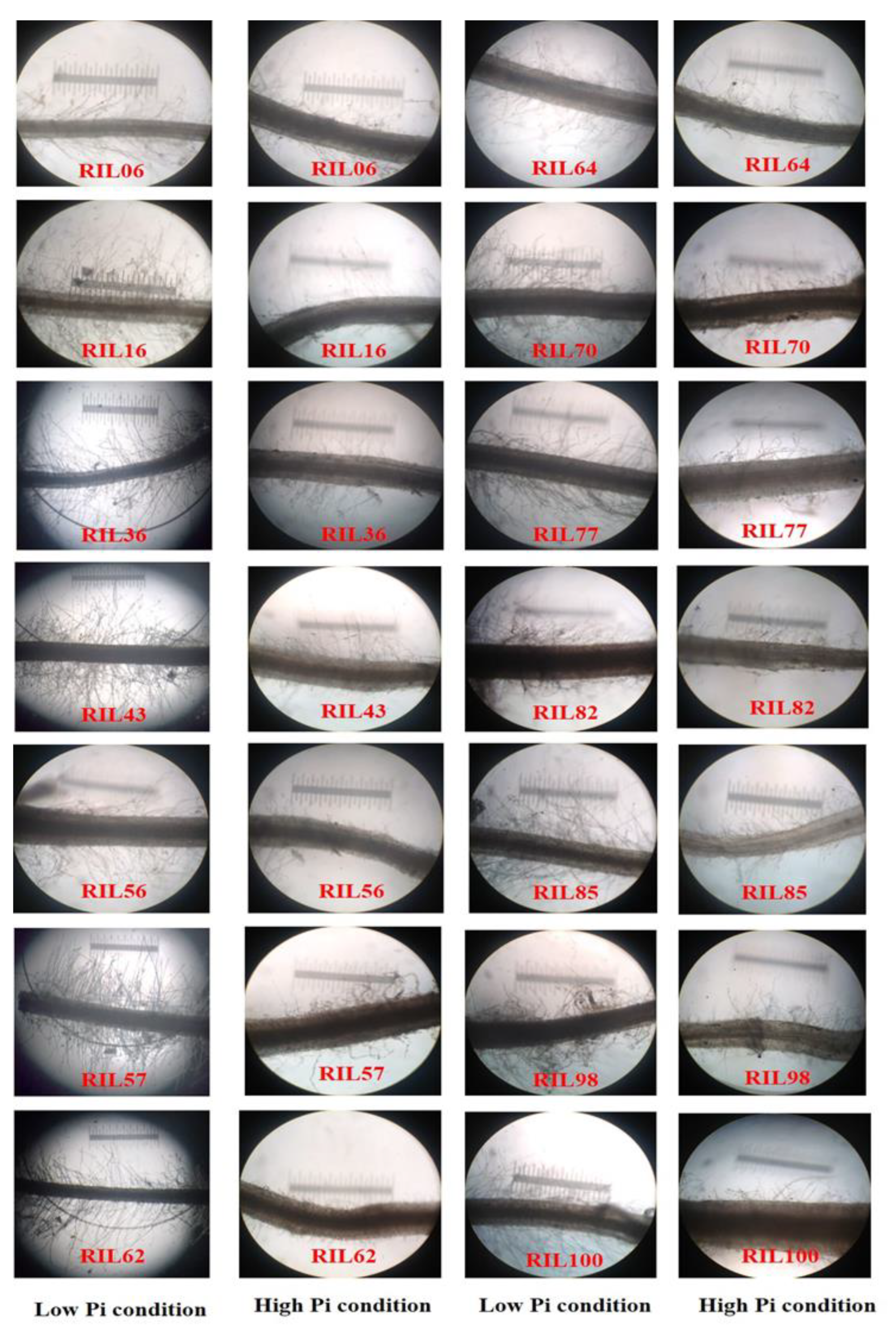
2.3. Agro-Morphological Trait Measurements
2.4. Analysis of Pi and TP Contents in Shoot and Root Tissues of RILs
2.5. Genotyping of F2 RILs for Linkage Mapping
2.6. QTL Mapping in RILs of Finger Millet for Agro-Morphological and P-Content Traits
2.7. In Silico Comparative Genomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of Agro-Morphological and P-Content Traits of Finger Millet under LP Conditions
3.2. Correlation Coefficient Variations among Agro-Morphological and P-Content Traits under LP and HP Conditions
3.3. Identification of QTL for Various Agro-Morphological, P-Content, and Root-Related Traits via Linkage Mapping under LP Conditions
3.4. Identification of QTL for Various Agro-Morphological Traits through Linkage Mapping under HP Conditions
3.5. Identification of Candidate Genes Linked to QTL from the Genome Sequences of Various Cereals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceasar, S.A.; Maharajan, T. The role of millets in attaining United Nation’s sustainable developmental goals. Plants People Planet 2022, 4, 345–349. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Ignacimuthu, S.; Ceasar, S.A. Improving the Nutrient-Use Efficiency in Millets by Genomics Approaches. In Omics of Climate Resilient Small Millets; Springer: Berlin/Heidelberg, Germany, 2022; pp. 205–220. [Google Scholar]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Mining Genes and Markers Across Minor Millets Using Comparative Genomics Approaches. In Omics of Climate Resilient Small Millets; Springer: Berlin/Heidelberg, Germany, 2022; pp. 185–203. [Google Scholar]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Nithiyanantham, S.; Kalaiselvi, P.; Mahomoodally, M.F.; Zengin, G.; Abirami, A.; Srinivasan, G. Nutritional and functional roles of millets—A review. J. Food Biochem. 2019, 43, e12859. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Finger millet [Eleusine coracana [L.] Gaertn]: An orphan crop with a potential to alleviate the calcium deficiency in the semi-arid tropics of Asia and Africa. Front. Sustain. Food Syst. 2021, 5, 684447. [Google Scholar] [CrossRef]
- Anitha, S.; Givens, D.I.; Botha, R.; Kane-Potaka, J.; Sulaiman, N.L.B.; Tsusaka, T.W. Calcium from Finger Millet—A Systematic Review and Meta-Analysis on Calcium Retention, Bone Resorption, and In Vitro Bioavailability. Sustainability 2021, 13, 8677. [Google Scholar] [CrossRef]
- Puranik, S.; Kam, J.; Sahu, P.P.; YadavM, R.; Srivastava, R.K.; Ojulong, H. Harnessing finger millet to combat calcium deficiency in humans: Challenges and prospects. Front. Plant Sci. 2017, 8, 1311. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.; Chandra, S.; Sharma, A.K. Review of Finger millet [Eleusine coracana [L.] Gaertn]: A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A. Finger Millet [Eleusine coracana [L.] Gaertn]: Nutritional Importance and Nutrient Transporters. Crit. Rev. Plant Sci. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Gull, A.; Jan, R.; Nayik, G.A.; Prasad, K.; Kumar, P. Significance of finger millet in nutrition, health and value added products: A review. Magnes 2014, 3, 1601–1608. [Google Scholar]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet [Eleusine coracana L.] polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Metwal, M.; Kaur, S.; Gupta, A.K.; Puranik, S.; Singh, S.; Yadav, R. Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front. Plant Sci. 2016, 7, 934. [Google Scholar] [CrossRef]
- Onipe, O.O.; Ramashia, S.E. Finger Millet Seed Coat—A Functional Nutrient-Rich Cereal By-Product. Molecules 2022, 27, 7837. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Maharajan, T.; Krishna, T.P.A.; RamakrishnanM, M.; Roch, G.V.; Satish, L. Finger millet [Eleusine coracana [L.] Gaertn.] improvement: Current status and future interventions of whole genome sequence. Front. Plant Sci. 2018, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ignacimuthu, S. Phosphate supply influenced the growth, yield and expression of PHT1 family phosphate transporters in seven millets. Planta 2019, 50, 1433–1448. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Ceasar, S.A.; Vinod, K.K.; Duraipandiyan, V.; Krishna, T.P.; Upadhyaya, H.D. Identification of putative QTLs for seedling stage phosphorus starvation response in finger millet [Eleusine coracana L. Gaertn.] by association mapping and cross species synteny analysis. PLoS ONE 2017, 12, e0183261. [Google Scholar] [CrossRef] [Green Version]
- Maharajan, T.; Ceasar, S.A.; Krishna, T.P.A.; Ramakrishnan, M.; Duraipandiyan, V.; Naif Abdulla, A. Utilization of molecular markers for improving the phosphorus efficiency in crop plants. Plant Breed. 2018, 137, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Roch, G.V.; Maharajan, T.; Ceasar, S.A.; Ignacimuthu, S. The Role of PHT1 Family Transporters in the Acquisition and Redistribution of Phosphorus in Plants. Crit. Rev. Plant Sci. 2019, 38, 171–198. [Google Scholar] [CrossRef]
- Baker, A.; Ceasar, S.A.; Palmer, A.J.; Paterson, J.B.; Qi, W.; Muench, S.P. Replace, reuse, recycle: Improving the sustainable use of phosphorus by plants. J. Exp. Bot. 2015, 66, 3523–3540. [Google Scholar] [CrossRef] [Green Version]
- Balyan, H.S.; Gahlaut, V.; Kumar, A.; Jaiswal, V.; Dhariwal, R.; Tyagi, S. Nitrogen and phosphorus use efficiencies in wheat: Physiology, phenotyping, genetics, and breeding. Plant Breed. Rev. 2016, 40, 167–234. [Google Scholar]
- Rose, T.J.; Liu, L.; Wissuwa, M. Improving phosphorus efficiency in cereal crops: Is breeding for reduced grain phosphorus concentration part of the solution? Front. Plant Sci. 2013, 4, 444. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Jewel, Z.A.; Mahender, A.; Anandan, A.; Hernandez, J.; Li, Z. Molecular genetics and breeding for nutrient use efficiency in rice. Int. J. Mol. Sci. 2018, 19, 1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, R.R.; Durga Rani, C.V.; Anila, M.; Mahadeva Swamy, H.K.; Bhadana, V.P.; Senguttuvel, P. Novel major QTLs associated with low soil phosphorus tolerance identified from the Indian rice landrace, Wazuhophek. PLoS ONE 2021, 16, e0254526. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Roch, G.V.; Ceasar, S.A. Recent advancements of molecular breeding and functional genomics for improving nitrogen-phosphorus-and potassium-use efficiencies in wheat. In Molecular Breeding in Wheat, Maize and Sorghum: Strategies for Improving Abiotic Stress Tolerance and Yield; CABI: Wallingford, UK, 2021; pp. 170–196. [Google Scholar]
- Kumar, K.; Yadava, P.; Gupta, M.; Choudhary, M.; Jha, A.K.; Wani, S.H. Narrowing down molecular targets for improving phosphorus-use efficiency in maize [Zea mays L.]. Mol. Biol. Rep. 2022, 49, 12091–12107. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Krishna, T.P.A.; Kiriyanthan, R.M.; Ignacimuthu, S.; Ceasar, S.A. Improving abiotic stress tolerance in sorghum: Focus on the nutrient transporters and marker-assisted breeding. Planta 2021, 254, 90. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, M.; Zheng, H.; Kong, F.; Guo, Y.; Zhao, Y. Detection of QTL for phosphorus efficiency and biomass traits at the seedling stage in wheat. Cereal Res. Commun. 2020, 48, 517–524. [Google Scholar] [CrossRef]
- Gao, S.; Xia, J.; Yuan, S.; Shen, Y.; Zhong, X.; Zhang, S. Novel QTL conferring phosphorus acquisition and utilization efficiencies in barley. Front. Genet. 2020, 11, 580452. [Google Scholar] [CrossRef]
- Gong, X.; Wheeler, R.; Bovill, W.D.; McDonald, G.K. QTL mapping of grain yield and phosphorus efficiency in barley in a Mediterranean-like environment. Theor. Appl. Genet. 2016, 129, 1657–1672. [Google Scholar] [CrossRef]
- Gong, X.; McDonald, G. QTL mapping of root traits in phosphorus-deficient soils reveals important genomic regions for improving NDVI and grain yield in barley. Theor. Appl. Genet. 2017, 130, 1885–1902. [Google Scholar] [CrossRef]
- Mahender, A.; Anandan, A.; Pradhan, S.K.; Singh, O.N. Traits-related QTLs and genes and their potential applications in rice improvement under low phosphorus condition. Arch. Agron. Soil Sci. 2018, 64, 449–464. [Google Scholar] [CrossRef]
- Ping, M.U.; Huang, C.; Jun Xia, L.I.; Li-Feng, L.I.U.; Zi-Chao, L.I. Yield trait variation and QTL mapping in a DH population of rice under phosphorus deficiency. Acta Agron. Sin. 2008, 34, 1137–1142. [Google Scholar]
- Shimizu, A.; Yanagihara, S.; Kawasaki, S.; Ikehashi, H. Phosphorus deficiency-induced root elongation and its QTL in rice [Oryza sativa L.]. Theor. Appl. Genet. 2004, 109, 1361–1368. [Google Scholar] [CrossRef]
- Ren, Y.; Qian, Y.; Xu, Y.; Zou, C.; Liu, D.; Zhao, X. Characterization of QTLs for root traits of wheat grown under different nitrogen and phosphorus supply levels. Front. Plant Sci. 2017, 8, 2096. [Google Scholar] [CrossRef] [Green Version]
- Su, J.Y.; Zheng, Q.; Li, H.W.; Li, B.; Jing, R.L.; Tong, Y.P. Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Sci. 2009, 176, 824–836. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Hassan, M.A.; Li, F.; Xia, X.; Shi, S. QTL mapping of root traits in wheat under different phosphorus levels using hydroponic culture. BMC Genom. 2021, 22, 174. [Google Scholar] [CrossRef]
- Bernardino, K.C.; Pastina, M.M.; Menezes, C.B.; de Sousa, S.M.; Maciel, L.S.; Guimarães, C.T. The genetic architecture of phosphorus efficiency in sorghum involves pleiotropic QTL for root morphology and grain yield under low phosphorus availability in the soil. BMC Plant Biol. 2019, 19, 87. [Google Scholar] [CrossRef]
- Uddin, M.S.; Azam, M.G.; Billah, M.; Bagum, S.A.; Biswas, P.L.; Khaldun, A.B.M. High-Throughput Root Network System Analysis for Low Phosphorus Tolerance in Maize at Seedling Stage. Agronomy 2021, 11, 2230. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Liu, H.; Liu, W.; Nie, Z.; Liu, D. QTL analysis of delayed maize flowering in response to low phosphate across multi-environments. Euphytica 2019, 215, 128. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Tahmasebi, A.; Richards, C. Comparative genomic analysis of quantitative trait loci associated with micronutrient contents, grain quality, and agronomic traits in wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 2142. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B.; Ravi, S.; Stevanato, P. Genomic analysis of ionome-related QTLs in Arabidopsis thaliana. Sci. Rep. 2021, 11, 19194. [Google Scholar] [CrossRef]
- David, R.H.A.; Ramakrishnan, M.; Maharajan, T.; BarathiKannan, K.; Babu, G.A.; Daniel, M.A. Mining QTL and genes for root traits and biochemical parameters under vegetative drought in South Indian genotypes of finger millet [Eleusine coracana [L.] Gaertn] by association mapping and in silico comparative genomics. Biocatal. Agric. Biotechnol. 2021, 32, 101935. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, S.; Panwar, P.; Gaur, V.S.; Sood, S. Identification of anchored simple sequence repeat markers associated with calcium content in finger millet [Eleusine coracana]. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 311–317. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Sood, S. Unraveling the genetics of calcium content in finger millet grains through association mapping. Indian J. Genet. Plant Breed. 2020, 80, 432–440. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Beynon, S.; Srivastava, R.K.; Sehgal, D.; Ojulong, H. Genome-wide association mapping and comparative genomics identifies genomic regions governing grain nutritional traits in finger millet [Eleusine coracana L. Gaertn.]. Plants People Planet 2020, 2, 649–662. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, A.; Sood, S.; Meher, P.K.; Kumar, A. Identification and validation of candidate genes for high calcium content in finger millet [Eleusine coracana [L.] Gaertn.] through genome-wide association study. J. Cereal Sci. 2022, 107, 103517. [Google Scholar] [CrossRef]
- Pendergast, I.V.T.H.; Qi, P.; Odeny, D.A.; Dida, M.M.; Devos, K.M. A high-density linkage map of finger millet provides QTL for blast resistance and other agronomic traits. Plant Genome 2022, 15, e20175. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Roch, G.V.; Ramakrishnan, M.; Ceasar, S.A.; Ignacimuthu, S. Hybridization and hybrid detection through molecular markers in finger millet [Eleusine coracana [L.] Gaertn.]. J. Crop Improv. 2020, 34, 335–355. [Google Scholar] [CrossRef]
- Li, M.; Guo, X.; Zhang, M.; Wang, X.; Zhang, G.; Tian, Y.; Wang, Z. Mapping QTLs for grain yield and yield components under high and low phosphorus treatments in maize (Zea mays L.). Plant Sci. 2010, 178, 454–462. [Google Scholar] [CrossRef]
- Vengadessan, V.; Rai, K.N.; Kannan Bapu, J.R.; Hash, C.T.; Bhattacharjee, R.; Senthilvel, S.; Nepolean, T. Construction of genetic linkage map and QTL analysis of sink-size traits in pearl millet (Pennisetum glaucum). Int. Sch. Res. Not. 2013, 2013, 471632. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Jamar, D.C.; Lou, P.; Wang, Y.; Wu, J.; Wang, X.; Vreugdenhil, D. Quantitative trait loci analysis of phytate and phosphate concentrations in seeds and leaves of Brassica rapa. Plant Cell Environ. 2008, 31, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Park, K.J.; Sa, K.J.; Koh, H.J.; Lee, J.K. QTL analysis for eating quality-related traits in an F2: 3 population derived from waxy corn× sweet corn cross. Breed. Sci. 2013, 63, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Turki, N.; Shehzad, T.; Harrabi, M.; Okuno, K. Mapping novel QTLs for tolerance to salt stress at the late vegetative stage in durum wheat (Triticum durum L.). J. King Saud. Uni. Sci. 2022, 35, 102506. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Hodge, A.; Baker, A.; Baldwin, S.A.; Öpik, M. Phosphate Concentration and Arbuscular Mycorrhizal Colonisation Influence the Growth, Yield and Expression of Twelve PHT1 Family Phosphate Transporters in Foxtail Millet [Setaria italica]. PLoS ONE 2014, 9, e108459. [Google Scholar] [CrossRef] [Green Version]
- Roch, G.V.; Maharajan, T.; Krishna, T.P.; Ignacimuthu, S.; Ceasar, S.A. Expression of PHT1 family transporter genes contributes for low phosphate stress tolerance in foxtail millet [Setaria italica] genotypes. Planta 2020, 252, 98. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1966; pp. 115–118. [Google Scholar]
- Chiou, T.; Aung, K.; Lin, S.I.; Wu, C.C.; Chiang, S.F.; Su, C. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell. 2006, 18, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Krishna, T.P.A.; Maharajan, T.; David, R.H.A.; Ramakrishnan, M.; Ceasar, S.A.; Duraipandiyan, V. Microsatellite markers of finger millet [Eleusine coracana [L.] Gaertn] and foxtail millet [Setaria italica [L.] Beauv] provide resources for cross-genome transferability and genetic diversity analyses in other millets. Biocatal. Agric. Biotechnol. 2018, 16, 493–501. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- McCouch, S.R. Gene nomenclature system for rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.; Wang, K.; Wang, J. GAHP: An integrated software package on genetic analysis with bi-parental immortalized heterozygous populations. Front. Genet. 2022, 13, 1021178. [Google Scholar] [CrossRef]
- Ceasar, S.A. Regulation of low phosphate stress in plants. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 123–156. [Google Scholar]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. [Google Scholar]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ramakrishnan, M.; Vinod, K.K.; Roch, G.V.; Upadhyaya, H.D.; Baker, A.; Ignacimuthu, S. Phenotypic responses of foxtail millet (Setaria italica) genotypes to phosphate supply under greenhouse and natural field conditions. PLoS ONE 2020, 15, e0233896. [Google Scholar] [CrossRef]
- Aluwihare, Y.C.; Ishan, M.; Chamikara, M.D.M.; Weebadde, C.K.; Sirisena, D.N.; Samarasinghe, W.L.G.; Sooriyapathirana, S.D.S.S. Characterization and selection of phosphorus deficiency tolerant rice genotypes in Sri Lanka. Rice Sci. 2016, 23, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Vejchasarn, P.; Lynch, J.P.; Brown, K.M. Genetic variability in phosphorus responses of rice root phenotypes. Rice 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari-Gowthem, G.; Kaur, S.; Sekhon, B.S.; Sharma, P.; Chhuneja, P. Genetic variation for phosphorus-use efficiency in diverse wheat germplasm. J. Crop Imp. 2019, 33, 536–550. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Root strategies for phosphorus acquisition. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 83–116. [Google Scholar]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging–an architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Xu, H.X.; Weng, X.Y.; Yang, Y. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ. J. Plant Physiol. 2007, 54, 741–748. [Google Scholar] [CrossRef]
- Hai-Bo, L.I.; Ming, X.I.A.; Ping, W.U. Effect of phosphorus deficiency stress on rice lateral root growth and nutrient absorption. J. Integr. Plant Biol. 2001, 43, 1154–1160. [Google Scholar]
- Rodriguez, D.; Maria, G.E.S.; Pomar, M.C. Phosphorus deficiency affects the early development of wheat plants. J. Agron. Crop Sci. 1994, 173, 69–72. [Google Scholar] [CrossRef]
- Su, J.; Xiao, Y.; Li, M.; Liu, Q.; Li, B.; Tong, Y. Mapping QTLs for phosphorus-deficiency tolerance at wheat seedling stage. Plant Soil 2006, 281, 25–36. [Google Scholar] [CrossRef]
- Sarker, B.C.; Karmoker, J.L.; Rashid, P. Effects of phosphorus deficiency on anatomical structures in maize [Zea mays L.]. Bangladesh J. Bot. 2010, 39, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Plenet, D.; Etchebest, S.; Mollier, A.; Pellerin, S. Growth analysis of maize field crops under phosphorus deficiency. Plant Soil 2000, 223, 119–132. [Google Scholar] [CrossRef]
- Carstensen, A.; Szameitat, A.E.; Frydenvang, J.; Husted, S. Chlorophyll a fluorescence analysis can detect phosphorus deficiency under field conditions and is an effective tool to prevent grain yield reductions in spring barley [Hordeum vulgare L.]. Plant Soil 2019, 434, 79–91. [Google Scholar] [CrossRef]
- Soleimani, B.; Sammler, R.; Backhaus, A.; Beschow, H.; Schumann, E.; Mock, H. Genetic regulation of growth and nutrient content under phosphorus deficiency in the wild barley introgression library S42IL. Plant Breed. 2017, 136, 892–907. [Google Scholar] [CrossRef]
- Gemenet, D.C.; Leiser, W.L.; Beggi, F.; Herrmann, L.H.; Vadez, V.; Rattunde, H.F.W. Overcoming phosphorus deficiency in West African pearl millet and sorghum production systems: Promising options for crop improvement. Front. Plant Sci. 2016, 7, 1389. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize [Zea mays L.] under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTL controlling root hair length in maize [Zea mays L.] under phosphorus deficiency. Plant Soil 2005, 270, 299–310. [Google Scholar] [CrossRef]
- Zhu, J.; Mickelson, S.M.; Kaeppler, S.M.; Lynch, J.P. Detection of quantitative trait loci for seminal root traits in maize [Zea mays L.] seedlings grown under differential phosphorus levels. Theor. Appl. Genet. 2006, 113, 1–10. [Google Scholar] [CrossRef]
- Wu, F.; Yang, X.; Wang, Z.; Deng, M.; Ma, J.; Chen, G.; Liu, Y. Identification of major quantitative trait loci for root diameter in synthetic hexaploid wheat under phosphorus-deficient conditions. J. Appl. Gene 2017, 58, 437–447. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Dai, A.; Liu, L.; Li, Z. Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. J. Gene Genom. 2009, 36, 173–183. [Google Scholar] [CrossRef]
- Yang, M.; Ding, G.; Shi, L.; Feng, J.; Xu, F.; Meng, J. Quantitative trait loci for root morphology in response to low phosphorus stress in Brassica napus. Theor. Appl. Gene 2010, 121, 181–193. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, X.; Mei, M.; Yan, X.; Liao, H. QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann. Bot. 2010, 106, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liao, H.; Yan, X.; Zhuang, B.; Dong, Y. Genetic variability for root hair traits as related to phosphorus status in soybean. Plant Soil 2004, 261, 77–84. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H. QTL mapping for traits related to P-deficient tolerance using three related RIL populations in wheat. Euphytica 2015, 203, 505–520. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Cai, Y.; Xu, J. QTL mapping of phosphorus efficiency and relative biologic characteristics in maize [Zea mays L.] at two sites. Plant Soil 2008, 313, 251–266. [Google Scholar] [CrossRef]
- Ming, F.; Zheng, X.; Mi, G.; He, P.; Zhu, L.; Zhang, F. Identification of quantitative trait loci affecting tolerance to low phosphorus in rice [Oryza Sativa L.]. Chin. Sci. Bull. 2000, 45, 520–525. [Google Scholar] [CrossRef]
- Li, Y.D.; Wang, Y.J.; Tong, Y.P.; Gao, J.G.; Zhang, J.S.; Chen, S.Y. QTL mapping of phosphorus deficiency tolerance in soybean [Glycine max L. Merr.]. Euphytica 2005, 142, 137–142. [Google Scholar] [CrossRef]
- Shi, R.; Li, H.; Tong, Y.; Jing, R.; Zhang, F.; Zou, C. Identification of quantitative trait locus of zinc and phosphorus density in wheat [Triticum aestivum L.] grain. Plant Soil 2008, 306, 95–104. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Ceasar, S.A.; Duraipandiyan, V.; Vinod, K.K.; Kalpana, K.; Al-Dhabi, N.A.; Ignacimuthu, S. Tracing QTLs for leaf blast resistance and agronomic performance of finger millet (Eleusine coracana (L.) Gaertn.) genotypes through association mapping and in silico comparative genomics analyses. PLoS ONE 2016, 11, e0159264. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Koizuka, N.; Martin, R.C.; Nonogaki, H. The BME3 [Blue Micropylar End 3] GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J. 2005, 44, 960–971. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Zubo, Y.O.; Tapken, W.; Kim, H.J.; Lavanway, A.M.; Howard, L. Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiol. 2012, 160, 332–348. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Han, X.; Tang, S.; Xia, X.; Yin, W. Poplar GATA transcription factor PdGNC is capable of regulating chloroplast ultrastructure, photosynthesis, and vegetative growth in Arabidopsis under varying nitrogen levels. Plant Cell Tissue Organ Cult. 2014, 119, 313–327. [Google Scholar] [CrossRef]
- An, Y.; Zhou, Y.; Han, X.; Shen, C.; Wang, S.; Liu, C. The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 2020, 71, 1969–1984. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, T.; Li, Z.; Huang, K.; Kim, N.E.; Ma, Z. OsGATA16, a GATA transcription factor, confers cold tolerance by repressing OsWRKY45–1 at the seedling stage in rice. Rice 2021, 14, 42. [Google Scholar] [CrossRef]
- Gupta, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Abiotic stresses cause differential regulation of alternative splice forms of GATA transcription factor in rice. Front. Plant Sci. 2017, 8, 1944. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Q.; Zeng, J.; He, X.; Liu, W. Genome-wide identification and characterization of GATA family genes in wheat. BMC Plant Biol. 2022, 22, 372. [Google Scholar] [CrossRef]
- Zeng, D.E.; Hou, P.; Xiao, F.; Liu, Y. Overexpressing a novel RING-H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice [Oryza sativa L.]. J. Plant Biol. 2014, 57, 357–365. [Google Scholar] [CrossRef]
- Song, J.; Xing, Y.; Munir, S.; Yu, C.; Song, L.; Li, H. An ATL78-Like RING-H2 finger protein confers abiotic stress tolerance through interacting with RAV2 and CSN5B in tomato. Front. Plant Sci. 2016, 7, 1305. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa drought-, heat-, and salt-induced RING finger protein 1 [OsDHSRP1] negatively regulates abiotic stress-responsive gene expression. Plant Mol. Biol. 2020, 103, 235–252. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Deng, D.; Chen, S.; Jiang, W.; Chen, J. Characterization and expression analysis of the maize RING-H2 finger protein gene ZmXERICO responsive to plant hormones and abiotic stresses. Acta Physiol. Plant. 2012, 34, 1529–1535. [Google Scholar] [CrossRef]
- Agarwal, P.; Khurana, P. Characterization of a novel zinc finger transcription factor [TaZnF] from wheat conferring heat stress tolerance in Arabidopsis. Cell Stress Chaperones 2018, 23, 253–267. [Google Scholar] [CrossRef]
- Han, G.; Qiao, Z.; Li., Y.; Yang., Z.; Wang., C.F.; Zhang, Y. RING Zinc Finger Proteins in Plant Abiotic Stress Tolerance. Front. Plant Sci. 2022, 13, 1055. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2. 4, an SNF1-type serine/threonine protein kinase of wheat [Triticum aestivum L.], confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceasar, S.A.; Maharajan, T.; Hillary., E.; Krishna., T.P.A. Insights to improve the plant nutrient transport by CRISPR/Cas system. Biotechnol. Adv. 2022, 59, 107963. [Google Scholar] [CrossRef] [PubMed]
- Maharajan, T.; Chellasamy, G.; Krishna, T.P.A.; Ceasar, S.A.; Yun, K. The role of metal transporters in phytoremediation: A closer look at Arabidopsis. Chemosphere 2022, 310, 136881. [Google Scholar] [CrossRef]
- Sathee, L.; Barman, D.; Nagar, S.; Tripathi., S.; Jha., S.K.; Chinnusamy., V. Genome editing targets for improving nutrient use efficiency and nutrient stress adaptation. Front. Genet. 2022, 13, 1427. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Ceasar, S.A. Application of CRISPR/Cas9 Genome Editing System to Reduce the Pre-and Post-Harvest Yield Losses in Cereals. Open Biotechnol. J. 2022, 16, e187407072205190. [Google Scholar] [CrossRef]
- Ceasar, A. Genome-editing in millets: Current knowledge and future perspectives. Mol. Biol. Rep. 2021, 49, 773–781. [Google Scholar] [CrossRef]
| Conditions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Low Pi | High Pi | ||||||||
| Min | Max | Mean | SD | CV% | Min | Max | Mean | SD | CV% | |
| PRL (cm) | 5.3 | 14.8 | 9.4 | 2.3 | 24.9 | 9.6 | 24.7 | 16.4 | 3.1 | 19.3 |
| SL (cm) | 0.9 | 5.0 | 2.7 | 0.8 | 31.6 | 3.3 | 8.1 | 5.91 | 1.0 | 17.3 |
| SFW (mg) | 57.3 | 356.3 | 192.9 | 0.08 | 43.1 | 0.9 | 4200 | 2700 | 0.7 | 28.5 |
| RFW (mg) | 29.1 | 146.7 | 76.2 | 0.03 | 41.3 | 372 | 1996 | 1017 | 0.3 | 37.3 |
| SDW (mg) | 23.8 | 196.4 | 97.16 | 0.04 | 49.1 | 366 | 1986 | 1219 | 0.3 | 36.3 |
| RDW (mg) | 9.4 | 61.7 | 29.4 | 0.01 | 45.1 | 105 | 496.6 | 296 | 0.1 | 34.5 |
| RHL (mm) | 0.006 | 0.01 | 0.01 | 0.002 | 22.1 | 0.003 | 0.01 | 0.006 | 0.002 | 31.4 |
| RHD | 24.6 | 48.6 | 33.1 | 5.5 | 16.7 | 10 | 36 | 21.7 | 5.9 | 27.2 |
| PiS ((µmoles g−1)) | 10.36 | 29.18 | 17.8 | 4.0 | 22.8 | 45.35 | 75.99 | 62.1 | 7.3 | 11.9 |
| PiR (µmoles g−1) | 6.37 | 22.81 | 12.3 | 2.7 | 22.5 | 34.90 | 65.14 | 51.7 | 7.2 | 13.9 |
| TPS (µmoles g−1) | 24.76 | 79.57 | 42.3 | 10.5 | 24.9 | 125.95 | 209.33 | 168.5 | 22.3 | 13.2 |
| TPR (µmoles g−1) | 17.25 | 56.20 | 28.8 | 7.4 | 25.8 | 97.47 | 185.77 | 142.5 | 20.5 | 14.4 |
| Trait | SL | SFW | RFW | SDW | RDW | RHL | RHD | PiS | PiR | TPS | TPR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRL | 0.735 *** | 0.566 *** | 0.531 *** | 0.493 *** | 0.502 *** | −0.037 | −0.058 | −0.108 | 0.007 | −0.126 | −0.036 |
| SL | 0.727 *** | 0.695 *** | 0.648 *** | 0.662 *** | −0.025 | −0.121 | −0.145 | −0.102 | −0.084 | −0.065 | |
| SFW | 0.917 *** | 0.864 *** | 0.833 *** | −0.011 | −0.025 | −0.159 | −0.151 | −0.004 | −0.098 | ||
| RFW | 0.884 *** | 0.943 *** | −0.030 | −0.103 | −0.131 | −0.157 | −0.011 | −0.074 | |||
| SDW | 0.868 *** | −0.061 | −0.045 | −0.147 | −0.132 | −0.014 | −0.058 | ||||
| RDW | −0.019 | −0.121 | −0.136 | −0.114 | −0.038 | −0.058 | |||||
| RHL | −0.025 | −0.085 | −0.119 | −0.026 | −0.013 | ||||||
| RHD | 0.087 | −0.004 | 0.243 * | 0.016 | |||||||
| PiS | 0.738 *** | 0.820 *** | 0.682 *** | ||||||||
| PiR | 0.561 *** | 0.865 *** | |||||||||
| TPS | 0.556 *** |
| Trait | SL | SFW | RFW | SDW | RDW | RHL | RHD | PiS | PiR | TPS | TPR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRL | 0.747 *** | 0.639 *** | 0.567 *** | 0.561 *** | 0.508 *** | 0.052 | 0.069 | 0.117 | 0.038 | 0.097 | 0.024 |
| SL | 0.816 *** | 0.700 *** | 0.711 *** | 0.656 *** | 0.044 | 0.042 | 0.122 | 0.077 | 0.122 | 0.055 | |
| SFW | 0.845 *** | 0.835 *** | 0.730 *** | 0.086 | 0.014 | 0.156 | 0.115 | 0.158 | 0.136 | ||
| RFW | 0.761 *** | 0.827 *** | 0.049 | −0.018 | 0.144 | 0.113 | 0.133 | 0.137 | |||
| SDW | 0.649 *** | 0.075 | −0.095 | 0.072 | 0.036 | 0.082 | 0.055 | ||||
| RDW | 0.025 | 0.008 | 0.015 | 0.078 | 0.050 | 0.058 | |||||
| RHL | −0.022 | 0.054 | 0.080 | 0.066 | 0.057 | ||||||
| RHD | 0.160 | 0.194 | 0.194 | 0.205 * | |||||||
| PiS | 0.866 *** | 0.952 *** | 0.841 *** | ||||||||
| PiR | 0.849 *** | 0.971 *** | |||||||||
| TPS | 0.818 *** |
| S. No. | Trait Name | QTL Name | Chromosome | Position | Left Marker | Right Marker | LOD | PVE (%) |
|---|---|---|---|---|---|---|---|---|
| 1. | PRL | qPRL-4-1 | 4 | 12 | ICECP24 | UGEP105 | 3.00 | 1.156 |
| 2. | SFW | qSFW-1-1 | 1 | 112 | UGEP12 | UGEP27 | 3.64 | 0.24 |
| qSFW-1-2 | 1 | 176 | UGEP27 | UGEP16 | 3.44 | 0.24 | ||
| qSFW-2-1 | 2 | 105 | UGEP28 | UGEP67 | 3.71 | 0.24 | ||
| qSFW-2-2 | 2 | 226 | UGEP67 | UGEP64 | 7.67 | 0.33 | ||
| qSFW-2-3 | 2 | 245 | UGEP67 | UGEP64 | 3.59 | 0.25 | ||
| qSFW-2-4 | 2 | 355 | UGEP64 | UGEP46 | 8.53 | 0.33 | ||
| qSFW-3-1 | 3 | 64 | UGEP95 | UGEP83 | 3.86 | 0.25 | ||
| qSFW-3-2 | 3 | 82 | UGEP95 | UGEP83 | 2.65 | 0.23 | ||
| qSFW-3-3 | 3 | 143 | UGEP83 | UGEP101 | 3.36 | 0.24 | ||
| qSFW-3-4 | 3 | 276 | UGEP101 | UGEP76 | 4.13 | 0.29 | ||
| qSFW-4-1 | 4 | 157 | ICECP8 | UGEP111 | 4.05 | 0.26 | ||
| qSFW-4-2 | 4 | 176 | ICECP8 | UGEP111 | 2.99 | 0.24 | ||
| qSFW-4-3 | 4 | 238 | UGEP111 | UGEP104 | 3.85 | 0.25 | ||
| qSFW-4-4 | 4 | 270 | UGEP111 | UGEP104 | 8.07 | 0.33 | ||
| qSFW-4-5 | 4 | 280 | UGEP111 | UGEP104 | 9.83 | 0.33 | ||
| 3. | RFW | qRFW-2-1 | 2 | 41 | UGEP69 | UGEP28 | 3.61 | 0.39 |
| qRFW-2-2 | 2 | 104 | UGEP28 | UGEP67 | 5.38 | 0.42 | ||
| qRFW-2-3 | 2 | 206 | UGEP67 | UGEP64 | 3.51 | 0.37 | ||
| qRFW-2-4 | 2 | 241 | UGEP67 | UGEP64 | 3.38 | 0.36 | ||
| qRFW-2-5 | 2 | 338 | UGEP64 | UGEP46 | 3.94 | 0.41 | ||
| qRFW-2-6 | 2 | 362 | UGEP64 | UGEP46 | 4.21 | 0.42 | ||
| qRFW-3-1 | 3 | 62 | UGEP95 | UGEP83 | 3.81 | 0.35 | ||
| qRFW-3-2 | 3 | 142 | UGEP83 | UGEP101 | 3.37 | 0.35 | ||
| qRFW-3-3 | 3 | 219 | UGEP101 | UGEP76 | 2.62 | 0.33 | ||
| qRFW-4-1 | 4 | 159 | ICECP8 | UGEP111 | 4.33 | 0.39 | ||
| qRFW-4-2 | 4 | 235 | UGEP111 | UGEP104 | 2.82 | 0.33 | ||
| qRFW-4-3 | 4 | 269 | UGEP111 | UGEP104 | 2.83 | 0.42 | ||
| qRFW-4-4 | 4 | 288 | UGEP111 | UGEP104 | 4.81 | 0.41 | ||
| 4. | SDW | qSDW-1-1 | 1 | 187 | UGEP27 | UGEP16 | 2.7 | 3.04 |
| qSDW-2-1 | 2 | 107 | UGEP28 | UGEP67 | 4.72 | 4.47 | ||
| qSDW-2-2 | 2 | 224 | UGEP67 | UGEP64 | 3.36 | 4.06 | ||
| qSDW-2-3 | 2 | 377 | UGEP64 | UGEP46 | 4.95 | 4.59 | ||
| qSDW-3-1 | 3 | 257 | UGEP101 | UGEP76 | 2.95 | 3.44 | ||
| qSDW-4-1 | 4 | 157 | ICECP8 | UGEP111 | 2.62 | 3.5 | ||
| qSDW-4-2 | 4 | 293 | UGEP111 | UGEP104 | 7.18 | 4.61 | ||
| 5. | RDW | qRDW-1-1 | 1 | 110 | UGEP12 | UGEP27 | 2.72 | 1.19 |
| qRDW-1-2 | 1 | 182 | UGEP27 | UGEP16 | 3.47 | 1.21 | ||
| qRDW-2-1 | 2 | 40 | UGEP69 | UGEP28 | 4.33 | 1.56 | ||
| qRDW-2-2 | 2 | 103 | UGEP28 | UGEP67 | 5.08 | 1.6 | ||
| qRDW-2-3 | 2 | 222 | UGEP67 | UGEP64 | 3.36 | 1.24 | ||
| qRDW-2-4 | 2 | 386 | UGEP64 | UGEP46 | 4.14 | 1.6 | ||
| qRDW-3-1 | 3 | 61 | UGEP95 | UGEP83 | 3.59 | 1.42 | ||
| qRDW-3-2 | 3 | 261 | UGEP101 | UGEP76 | 3.14 | 1.26 | ||
| qRDW-4-1 | 4 | 160 | ICECP8 | UGEP111 | 3.62 | 1.37 | ||
| qRDW-4-2 | 4 | 298 | UGEP111 | UGEP104 | 4.76 | 1.35 | ||
| 6. | RHD | qRHD-2-1 | 2 | 254 | UGEP67 | UGEP64 | 4.37 | 4.17 |
| qRHD-2-2 | 2 | 311 | UGEP64 | UGEP46 | 4.28 | 4.21 | ||
| qRHD-3-1 | 3 | 217 | UGEP101 | UGEP76 | 3.51 | 3.79 | ||
| qRHD-3-2 | 3 | 305 | UGEP101 | UGEP76 | 3.12 | 4.2 | ||
| 7. | PiS | qPiS-1-1 | 1 | 163 | UGEP27 | UGEP16 | 3.72 | 8.39 |
| qPiS-3-1 | 3 | 68 | UGEP95 | UGEP83 | 2.52 | 9.45 | ||
| 8. | PiR | qPiR-3-1 | 3 | 18 | UGEP78 | UGEP95 | 2.58 | 10.72 |
| 9. | TPS | qTPS-1-1 | 1 | 123 | UGEP12 | UGEP27 | 5.01 | 8.82 |
| qTPS-1-2 | 1 | 162 | UGEP27 | UGEP16 | 5.16 | 8.82 |
| S. No. | Trait Name | QTL Name | Chromosome | Position | Left Marker | Right Marker | LOD | PVE (%) |
|---|---|---|---|---|---|---|---|---|
| 1. | PRL | qPRL-4-1 | 4 | 204 | UGEP111 | UGEP104 | 3.18 | 13.65 |
| 2. | RFW | qRFW-1-1 | 1 | 50 | UGEP5 | UGEP12 | 23.08 | 1.96 |
| qRFW-1-2 | 1 | 103 | UGEP12 | UGEP27 | 25.13 | 1.96 | ||
| qRFW-1-3 | 1 | 185 | UGEP27 | UGEP16 | 25.64 | 2 | ||
| qRFW-2-1 | 2 | 26 | UGEP69 | UGEP28 | 21.89 | 1.86 | ||
| qRFW-2-2 | 2 | 120 | UGEP28 | UGEP67 | 11.11 | 1.85 | ||
| qRFW-2-3 | 2 | 221 | UGEP67 | UGEP64 | 30.34 | 2 | ||
| qRFW-2-4 | 2 | 339 | UGEP64 | UGEP46 | 20.68 | 1.86 | ||
| qRFW-3-1 | 3 | 74 | UGEP95 | UGEP83 | 20.58 | 1.86 | ||
| qRFW-3-2 | 3 | 148 | UGEP83 | UGEP101 | 24.26 | 1.98 | ||
| qRFW-3-3 | 3 | 221 | UGEP101 | UGEP76 | 21.24 | 1.86 | ||
| qRFW-4-1 | 4 | 46 | UGEP105 | UGEP109 | 18.73 | 1.86 | ||
| qRFW-4-2 | 4 | 154 | ICECP8 | UGEP111 | 20.83 | 1.88 | ||
| qRFW-4-3 | 4 | 269 | UGEP111 | UGEP104 | 20.75 | 1.86 | ||
| 3. | SDW | qSDW-1-1 | 1 | 102 | UGEP12 | UGEP27 | 29.4 | 0.29 |
| qSDW-2-1 | 2 | 28 | UGEP69 | UGEP28 | 9.94 | 0.21 | ||
| qSDW-2-2 | 2 | 118 | UGEP28 | UGEP67 | 29.71 | 0.29 | ||
| qSDW-2-3 | 2 | 229 | UGEP67 | UGEP64 | 36.46 | 0.31 | ||
| qSDW-2-4 | 2 | 387 | UGEP64 | UGEP46 | 30.13 | 0.29 | ||
| qSDW-3-1 | 3 | 75 | UGEP95 | UGEP83 | 34.66 | 0.31 | ||
| qSDW-3-2 | 3 | 138 | UGEP83 | UGEP101 | 30.8 | 0.3 | ||
| qSDW-3-3 | 3 | 158 | UGEP83 | UGEP101 | 30.16 | 0.29 | ||
| qSDW-3-4 | 3 | 238 | UGEP101 | UGEP76 | 33.41 | 0.31 | ||
| qSDW-4-1 | 4 | 47 | UGEP105 | UGEP109 | 26.46 | 0.29 | ||
| qSDW-4-2 | 4 | 101 | UGEP109 | ICECP8 | 30.68 | 0.3 | ||
| qSDW-4-3 | 4 | 165 | ICECP8 | UGEP111 | 33.41 | 0.3 | ||
| qSDW-4-4 | 4 | 248 | UGEP111 | UGEP104 | 36.81 | 0.31 | ||
| 4. | RHL | qRHL-1-1 | 1 | 24 | UGEP5 | UGEP12 | 2.7 | 14.28 |
| 5. | PiS | qPiS-3-1 | 3 | 286 | UGEP101 | UGEP76 | 3.44 | 12.97 |
| qPiS-4-1 | 4 | 24 | ICECP24 | UGEP105 | 2.69 | 2.58 | ||
| 6. | PiR | qPiR-1-1 | 1 | 183 | UGEP27 | UGEP16 | 2.97 | 8.76 |
| 7. | TPS | qTPS-1-1 | 1 | 199 | UGEP27 | UGEP16 | 3.72 | 6.57 |
| qTPS-2-1 | 2 | 125 | UGEP28 | UGEP67 | 2.56 | 7.87 | ||
| qTPS-3-1 | 3 | 282 | UGEP101 | UGEP76 | 3.04 | 7.98 | ||
| qTPS-4-1 | 4 | 36 | UGEP105 | UGEP109 | 3.67 | 3.59 | ||
| qTPS-4-2 | 4 | 286 | UGEP111 | UGEP104 | 3.32 | 3.32 | ||
| 8. | TPR | qTPR-1-1 | 1 | 192 | UGEP27 | UGEP16 | 3.48 | 3.48 |
| Name of the Marker | Species Name | Chromosome | E Value | Score | Transcript ID | Name of the Gene | Location Position |
|---|---|---|---|---|---|---|---|
| UGEP16 | Panicum virgatum | Chr01 | 6.2 × 10−2 | 41.0 | Pavir.Aa02795.1 | IgA-specific serine endopeptidase | 10.7 kb [US] |
| UGEP27 | Panicum hallii | Chr05 | 2.7 × 10−9 | 66.2 | Pahal.E03230.1 | Myb/SANT-like DNA-binding domain [Myb_DNA-bind_3] | 21.48 kb [DS] |
| Chr08 | 2.1 × 10−4 | 50.0 | Pahal.E03231.1 | ATP-binding cassette, subfamily B [MDR/TAP], member 1 [ABCB1] | 53.7 kb [DS] | ||
| Chr08 | 3.1 × 10−2 | 42.8 | Pahal.E03229.1 | Transferase family | 53.7 kb [US] | ||
| Setaria italica | Chr09 | 1.5 × 10−4 | 50 | Seita.9G325200.1 | Gata transcription factor 2 | 5.37 Kb | |
| Chr08 | 5.4 × 10−7 | 59 | Sobic.008G098300.1 | Ubiquitin carboxyl-terminal hydrolase 48 | 1.07 Kb [US] | ||
| UGEP67 | Setaria italica | Chr09 | 5.8 × 10−4 | 46.4 | Seita.9G146300.1 | BTB/POZ domain [BTB]//NPR1/NIM1 like defense protein C terminal [NPR1-like-C] | 5.37 Kb [US] |
| Panicum hallii | Chr06 | 1.1 × 10−14 | 82.4 | Pahal.F01859.1 | RING-H2 finger protein ATL13-related | 5.37 kb [DS] | |
| Chr03 | 1.3 × 10−13 | 78.8 | Pahal.C00565.1 | Serine/yhreonine-protein kinase | 1.07 kb [DS] | ||
| Brachypodium distachyon | Chr03 | 9.1 × 10−6 | 51.8 | Bradi3g33410.1 | Homeodomain-Leucine Zipper II family protein | 21.48 kb [DS] | |
| UGEP 95 | Setaria italica | Chr02 | 9.8 × 10−2 | 39.2 | Seita.2G255900.1 | Heat shock protein 70KDA | 53.7 kb [US] |
| Chr02 | 9.8 × 10−2 | 39.2 | Seita.2G255700.1 | Calcium-binding EGF domain [EGF-CA] | 53.7 kb [DS] | ||
| Zea mays | Chr04 | 1.8 × 10−6 | 57.2 | Grmzm2g173903_T01 | Beta catenin-related armadillo repeat-containing | 21.48 kb [DS] | |
| UGEP101 | Zea mays | Chr03 | 1.8 × 10−5 | GRMZM2G130864_T01 | 1,4-β-Glucanase | 21.82 [DS] | |
| UGEP104 | Brachypodium distachyon | Chr01 | 1.0 × 10−47 | Bradi1g38238.1 | Ethylene-responsive transcription factor | 58.45 [US] | |
| Chr05 | 2.8 × 10−4 | Bradi5g11270.1 | MADS TF | 1.26 [US] | |||
| Panicum virgatum | Chr03a | 2.2 × 10−46 | Pavir.Ca00579.1 | MADS box protein | 82.64 [US] | ||
| Setaria italica | Chr07 | 6.1 × 10−2 | Seita.7G132800.1 | C2H2-type zinc finger | 26.66 [US] | ||
| Chr04 | 6.5 × 10−2 | Seita.4G278800.1 | Ser/Thr Protein kinase | 6.29 [DS] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharajan, T.; Ajeesh Krishna, T.P.; Rakkammal, K.; Ramakrishnan, M.; Ceasar, S.A.; Ramesh, M.; Ignacimuthu, S. Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping. Agriculture 2023, 13, 262. https://doi.org/10.3390/agriculture13020262
Maharajan T, Ajeesh Krishna TP, Rakkammal K, Ramakrishnan M, Ceasar SA, Ramesh M, Ignacimuthu S. Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping. Agriculture. 2023; 13(2):262. https://doi.org/10.3390/agriculture13020262
Chicago/Turabian StyleMaharajan, Theivanayagam, Thumadath Palayullaparambil Ajeesh Krishna, Kasinathan Rakkammal, Muthusamy Ramakrishnan, Stanislaus Antony Ceasar, Manikandan Ramesh, and Savarimuthu Ignacimuthu. 2023. "Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping" Agriculture 13, no. 2: 262. https://doi.org/10.3390/agriculture13020262
APA StyleMaharajan, T., Ajeesh Krishna, T. P., Rakkammal, K., Ramakrishnan, M., Ceasar, S. A., Ramesh, M., & Ignacimuthu, S. (2023). Identification of QTL Associated with Agro-Morphological and Phosphorus Content Traits in Finger Millet under Differential Phosphorus Supply via Linkage Mapping. Agriculture, 13(2), 262. https://doi.org/10.3390/agriculture13020262









