Abstract
Seaweeds could be alternative feedstuffs for ruminants, but their utilization in practical feeding is difficult because they deteriorate rapidly. We investigated the possibility of preserving Saccharina latissima and Porphyra umbilicalis in multinutrient blocks (MB), which is a cost-effective preservation method for high-moisture feeds. Three different MB were prepared: without seaweed (control; CMB), with 25% of fresh S. latissima (SLMB), and with 36% of fresh P. umbilicalis (PUMB). Both seaweeds and MB were fermented in vitro with ruminal fluid from sheep. The nitrogen and fiber content of P. umbilicalis was 4- and 4.5-fold greater, respectively, than in S. latissima, but P. umbilicalis produced less gas than S. latissima. Both CMB and PUMB had similar in vitro dry matter degradability (65.8 and 65.1%, respectively), but SLMB had lower values (p < 0.05; 58.2%). There were no differences among MB in methane and total VFA production, but the VFA pattern was shifted to acetate in SLMB and to butyrate in PUMB. The results indicate that multinutrient blocks could be a feasible option to preserve and store seaweeds for ruminant feeding without compromising ruminal fermentation, but in vivo studies are needed to assess the effects on intake and animal performance.
1. Introduction
The world population is expected to reach more than 9 billion people by the year 2050 [1] and consequently global demands of high-quality food, such as meat and milk, will likely also increase. However, current livestock systems rely on feedstuffs such as cereals and soybean meal, which could be used for human consumption instead. In addition, these feedstuffs are frequently imported from other countries, thus increasing the carbon footprint of animal products for human consumption. For that reason, it is important to find and utilize alternative sources of nutrients for livestock feeding, such as seaweeds [2]. Although seaweeds have been used in animal feeding for a long time, their utilization has always been controversial [3]. Seaweeds are rich in microminerals, complex carbohydrates, pigments, polyunsaturated fatty acids, and bioactive compounds [4,5], but they can also contain considerable amounts of non-digestible dietary fiber [6]; thus, fiber-rich seaweeds might be more suitable for ruminant than for monogastric feeding, as ruminal microbiota can degrade and ferment dietary fiber [7]. Red seaweeds, such as Porphyra spp., can contain up to 347 g/kg of high-quality protein [8] including all the essential amino acids [9]. On the other hand, brown seaweeds such as Saccharina latissima have a relatively low protein content (50–150 g/kg dry matter (DM)), but a considerable amount of easily fermentable carbohydrates such as mannitol and laminarin [10]. Moreover, S. latissima could be an important supply of nutrients for animal feeding due to its high biomass yield and ease of cultivation [11]. Seaweeds might potentially replace protein concentrates, cereals or other fibrous feedstuffs commonly used in ruminant diets [8]. Indeed, diets containing up to 90% of brown seaweeds have been used to feed sheep in coastal regions [12]. In addition, seaweed cultivation does not compete with terrestrial crops, do not require fresh water and could potentially decrease atmospheric CO2 levels, and thus their utilization in ruminant feeding might contribute to the sustainability of the livestock sector [6]. In recent years, the interest in utilizing seaweeds for ruminant feeding has increased because it can help to fulfill the ‘Blue Growth’ objectives and to alleviate the shortage of feedstuffs in certain situations [13], as well as to decrease ruminants’ enteric methane emissions [14].
However, utilization of seaweeds for ruminant feeding can be difficult as they start to decompose shortly after harvest because of their high water content [15], and therefore low-cost conservation and storage methods are needed. Recently, several authors have evaluated different ensiling procedures for seaweeds [15,16,17], but this process demands optimization due to seaweeds characteristics that challenge the silage process [15,18]. Another cost-effective preservation method that might facilitate the inclusion of seaweeds in diets for ruminants are the multinutrient blocks (MB). Multinutrient blocks are a solid mixture of different feeds and usually include urea, a binder agent, salt and a mineral- vitamin supplement [19], and they have previously been used to preserve different wet feedstuffs or by-products [20,21,22]. In the practice, MB including different ingredients can be used to partially replace the concentrate given to animals without compromising ruminal fermentation and production level [23,24]. However, to our best knowledge, there is no information available regarding the feasibility of MB as a preservation method for seaweeds. Therefore, the objective of this study was to analyze the possibility of preserving two commercially interesting seaweed species; Saccharina latissima and Porphyra umbilicalis, by including them in MB as replacement of conventional feedstuffs, and to assess the in vitro fermentation and methane production when the MB were incubated with ruminal fluid from sheep.
2. Materials and Methods
The sheep used in this study as donors of ruminal fluid for the in vitro trials were handled and cared for following the European guidelines for experimental animal protection. The experimental procedures for rumen content sampling were approved by the Institutional Animal Care and Use Committee of the Comunidad Autónoma de Madrid of Spain (Approval number PROEX 035/17) before starting the in vitro trials.
2.1. Seaweeds Samples and Reference Feeds
Biomass of P. umbilicalis and S. latissima was collected by hand from wild stocks at the coast of Bodø (Norway; 67°16′57″ N 14°22′30″ E). Seaweeds were sampled in middle June based on previous studies showing that nitrogen (N) content was greater and neutral detergent fiber (NDF) content was lower in seaweeds collected in spring compared with those collected in autumn [6]. Seaweed samples were maintained in running-water tanks and processed within 48 h after collection. Firstly, the seaweeds were washed 3 times in water with decreasing salinity (seawater, 70:30 seawater: fresh water mixture, and finally fresh water) and hand squeezed. Then, samples were manually cut into pieces of approximately 2 × 3 cm using scissors before including them into MB.
In addition, a sample of 3 feedstuffs widely used in ruminant feeding (grass hay, corn grains and sunflower meal) were obtained from a local animal feed factory to be used as reference in the in vitro incubations.
2.2. Multinutrient Block Manufacturing
The MB were prepared as described by Molina-Alcaide et al. [23]. Briefly, the procedure consisted of mixing thoroughly all feedstuffs and other ingredients before packing the resultant mixture into round metal molds of 30 cm diameter. Molasses, urea, calcium carbonate, sepiolite, salt and the vitamin-mineral mixture were initially mixed with water, and then the rest of the feedstuffs were added. The mixture was heavily packed within the molds and then it was removed from the molds. The compacted MB were stored in a dark ventilated temperature-controlled room at 20 °C until performing the analyses (Figure 1). Once a week, the MB were turned to accelerate the drying process.

Figure 1.
Multinutrient blocks (MB) stored at room temperature (20 °C).
The ingredient composition of the experimental MB is given in Table 1. The control MB (CMB) contained feedstuffs commonly used in ruminant feeding. The amounts of S. latissima and P. umbilicalis in the MB including seaweeds were selected to fully replace corn and soyabean meal, respectively, in the control MB while maintaining similar N and NDF composition. The MB including S. latissima (SLMB) contained 25% of fresh seaweed and did not include corn grains. The MB including P. umbilicalis (PUMB) contained 36% of fresh seaweed and did not include soybean meal and pea hull fiber. The amount of other ingredients was also modified in SLMB and PUMB. Urea was included in the PUMB to reach similar N content. In both SLMB and PUMB, grass hay and molasses were used to help compaction, and the content of calcium carbonate, salt and vitamin-mineral premix was reduced to half of the CMB due to the high ash content of the seaweeds. After 2 months of storage, 4 MB of each type were randomly selected and sampled for conducting the analysis of chemical composition and in vitro incubations.

Table 1.
Ingredient composition (g/kg fresh matter) of multinutrient blocks including no seaweed (Control; CMB), fresh Saccharina latissima (SLMB) or fresh Porphyra umbilicalis (PUMB).
2.3. Experimental Procedures for Measuring Gas Production Kinetics
Ruminal fluid for the in vitro incubations was obtained from 4 Lacaune sheep (64.0 ± 1.85 kg body weight) provided with a permanent rumen cannula. The animals were housed in floor pens with straw bedding, had free access to fresh water and were fed twice daily (8:00 and 18:00) a diet of grass hay and concentrate in 2:1 proportion. The diet was supplied at a rate of 45 g DM/kg body weight0.75 to prevent feed selection.
The in vitro incubations were performed as described by Tejido et al. [25]. Samples of the both seaweeds, the 3 reference feedstuffs, and the MB were accurately weighed (500 mg) in glass vials of 120 mL. Ruminal content was obtained from each sheep before the morning feeding and strained through four layers of cheesecloth. The obtained fluid was mixed (1:4 ratio) with the buffer solution of Goering and Van Soest [26] at 39 °C and under continuous CO2 flushing. Fifty mL of the mixture were added to each vial using a peristaltic pump. The ruminal fluid of each sheep was independently mixed with the culture medium to obtain 4 replicates for each sample. Vials were capped with rubber stops and incubated at 39 °C for 120 h to measure the gas production kinetics. The amount of gas produced was measured at 2.5, 5, 7.5, 10, 15, 21, 26, 31, 36, 48, 60, 72, 96, and 120 h using a Delta Ohm DTP704-2BGI pressure transducer (Herter Instruments SL, Barcelona, Spain) and a plastic syringe. After each measurement the gas was released. In addition, 2 vials without substrate (blanks) were incubated for each ruminal inoculum to correct for the gas produced from the substrates added with the ruminal fluid.
2.4. Experimental Procedures for Measuring Ruminal Fermentation Parameters and Methane Production of Multinutrient Blocks
The 4 samples of each type of MB (CMB, SLMB and PUMB; 12 samples in total) were incubated in vitro following the same methodology described previously for measuring the gas production kinetics, with the exception that incubations lasted 24 h. After 24 h, the amount of gas produced in each vial was measured as described previously and a 12 mL sample of gas was collected in vacuum tubes (Terumo Europe N.V., Leuven, Belgium) that were maintained at room temperature before the analysis of CH4 concentration. The content of the vials (mixture of the MB sample, ruminal fluid and buffer) was homogenized by hand-shaken before opening and measuring the pH of the vials content (Crison Basic 20 pHmeter; Crison Instruments, Barcelona, Spain). The vials were then introduced in an iced-water bath and 3 mL of the content were sampled and mixed with 3 mL of 0.5 M HCl. Samples were stored frozen (−20 °C) and used for the analysis of volatile fatty acids (VFA) and NH3-N concentrations. As in the previous incubations, the ruminal fluid from each sheep was used independently as inoculum to obtain 4 replicates for each MB sample, making a total of 16 replicates for each MB type (CMB, SLMB and PUMB).
The in vitro DM degradability (IVDMD) of the MB was measured using a Ankom Daisy II incubator (Ankom Technology Corp., Fairport, NY, USA) as described by Marcos et al. [27]. In brief, 300 mg were weighed in triplicate into polyester bags (30 µm pore size; Ankom Corp #57, Ankom Technology Corp., Fairport, NY, USA) and bags were heat-sealed. Bags were incubated at 39 °C in the 1:4 mixture of ruminal fluid and the incubation medium of Goering and Van Soest [26] previously described for 48 h under continuous rotation. After 48 h, bags were washed with cold water, dried at 60 °C for 48 h, and weighed to calculate the IVDMD.
2.5. Chemical Composition Analyses
The chemical composition of seaweeds, reference feedstuffs and MB were analysed as described by Marcos et al. [28] and all analyses were performed in duplicate. Briefly, the procedures of the Association of Official Analytical Chemists [29] were used to analyse the content in DM, ash and ether extract (EE), whereas the content in neutral detergent fiber (NDF) and acid detergent fiber (ADF) was determined according to Van Soest et al. [30]. The analysis of NDF involved the use of α-amylase, and both NDF and ADF values were expressed exclusive of ash. Nitrogen content was measured by the Dumas combustion method. The amount of N insoluble in acid detergent solution (ADIN) was determined by analyzing the N content in the ADF residue.
In the 24 h in vitro trials, concentrations of NH3-N in the vials content were determined by the phenol-hypochlorite method [31]. Concentrations of both VFA and CH4 were analysed by gas chromatography as described by García-Martínez et al. [32] and Martínez et al. [33], respectively.
2.6. Calculations and Statistical Analyses
The gas production data for each sample (seaweeds, reference feedstuffs and MB) were fitted with time using the model of Krishnamoorthy et al. [34], Gas = PGP × (1 − e (−c × (t − lag))), in which PGP is the potential gas production, c is the fractional rate of gas production, lag is the moment when gas production starts, and t is the time of gas measurement. The fitting of data to the model was performed with the NLIN PROC of SAS [35] using an iterative least squares procedure. The estimated gas production parameters were used to calculate AGPR (average gas production rate) using the equation proposed by France et al. [36]: AGPR = PGP × c ÷ [2 × (ln2 + c × lag)]. Finally, the content in metabolizable energy (ME) of the MB was estimated from chemical composition (CP and EE, both expressed as g/kg DM) and the gas produced at 24 h of incubation (Gas24; mL/300 mg DM incubated) using the equation proposed by Menke and Steingass [37]: ME = 2.43 + 0.1206 × Gas24 + 0.0069 × CP + 0.0187 × EE.
3. Results and Discussion
3.1. Chemical Composition and Gas Production Kinetics of Seaweeds and Reference Feedstuffs
The chemical composition and gas production parameters of S. latissima and P. umbilicalis and reference feedstuffs are shown in Table 2. The dry matter content of S. latissima was similar to that reported previously [6,17], but the DM of P. umbilicalis was somewhat greater than previous values observed for Porphyra spp. [6,8]. As expected, the DM content of both seaweeds was considerably lower than in the reference feedstuffs. Ash content was 68% larger in S. latissima than in P. umbilicalis, but both values agree well with previous studies on both seaweeds harvested in spring in the same area and washed in a similar way [38], although others [11,17] have reported lower ash contents. The chemical composition of seaweeds is strongly affected by seasonal variation during the year [39], but other factors as water salinity and pH, location, daylight, mineral concentration, or waves can also affect chemical composition [40,41,42].

Table 2.
Chemical composition and in vitro gas production parameters of the samples of Saccharina latissima and Porphyra umbilicalis and of samples of grass hay, corn grains and sunflower meal used as reference feedstuffs.
Both seaweeds differed substantially in N content (Table 2), which was 4-fold greater in P. umbilicalis compared with S. latissima. Some authors have proposed that Porphyra spp. could replace protein sources (i.e., soybean meal) in ruminant diets due to its large content in high-digestible protein [8,11,43]. The N content of the P. umbilicalis used in our study was lower than that reported previously [6,8,39] for Porphyra spp., as well as for both sunflower and soybean meal. Values of ADIN in both seaweeds were lower than in sunflower meal and represented 23.8 and 6.87% of total N for S. latissima and P. umbilicalis, respectively. The low ADIN content in P. umbilicalis would suggest high protein digestibility, as this parameter is considered an indicator of unavailable N, although the validity of this analysis has been questioned [44].
The NDF content of P. umbilicalis was 4.5-fold greater than in S. latissima (Table 2), whereas the ADF content was 2.6-fold lower. There is a huge disparity in the available results in the literature regarding the NDF and ADF content of both seaweeds [6,8,11,17,38,43,45]. Besides the factors that can influence the chemical composition of seaweeds, it is also probable that the sequential fiber analysis used in most studies is not fully adequate due to both the complexity of seaweeds carbohydrates and differences with the carbohydrates of terrestrial crops for which this analysis was intended. In addition, the NDF differences between S. latissima and P. umbilicalis (brown and red seaweeds, respectively) might be attributed to the particular carbohydrates in each seaweed, as the main storage carbohydrate is floridean starch in red seaweeds, and laminarin in brown seaweeds [46,47]. It is possible that NDF and ADF analysis do not capture these fractions equally, and this contributes to observed differences between the two seaweeds. Whereas the NDF content in S. latissima was similar to that in corn grains, P. umbilicalis had a NDF content greater than that in sunflower meal but lower than in grass hay. In contrast, both seaweeds had ADF content markedly lower compared with grass hay and sunflower meal.
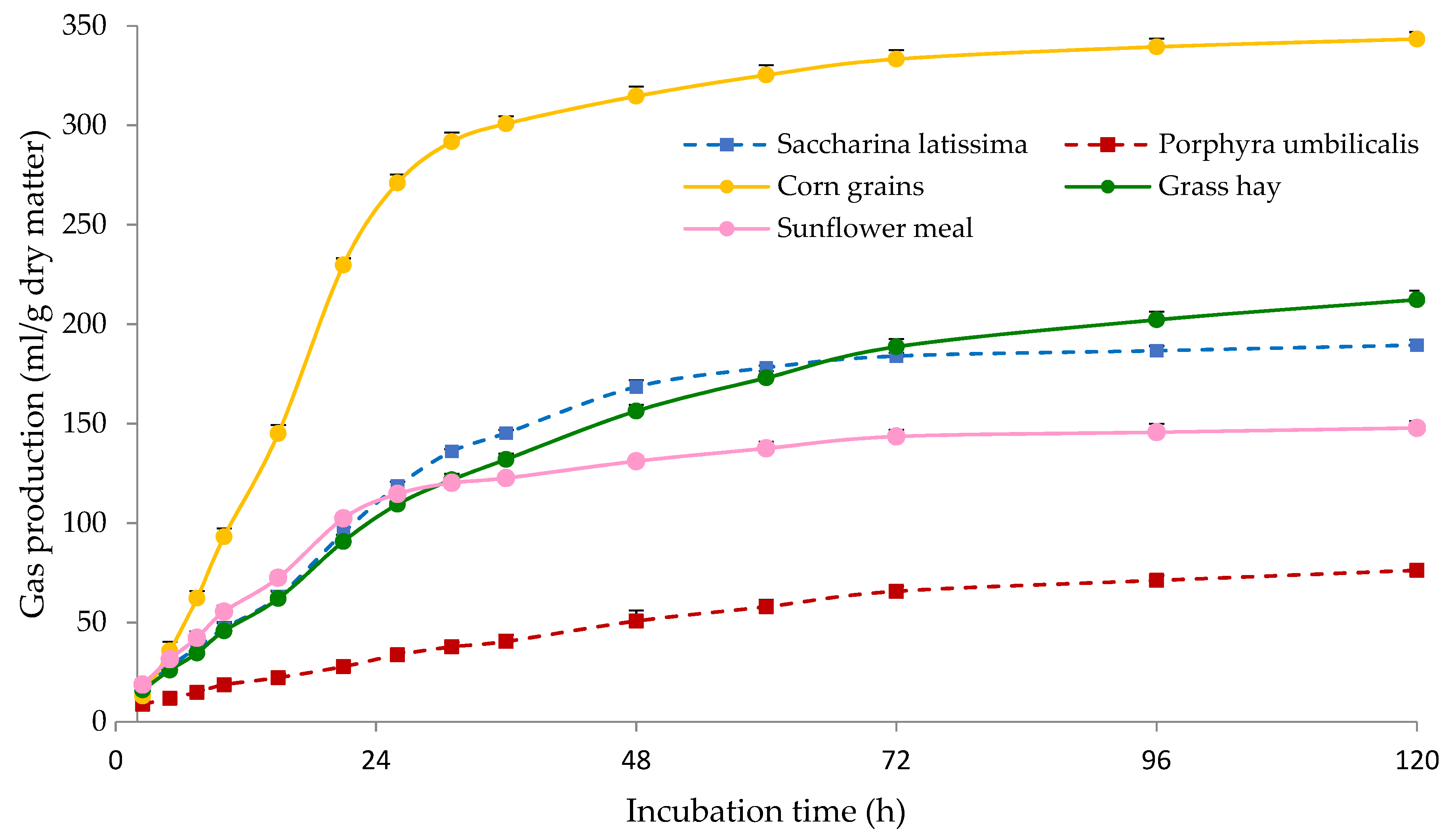
The gas produced during the in vitro incubations reflects closely the organic matter fermented [48], and therefore gas parameters are indicative of the rate and extent of ruminal fermentation of the incubated substrate. As shown in Figure 2, corn grains showed the highest PGP, c and AGPR values among all tested samples, which can be explained by the rapid and extensive fermentation of starch. Saccharina latissima, grass hay and sunflower meal had similar gas production at 24 h of incubation, but afterwards the gas production of sunflower meal was lower than in the seaweed and hay samples. It should be taken into account that protein fermentation generates less gas compared with carbohydrates [49], which could help to explain the lower value of sunflower meal. This can also be related to the low gas production observed for P. umbilicalis, which showed the lowest PGP, c and AGPR values.
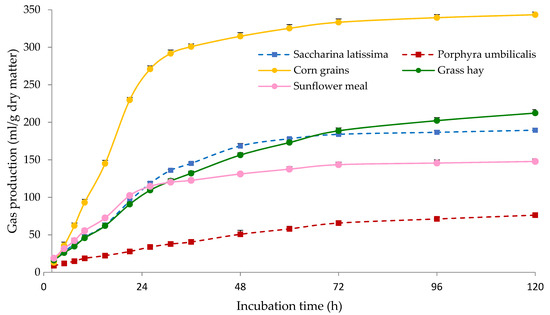
Figure 2.
Gas production kinetics of Saccharina latissima, Porphyra umbilicalis, grass hay, corn grains and sunflower meal. The bars indicate the standard error of the mean (n = 4).
The NDF fraction is less fermentable than water-soluble and other non-structural carbohydrates (i.e., starch, oligosaccharides), and ADF is often negatively associated with ruminal degradability [7]. The NDF content of S. latissima was similar to that in corn grains (Table 2), but its gas production was much lower (Figure 2). This supports the idea of Bikker et al. [45] that NDF and ADF analyses are not adequate for seaweeds, as these chemical fractions do not provide detailed information on specific polysaccharides and the actual nutritional value of the seaweeds. Similarly, the ADF content of P. umbilicalis was considerably low but it showed the lowest gas production among all tested samples. In addition, ruminal fermentation of seaweeds might be limited by the presence of complex carbohydrates and biocompounds, as specific enzymes might be needed for their degradation [50]. The structure of the cell wall of seaweeds can also limit the access of microorganisms and enzymes [51].
3.2. Chemical Composition and Ruminal Fermentation of Multinutrient Blocks
The chemical composition of MB blocks is shown in Table 3. Multinutrient blocks were formulated to fully replace either corn (SLMB) or soybean meal (PUMB). Although the MB were formulated to have similar N content, the N level of PUMB was lower compared with CMB, as the N content of the P. umbilicalis sample was lower than the expected value close to 60.0 g/kg observed in our previous studies for Porphyra spp. samples harvested in spring from the same area [6,39]. The EE content was slightly greater in SLMB than in CMB and PUMB, which could explain the small differences in gross energy content (17.5 vs. 17.0 MJ/kg).

Table 3.
Chemical composition (g/kg dry matter (DM)) and gross energy content of multinutrient blocks including no seaweed (Control; CMB), Saccharina latissima (SLMB) or Porphyra umbilicalis (PUMB).
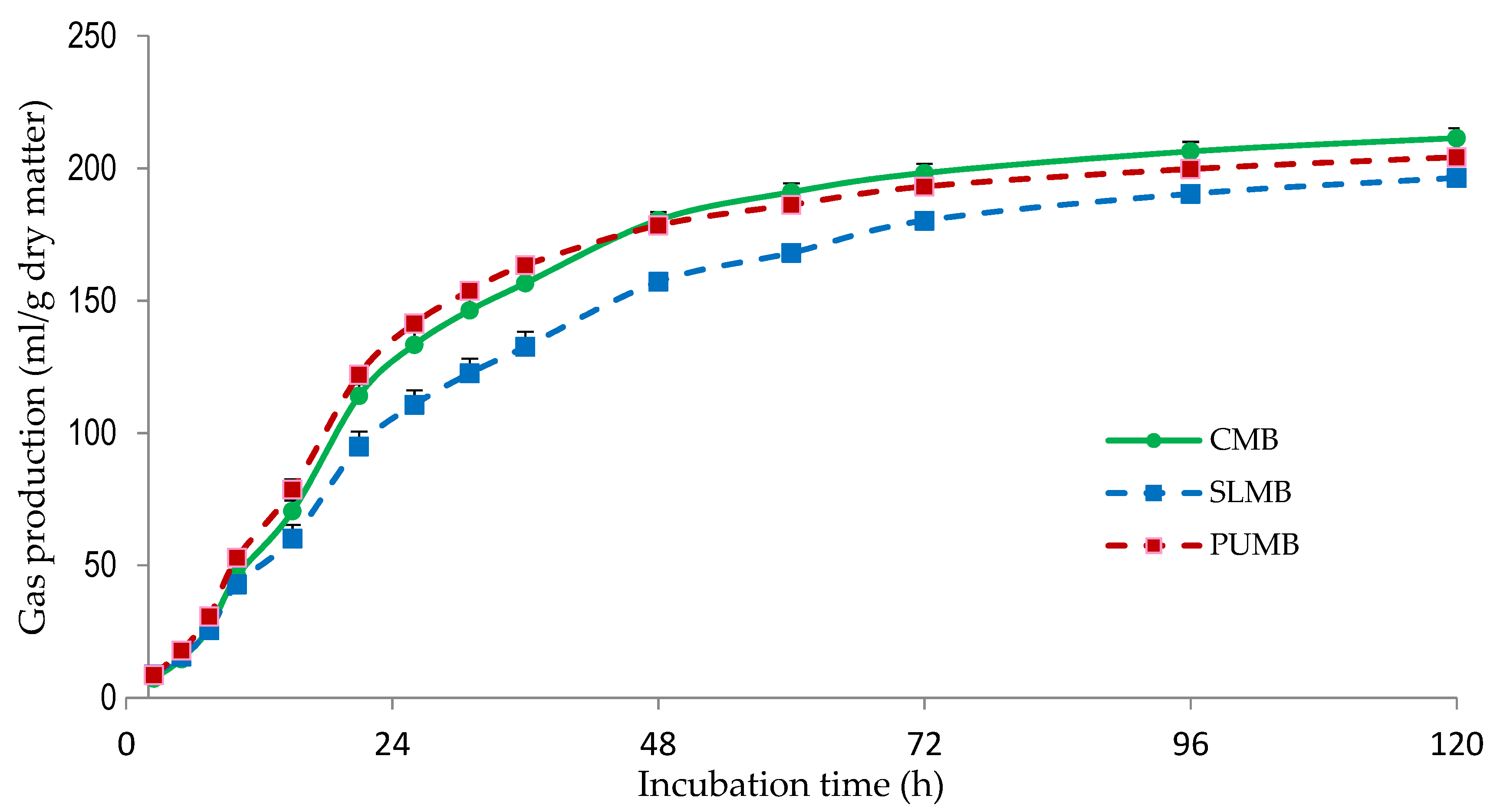
Table 4 shows the gas production parameters and the IVDMD of the MB. Potential gas production was greater (p < 0.05) for CMB compared with SLMB and PUMB, probably due the higher amount of corn, which is extensively fermented (Figure 2). The SLMB showed the lowest (p < 0.05) PGP, which is in agreement with the greater NDF and ADF content of this MB (Table 3), as NDF and ADF are less degradable in the rumen than other feed fractions (i.e., starch, oligosaccharides). The lower gas production observed at 48 h of incubation observed for SLMB compared with CMB and PUMB (Figure 3) is in agreement with the lesser (p < 0.05) IVDMD determined for SLMB (Table 4). Similarly, the estimated metabolizable energy content of the SLMB was lower (p < 0.05) than in the CMB and PUMB. Even though significant, differences between CMB and PUMB were small.

Table 4.
Parameters of in vitro gas production kinetics, in vitro dry matter degradability (IVDMD) and estimated metabolizable energy content of multinutrient blocks including no seaweed (Control; CMB), Saccharina latissima (SLMB) or Porphyra umbilicalis (PUMB).
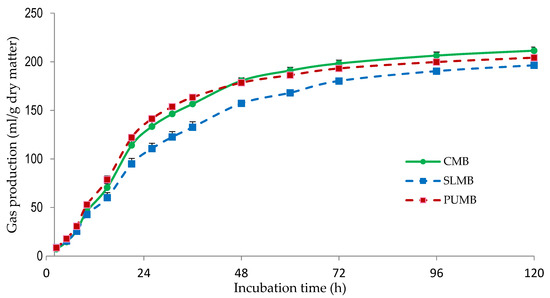
Figure 3.
Gas production kinetics of multinutrient blocks without including seaweeds (CMB; control) and including Saccharina latissima (SLMB) or Porphyra umbilicalis (PUMB). The bars indicate the standard error of the mean (n = 16).
Both c and AGPR values were greatest (p < 0.05) for PUMB and lowest (p < 0.05) for SLMB, with CMB showing intermediate values. The lack of corn grains in the SLMB, which are rapid and highly fermented (Figure 2), and the inclusion of S. latissima can explain the lower degradation rate of the SLMB. The PUMB included no pea hull fiber, which is poorly degraded [52]. Furthermore, PUMB contained a greater concentration of molasses that are rapidly degraded by ruminal microorganisms [7], which can help to explain the greater degradation of PUMB. Likewise, the greater content in molasses in SLMB and PUMB compared with CMB could be responsible for the lower (p < 0.05) Lag values observed for both MB including seaweeds. Molasses are frequently used as binder in MB, and they have also the added benefit of improving palatability. Previous studies have reported the low palatability of seaweeds and suggested that the inclusion of molasses might improve it [11].
Other authors have already evaluated in vitro the effects of including either S. latissima or Porphyra spp. in different diets on ruminal fermentation. De la Moneda et al. [6] observed that including up to 84 g/kg of dried Porphyra spp. partially replacing sunflower and soybean meal the concentrate of a mixed diet (50:50 oat hay:concentrate) resulted in increased PGP and decreased both c and AGPR after in vitro incubation with ruminal fluid from goats. In the same study, the partial replacement of corn with 150 g/kg of dried S. latissima in the concentrate had no effect on PGP, but in agreement with our results decreased both c and AGPR. In contrast, Maia et al. [53] observed that including 25% of S. latissima (DM basis) in a total mixed diet incubated in a Rusitec system had no negative effects on the organic matter degradability of the diet. Lind et al. [43] reported that replacing 96 g of soybean meal with Porphyra spp. (fresh matter basis) in a diet based on grass silage and crushed oats had no effect on PGP, c or Lag after 72 h of in vitro incubation. Pandey et al. [38] analysed the effects of including up to 20% of the total DM of different seaweeds to maize silage after 48 h in vitro fermentation and observed that S. latissima had no effect, but P. umbilicalis decreased the amount of gas produced, which is in agreement with the lower fermentation observed in our study for S. latissima compared with P. umbilicalis (Figure 2).
Discrepancies in the results of the different studies could be attributed to the variable chemical composition of seaweeds, the animals used as donors of ruminal fluid and their diets, seaweeds inclusion levels, or even measurement time. In fact, in our study the gas production of the 3 MB was similar over the first 15 h of incubation but afterwards SLMB produced less gas compared with CMB and PUMB (Figure 3).
Ruminal parameters determined after 24 h of incubation for all MB are shown in Table 5. The greater pH (p < 0.05) of SLMB compared with CMB is consistent with the lower IVDMD observed for SLMB (Table 4), as these parameters are negatively related. However, no differences (p = 0.445) among MB were observed in total VFA production. Gas production is strongly correlated with total VFA production [54], and gas production was similar for all MB over the first 15 h of incubation. After 24 h, SLMB produced less gas than CMB and PUMB (Figure 3), and therefore differences in total VFA production would be expected. However, protein degradation leads to less short chained fatty acids production compared with carbohydrates, and its production is dependent upon amino acid composition and the extent of its ruminal deamination [54]. Different rates of protein degradation, as well as differences in the amino acid profile of the MB, could help to explain these results.

Table 5.
Ruminal parameters measured after 24 h of in vitro incubations of multinutrient blocks including no seaweed (Control; CMB), Saccharina latissima (SLMB) or Porphyra umbilicalis (PUMB) with ruminal fluid from sheep.
In agreement with our results, other authors observed no changes in total VFA production by including S. latissima or Porphyra spp. in diets that were incubated in vitro with ruminal fluid [6,38,43,53]. In our study, there were significant differences among MB in the molar proportions of individual VFA. The SLMB had the greatest (p < 0.05) acetate proportion, whereas the greatest (p < 0.05) propionate and butyrate proportions were observed for CMB and PUMB, respectively. As a consequence, the acetate:propionate ratio was lower (p < 0.05) for CMB compared with SLMB and PUMB. Fermentation of easily fermentable carbohydrates (i.e., starch) within the rumen are generally correlated with higher propionate proportions, while fermentation of fiber is associated with greater acetate proportions [7]. The greater NDF content of the SLMB compared with CMB and PUMB agrees well with the larger acetate proportion observed, whereas the high propionate production of the CMB is in accordance with their high cereal content (402, 152 and 282 g/kg for CMB, SLMB and PUMB, respectively). The high proportion of butyrate of the PUMB (14.8%) might be related with the higher level of molasses inclusion (Table 1) that are rapidly fermented in the rumen resulting in increased butyrate proportions [55].
High proportions of isobutyrate, isovalerate and valerate are often associated with extensive ruminal protein degradation [7]. The SLMB showed the greatest (p < 0.05) proportions of these three VFA, and CMB had the lowest proportions (p < 0.05) of isobutyrate and isovalerate. In agreement with these results, NH3-N concentrations were greater (p < 0.05) for SLMB, indicating either a greater protein degradation or a lower capture of NH3-N by ruminal microorganisms for the MB with S. latissima. Maia et al. [53] observed that including 25% of dried S. latissima in a total mixed diet improved the protein digestibility of the diet in a Rusitec system. Similarly, Ramin et al. [56] incubated in vitro increasing quantities of a protein-enriched fraction of S. latissima together with grass silage using ruminal fluid from cows as inoculum and observed an increase in the organic matter and protein degradability. It is worth noting that NH3-N concentrations for all MB were adequate for the growth of ruminal microorganisms in vitro [57].
Although Tayyab et al. [8] reported that protein degradability of Porphyra spp. was lower than that of soybean meal in 24 h in vitro incubations, no differences between CMB and PUMB were observed on NH3-N concentrations in our study. In accordance with our results, no negative effects on the growth rate of lambs and total tract digestibility of adult sheep have been observed when replacing soybean meal with an equivalent DM amount of Porphyra spp. in in vivo trials [11,43].
No differences (p > 0.05) among MB were detected either in methane or the methane/VFA ratio. Some seaweeds contain a wide range of compounds with the potential to decrease methane emissions [58]. Similar to our results, Lind et al. [43] did not observe significant differences on CH4 production after replacing soybean meal with Porphyra spp. in both in vitro and in vivo trials, and Maia et al. [53] reported that including dried S. latissima in a total mixed diet had no effect on methane production in Rusitec fermenters. In a recent study, Pandey et al. [38] reported a decrease in methane production of 9 and 29% after replacing 20% of the DM of a substrate composed only by maize silage with S. latissima and P. umbilicalis, respectively, after 48 h of in vitro incubation, but differences were not statistically signficant. Altogether, the results seem to indicate that neither S. latissima nor P. umbilicalis contain compounds with antimethanogenic activity.
4. Conclusions
The results indicate that multinutrient blocks could be a feasible option to preserve and store seaweeds for ruminant feeding. Furthermore, the inclusion of Saccharina latissima and Porphyra umbilicalis in the multinutrient blocks formulated in our study had no negative impact on ruminal fermentation, and only slightly shifts in ruminal fermentation pattern were noticed. In vivo studies are needed to effectively determine whether multinutrient blocks including seaweeds can be used in ruminant production systems with no detrimental effects on feed intake and animal performance. Additionally, microbiological and toxicological analyses of seaweeds should be conducted before including them in multinutrient blocks.
Author Contributions
Conceptualization, E.M.-A., M.N.-G., M.R.W. and M.D.C.; methodology, E.M.-A., M.N.-G., M.R.W. and M.D.C.; formal analysis, C.N.M. and T.d.E.; investigation, E.M.-A., M.N.-G., M.R.W., M.D.C., C.N.M. and T.d.E.; resources, M.N.-G. and M.D.C.; writing—original draft preparation, C.N.M. and M.D.C.; writing—review and editing, E.M.-A., M.N.-G., M.R.W. and T.d.E.; supervision, M.N.-G., M.D.C. and E.M.-A.; project administration, M.N.-G.; funding acquisition, M.N.-G. and M.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Norwegian Regional Research Fund-North (Grant number 248210), and the Spanish State Research Agency (AEI) and the European Regional Development Fund (Project AGL2016-75322-C2-1-R).
Institutional Review Board Statement
The experimental protocols of this study were approved by the Institutional Animal Care and Use Committee of the Comunidad Autónoma de Madrid of Spain (Approval number PROEX 035/17).
Data Availability Statement
All data are publicly available.
Acknowledgments
We thank Heidi Hovland Ludviksen from the Faculty of Biosciences and Aquaculture at Nord University for her help in harvesting the seaweed biomass, and to Per Magnus Hansen from the Norwegian Institute of Bioeconomy Research for his help in harvesting and processing the seaweed biomass and for making the MB. C.N.M. and T.d.E. received a Margarita Salas grant from the Ministerio de Universidades of the Spanish Government financed by the European Union-Next Generation EU (UP2021-035).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A Seaweed Aquaculture Imperative to Meet Global Sustainability Targets. Nat. Sustain. 2022, 5, 185–193. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review: Feed Demand Landscape and Implications of Food-Not Feed Strategy for Food Security and Climate Change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef]
- Evans, F.D.; Critchley, A.T. Seaweeds for Animal Production Use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological Activities and Potential Health Benefits of Sulfated Polysaccharides Derived from Marine Algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for Livestock Diets: A Review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- de la Moneda, A.; Carro, M.D.; Weisbjerg, M.R.; Roleda, M.Y.; Lind, V.; Novoa-Garrido, M.; Molina-Alcaide, E. Variability and Potential of Seaweeds as Ingredients of Ruminant Diets: An in vitro study. Animals 2019, 9, 851. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Tayyab, U.; Novoa-Garrido, M.; Roleda, M.Y.; Lind, V.; Weisbjerg, M.R. Ruminal and Intestinal Protein Degradability of Various Seaweed Species Measured in Situ in Dairy Cows. Anim. Feed Sci. Technol. 2016, 213, 44–54. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Horn, S.J. Seaweed Biofuels: Production of Biogas and Bioethanol from Brown Macroalgae; VDM Publishing: Saarbrücken, Germany, 2009. [Google Scholar]
- Özkan-Gülzari, S.; Lind, V.; Aasen, I.M.; Steinshamn, H. Effect of Supplementing Sheep Diets with Macroalgae Species on In Vivo Nutrient Digestibility, Rumen Fermentation and Blood Amino Acid Profile. Animal 2019, 13, 2792–2801. [Google Scholar] [CrossRef]
- Balasse, M.; Tresset, A.; Dobney, K.; Ambrose, S.H. The Use of Isotope Ratios to Test for Seaweed Eating in Sheep. J. Zool. 2005, 266, 283–291. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.L.; Jacquemin, B.; Rebours, C.; Barbier, M.; Charrier, B. Pegasus-Phycomorph European Guidelines for a Sustainable Aquaculture of Seaweeds; COST Action FA1406: Roscoff, France, 2019. [Google Scholar]
- Belanche, A.; Jones, E.; Parveen, I.; Newbold, C.J. A Metagenomics Approach to Evaluate the Impact of Dietary Supplementation with Ascophyllum nodosum or Laminaria digitata on Rumen Function in Rusitec Fermenters. Front. Microbiol. 2016, 7, 299. [Google Scholar] [CrossRef]
- Novoa-Garrido, M.; Marcos, C.N.; Carro, M.D.; Molina-Alcaide, E.; Larsen, M.; Weisbjerg, M.R. Preserving Porphyra umbilicalis and Saccharina latissima as Silages for Ruminant Feeding. Animals 2020, 10, 1957–1974. [Google Scholar] [CrossRef]
- Cabrita, A.; Maia, M.R.G.; Sousa-Pinto, I.; Fonseca, A. Ensilage of Seaweeds from an Integrated Multi-trophic Aquaculture System. Algal Res. 2017, 24, 290–298. [Google Scholar] [CrossRef]
- Campbell, M.; Ortuño, J.; Ford, L.; Davies, D.R.; Koidis, A.; Walsh, P.J.; Theodoridou, K. The Effect of Ensiling on the Nutritional Composition and Fermentation Characteristics of Brown Seaweeds as a Ruminant Feed Ingredient. Animals 2020, 10, E1019. [Google Scholar] [CrossRef]
- Yen, Y.; Weisbjerg, M.R.; Rautenberger, R.; Fečkaninová, A.; Novoa-Garrido, M. Improving Fermentation of Saccharina latissima and Alaria esculenta Silages with Additives for Preserving Biomass and Antioxidants. J. Appl. Phycol. 2022, 34, 625–636. [Google Scholar] [CrossRef]
- Ben-Salem, H.; Nefzaoui, A. Feed Blocks as Alternative Supplements for Sheep and Goats. Small Rumin. Res. 2003, 49, 275–288. [Google Scholar] [CrossRef]
- Romero-Huelva, M.; Ramos-Morales, E.; Molina-Alcaide, E. Nutrient Utilization, Ruminal Fermentation, Microbial Abundances, and Milk Yield and Composition in Dairy Goats Fed Diets Including Tomato and Cucumber Waste Fruits. J. Dairy Sci. 2012, 95, 6015–6026. [Google Scholar] [CrossRef]
- de Evan, T.; Carro, M.D.; Fernández-Yepes, J.E.; Haro, A.; Arbesú, L.; Romero-Huelva, M.; Molina-Alcaide, E. Effects of Feeding Multinutrient Blocks Including Avocado Pulp and Peels to Dairy Goats on Feed Intake and Milk Yield and Composition. Animals 2020, 10, 194. [Google Scholar] [CrossRef]
- Marcos, C.N.; Carro, M.D.; Fernández-Yepes, J.E.; Arbesu, L.; Molina-Alcaide, E. Utilization of Avocado and Mango Fruit Wastes in Multi-Nutrient Blocks for Goats Feeding: In Vitro Evaluation. Animals 2020, 10, 2279. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Morales-García, E.Y.; Martín-García, A.I.; Ben-Salem, H.; Nefzaoui, A.; Sanz-Sampelayo, M.R. Effects of Partial Replacement of Concentrate with Feed Blocks on Nutrient Utilization, Microbial N Flow, and Milk Yield and Composition in Goats. J. Dairy Sci. 2010, 93, 2076–2087. [Google Scholar] [CrossRef]
- de Evan, T.; Carro, M.D.; Fernández-Yepes, J.E.; Haro, A.; Arbesú, L.; Romero-Huelva, M.; Molina-Alcaide, E. Feeding Mango Wastes to Dairy Goats: Effects on Diet Digestibility, Ruminal Fermentation and Milk Yield and Composition. Anim. Feed Sci. Technol. 2022, 286, 115252. [Google Scholar] [CrossRef]
- Tejido, M.L.; Ranilla, M.J.; García-Martínez, R.; Carro, M.D. In vitro Microbial Growth and Rumen Fermentation of Different Diets as Affected by the Addition of Disodium Malate. Anim. Sci. 2005, 81, 31–38. [Google Scholar] [CrossRef]
- Goering, M.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications). In Agricultural Handbook; Agriculture Handbook No. 379; Agricultural Research Services: Washington, DC, USA, 1970. [Google Scholar]
- Marcos, C.N.; de Evan, T.; Molina-Alcaide, E.; Carro, M.D. Nutritive Value of Tomato Pomace for Ruminants and its Influence on In Vitro Methane Production. Animals 2019, 9, 343. [Google Scholar] [CrossRef]
- Marcos, C.N.; García-Rebollar, P.; de Blas, C.; Carro, M.D. Variability in the Chemical Composition and In Vitro Ruminal Fermentation of Olive Cake By-products. Animals 2019, 9, 109. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber and Non Starch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- García-Martínez, R.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Effects of Disodium Fumarate on In Vitro Rumen Microbial Growth, Methane Production and Fermentation of Diets Differing in their Forage Concentrate Ratio. Br. J. Nutr. 2005, 94, 71–77. [Google Scholar] [CrossRef]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. The Effect of the Diet Fed to Donor Sheep on In Vitro Methane Production and Ruminal Fermentation of Diets of Variable Composition. Anim. Feed Sci. Technol. 2010, 158, 126–135. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Soller, H.; Steingass, H.; Menke, K.H. A Comparative Study on Rumen Fermentation of Energy Supplements In Vitro. J. Anim. Physiol. Anim. Nutr. 1991, 65, 28–35. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT® Users Guide; Version 9.3; SAS Inst. Inc.: Cary, NC, USA, 2017. [Google Scholar]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of their Gas Production Profiles observed In Vitro: Derivation of Models and other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and In Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Pandey, D.; Hansen, H.H.; Dhakal, R.; Aryal, N.; Rai, S.P.; Sapkota, R.; Nielsen, M.O.; Novoa-Garrido, M.; Khanal, P. Interspecies and Seasonal Variations in Macroalgae from the Nordic Region: Chemical Composition and Impacts on Rumen Fermentation and Microbiome Assembly. J. Clean. Prod. 2022, 363, 132456. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Carro, M.D.; Roleda, M.Y.; Weisbjerg, M.R.; Lind, V.; Novoa-Garrido, M. In Vitro Ruminal Fermentation and Methane Production of Different Seaweed Species. Anim. Feed Sci. Technol. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- Handå, A.; Forbord, S.; Wang, X.; Broch, O.J.; Dahle, S.W.; Størseth, T.R.; Reitan, K.I.; Olsen, Y.; Skjermo, J. Seasonal and Depth Dependent Growth of Cultivated Kelp (Saccharina latissima) in Close Proximity to Salmon (Salmo salar) Aquaculture in Norway. Aquaculture 2013, 414–415, 191–201. [Google Scholar] [CrossRef]
- Schiener, P.; Zhao, S.; Theodoridou, K.; Carey, M.; Mooney-McAuley, K.; Greenwell, C. The Nutritional Aspects of Biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata. Biomass Convers. Biorefin. 2016, 7, 221–235. [Google Scholar] [CrossRef]
- Marinho, G.S.; Holdt, S.L.; Angelidaki, I. Seasonal Variations in the Amino Acid Profile and Protein Nutritional Value of Saccharina latissima Cultivated in a Commercial IMTA System. J. Appl. Phycol. 2015, 27, 1991–2000. [Google Scholar] [CrossRef]
- Lind, V.; Weisbjerg, M.R.; Jørgensen, G.M.; Fernandez-Yepes, J.E.; Arbesú, L.; Molina-Alcaide, E. Ruminal Fermentation, Growth Rate and Methane Production in Sheep Fed Diets Including White Clover, Soybean Meal or Porphyra sp. Animals 2020, 10, 79. [Google Scholar] [CrossRef]
- Marcos, C.N.; Carro, M.D.; García, S.; González, J. The ADIN Analysis Overestimates the Amount of N Associated to ADF. Anim. Feed Sci. Technol. 2018, 244, 36–41. [Google Scholar] [CrossRef]
- Bikker, P.; Stokvis, L.; van Krimpen, M.M.; van Wikselaar, P.G.; Cone, J.W. Evaluation of Seaweeds from Marine Waters in Northwestern Europe for Application in Animal Nutrition. Anim. Feed Sci. Technol. 2020, 263, 114460. [Google Scholar] [CrossRef]
- Yu, S.; Blennow, A.; Bojko, M.; Madsen, F.; Olsen, C.E.; Engelsen, S.B. Physico-chemical Characterization of Floridean Starch of Red Algae. Starch 2002, 54, 66–74. [Google Scholar] [CrossRef]
- Becker, S.; Tebben, J.; Coffinet, S.; Wiltshire, K.; Iversen, M.H.; Harder, T.; Hinrichs, K.-U.; Hehemann, J.-H. Laminarin is a Major Molecule in the Marine Carbon Cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production when they are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Cone, J.W.; Van Gelder, A.H. Influence of Protein Fermentation on Gas Production Profiles. Anim. Feed Sci. Technol. 1999, 76, 251–264. [Google Scholar] [CrossRef]
- Øverland, M.; Myand, L.T.; Skrede, A. Marine Macroalgae as Sources of Protein and Bioactive Compounds in Feed for Monogastric Animals. J. Sci. Food Agric. 2018, 99, 13–24. [Google Scholar] [CrossRef]
- Baldan, B.; Andolfo, P.; Navazio, L.; Tolomio, C.; Mariani, P. Cellulose in Algal Cell Wall: An “In Situ” Localization. Eur. J. Histochem. 2001, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mekasha, Y.; Tegegne, A.; Yami, A.; Umunna, N.N. Evaluation of Non-Conventional Agro-Industrial By-Products as Supplementary Feeds for Ruminants: In Vitro and Metabolism Study with Sheep. Small Rumin. Res. 2002, 44, 25–35. [Google Scholar] [CrossRef]
- Maia, M.R.; Fonseca, A.J.; Cortez, P.P.; Cabrita, A.R. In vitro Evaluation of Macroalgae as Unconventional Ingredients in Ruminant Animal Feeds. Algal Res. 2019, 40, 101481. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J. Relationships Between Chemical Composition, Dry Matter Degradation and In Vitro Gas Production of Several Ruminant Feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Ferraro, S.; Mendoza, G.; Miranda, A.; Gutierrez, C. In Vitro Gas Production and Ruminal Fermentation of Glycerol, Propylene Glycol and Molasses. Anim. Feed Sci. Technol. 2009, 154, 112. [Google Scholar] [CrossRef]
- Ramin, M.; Franco, M.; Roleda, M.Y.; Aasen, I.M.; Hetta, M.; Steinshamn, H. In Vitro Evaluation of Utilisable Crude Protein and Methane Production for a Diet in which Grass Silage was Replaced by Different Levels and Fractions of Extracted Seaweed Proteins. Anim. Feed Sci. Technol. 2019, 255, 114225. [Google Scholar] [CrossRef]
- Satter, L.D.; Slyter, L.L. Effect of Ammonia Concentration on Rumen Microbial Protein Production In Vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Grondahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Krizsan, S.J.; Kirwan, S.F.; et al. Seaweed and Seaweed Bioactives for Mitigation of Enteric Methane: Challenges and Opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).