Searching for Novel Oat Crown Rust Resistance in Diploid Oat Avena strigosa Schreb. Reveals the Complexity and Heterogeneity of the Analyzed Genebank Accessions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Virulence Assessment
2.2. Data Mining and Analysis
| No. | Plant ID | Origin | Puccinia coronata Race 1 | Infection Profile (Number of Resistant Seedlings) 2 | Diversity 3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 51(22) | 94(63) | CR230 | CR241 | CR257 | Sh | Hs | ||||
| 1 | 51022 | Brazil | HS, S | MS, R | S, MS, R | S, R | HS, R | 0 (2), 1.2 (6), 2.5 (1), 4.1 (1) | 0.473 | 0.200 |
| 2 | 51199 | Bulgaria | HS, S | HS, S, MS | S, MS, R | S, HS | HS, S, MS | 0 (9), 1.3 (1) | 0.141 | 0.036 |
| 3 | 51326 | unknown | S | S, MS | MS, MR, R | HS | HS | 0 (7), 1.3 (3) | 0.265 | 0.084 |
| 4 | 51518 | Poland | HS, S, MS, R | HS, MS | HS | HS, S, MS | HS | 0 (7), 1.1 (3) | 0.265 | 0.084 |
| 5 | 51520 | Poland | HS, S, R | HS, MS, R | HS | HS, S, MS, MR | HS | 0 (4), 1.1 (2), 1.2 (2), 1.4 (1), 2.1 (1) | 0.639 | 0.204 |
| 6 | 51523 | Poland | HS, S, MS, R | HS, MS, R | HS | HS, MS, R | HS, S, MR, R | 1 (3), 1.2 (1), 1.5 (2), 2.4 (2), 2.8 (1), 3.7 (1) | 0.736 | 0.280 |
| 7 | 51524 | Poland | HS, S, MS, R | HS, MS, R | HS | HS, MS, MR, R | HS, S, MS | 0 (3), 1.1 (3), 1.2 (1), 1.4 (1), 2.3 (1), 2.4 (1) | 0.714 | 0.236 |
| 8 | 51575 | Holland | S | MR, R | MR | HS, MS, R | HS, R | 2.5 (2), 3.2 (5), 4.1 (3) | 0.447 | 0.148 |
| 9 | 51578 | Uruguay | HR | HR | R | R | HS, S, MS, MR | 4.4 (9), 5 (1) | 0.141 | 0.036 |
| 10 | 51579 | Russia | HS, S, R | HS, S, MS | HS | HS, S, MS, R | HS | 0 (4), 1.1 (5), 1.4 (1) | 0.410 | 0.136 |
| 11 | 51581 | Russia | HS, S, MS, R | HS, S, MS, R | HS | HS, S, MS | HS | 0 (9), 2.1 (1) | 0.141 | 0.072 |
| 12 | 51582 | Spain | HS | R | MS, MR | S | MS | 2 (1), 2.5 (9) | 0.141 | 0.036 |
| 13 | 51583 | Spain | R | MR, R | HS, R | R | HS, R | 3.8 (1), 4.2 (1), 5 (8) | 0.278 | 0.100 |
| 14 | 51584 | France | HS, R | HS | HS | S, MS, R | HS, S | 0 (8), 1.1 (1), 2.3 (1) | 0.278 | 0.100 |
| 15 | 51585 | Poland | HS, S, MS, R | HS, S | MR, R | S, MS, R | MS, R | 3 (1), 2.7 (6), 3.1 (2), 3.6 (1) | 0.473 | 0.136 |
| 16 | 51586 | Poland | HS, S, MS, MR, R | HS, S, MR, R | HS, S, MR, R | HS, S, MS, R | HS, R | 0 (3), 1.1 (2), 1.2 (2), 2.1 (1), 2.3 (1), 5 (1) | 0.736 | 0.332 |
| 17 | 51596 | Chile | HR | R | R | R | HS, HR | 4.4 (9), 5 (1) | 0.141 | 0.036 |
| 18 | 51597 | Chile | HS, S, MS, MR, R | R | R | R | S | 3.5 (3), 4.4 (7) | 0.265 | 0.084 |
| 19 | 51598 | Poland | S, MS | MS, MR, R | HS, S | S | HS, S, MS, R | 0 (1), 1.2 (8), 1.5 (1) | 0.278 | 0.100 |
| 20 | 51613 | Poland | HS, R | S, MS, R | HS | HS, S | HS, S | 0 (9), 2.1 (1) | 0.141 | 0.072 |
| 21 | 51630 | Poland | S, MR | MS, MR | HS | HS | HS | 0 (8), 1.2 (1), 2.1 (1) | 0.278 | 0.100 |
| 22 | 51732 | Poland | S, MS, MR | MR, R | MR, R | MS, MR, R | S, MS, MR, R | 2.5 (5), 3.2 (1), 3.5 (2), 4.1 (1), 4.4 (1) | 0.590 | 0.196 |
| 23 | 51750 | Poland | S, MS, MR, R | MR, R | MS, MR, R | S, MS, R | HS, MS, MR, R | 2.9 (1), 2.8 (2), 3.2 (1), 4.1 (2), 4.4 (3), 5 (1) | 0.736 | 0.360 |
| 24 | 51751 | Poland | HS, S, MS, R | HS | HS | HS, MS | HS, R | 0 (5), 1.1 (4), 1.5 (1) | 0.410 | 0.132 |
| 25 | 51753 | Poland | S, MS, MR | MR, R | MS, R | MS | MS, MR, R | 2 (1), 2.5 (1), 2.9 (5), 3.2 (1), 4.3 (2) | 0.590 | 0.224 |
| 26 | 51754 | Poland | S, MS, MR | S, MS, MR | MR, R | S | HS | 3 (7), 2.5 (2), 3.4 (1) | 0.348 | 0.120 |
| 27 | 51755 | Poland | MS, R | MS, MR, R | MS, R | S, MS | HS | 0 (1), 1.2 (7), 2.1 (1), 3.4 (1) | 0.408 | 0.136 |
| 28 | 51887 | Poland | MR, R | MR, R | R | MR, R | MR, R | 5 (10) | 0.000 | 0.000 |
| 29 | 51987 | Poland | S, R | MS, MR, R | S, MS, R | S, MS, R | HS, S, MS, R | 0 (1), 1.2 (1), 2.9 (2), 4.4 (2), 5 (4) | 0.639 | 0.420 |
| 30 | 52339 | Poland | S, MS | S, MS, MR, R | S, MS | S, MS, MR, R | HS | 0 (3), 1.2 (2), 1.4 (3), 2.6 (2) | 0.593 | 0.196 |
| 31 | 52340 | Poland | S | MR, R | S, MS | S, R | HS, S, MS, R | 2 (6), 2.6 (2), 2.9 (1), 3.3 (1) | 0.473 | 0.148 |
| 32 | 52341 | Poland | MR, R | S, MS | MS, MR, R | MS | S, MS, MR, R | 7 (2), 2.4 (3), 3.1 (5) | 0.447 | 0.148 |
| 33 | 52342 | Poland | MS, MR, HR | MS, MR, HR | MR, HR | MS, MR, R | MS, R | 3.1 (1), 3.5 (1), 4.3 (2), 5 (6) | 0.473 | 0.192 |
| 34 | 501048 | Poland | HS, S, MR, R | HS | HS, MS | HS | HS | 0 (8), 1.1 (2) | 0.217 | 0.064 |
| 35 | 502855 | United Kingdom | MS, MR, HR | S | S, MS, MR, R | S | HS | 1 (4), 1.3 (1), 2.2 (5) | 0.410 | 0.132 |
| 36 | 502856 | United Kingdom | MR, R | MS, R | MS, MR, R | MS, MR | HS, MS, MR, R | 1 (5), 2.3 (1), 2.4 (1), 2.8 (1), 3.1 (1), 5 (1) | 0.651 | 0.288 |
| 37 | 502857 | United Kingdom | MS, MR | HS, R | MS | S, MS | HS, R | 0 (8), 1.5 (1), 2.8 (1) | 0.278 | 0.136 |
| 38 | 502858 | United Kingdom | S, MS, MR | HS | S | MS | HS | 0 (9), 1.1 (1) | 0.141 | 0.036 |
| 39 | 502859 | United Kingdom | S, MS | HS | S, MS, MR | S | HS | 0 (9), 1.3 (1) | 0.141 | 0.036 |
| Puccinia coronata Race | Oat Differential Line with a Corresponding Phenotype | |||||
|---|---|---|---|---|---|---|
| IP | 51(22) | 94(63) | CR230 | CR241 | CR257 | |
| 0 | H 1 | H | H | H | H | - |
| 1.1 | L | H | H | H | H | Pc36, Pc39, Pc55, Pc61, Pc70, Pc71 |
| 1.2 | H | L | H | H | H | - |
| 1.3 | H | H | L | H | H | - |
| 1.4 | H | H | H | L | H | - |
| 1.5 | H | H | H | H | L | - |
| 2.1 | L | L | H | H | H | Pc38, Pc63 |
| 2.2 | L | H | L | H | H | - |
| 2.3 | L | H | H | L | H | - |
| 2.4 | L | H | H | H | L | - |
| 2.5 | H | L | L | H | H | - |
| 2.6 | H | L | H | L | H | - |
| 2.7 | H | H | L | H | L | - |
| 2.8 | L | L | H | H | L | - |
| 2.9 | H | L | H | H | L | Pc14 |
| 3.1 | L | H | L | H | L | Pc48, Pc103-1 |
| 3.2 | H | L | L | H | L | - |
| 3.3 | H | L | H | L | L | Pc35 |
| 3.4 | L | L | L | H | H | - |
| 3.5 | H | L | L | L | H | - |
| 3.6 | H | H | L | L | L | Pc54, Pc62, Pc64, Pc96, Pc97, Pc98 |
| 3.7 | L | H | H | L | L | - |
| 3.8 | L | L | H | L | H | - |
| 4.1 | H | L | L | L | L | Pc45, Pc51, Pc101, Pc104 |
| 4.2 | L | L | H | L | L | Pc59, Pc60, Pc91 |
| 4.3 | L | L | L | H | L | Pc52 |
| 4.4 | L | L | L | L | H | Pc56, Pc68 |
| 5 | L | L | L | L | L | - |
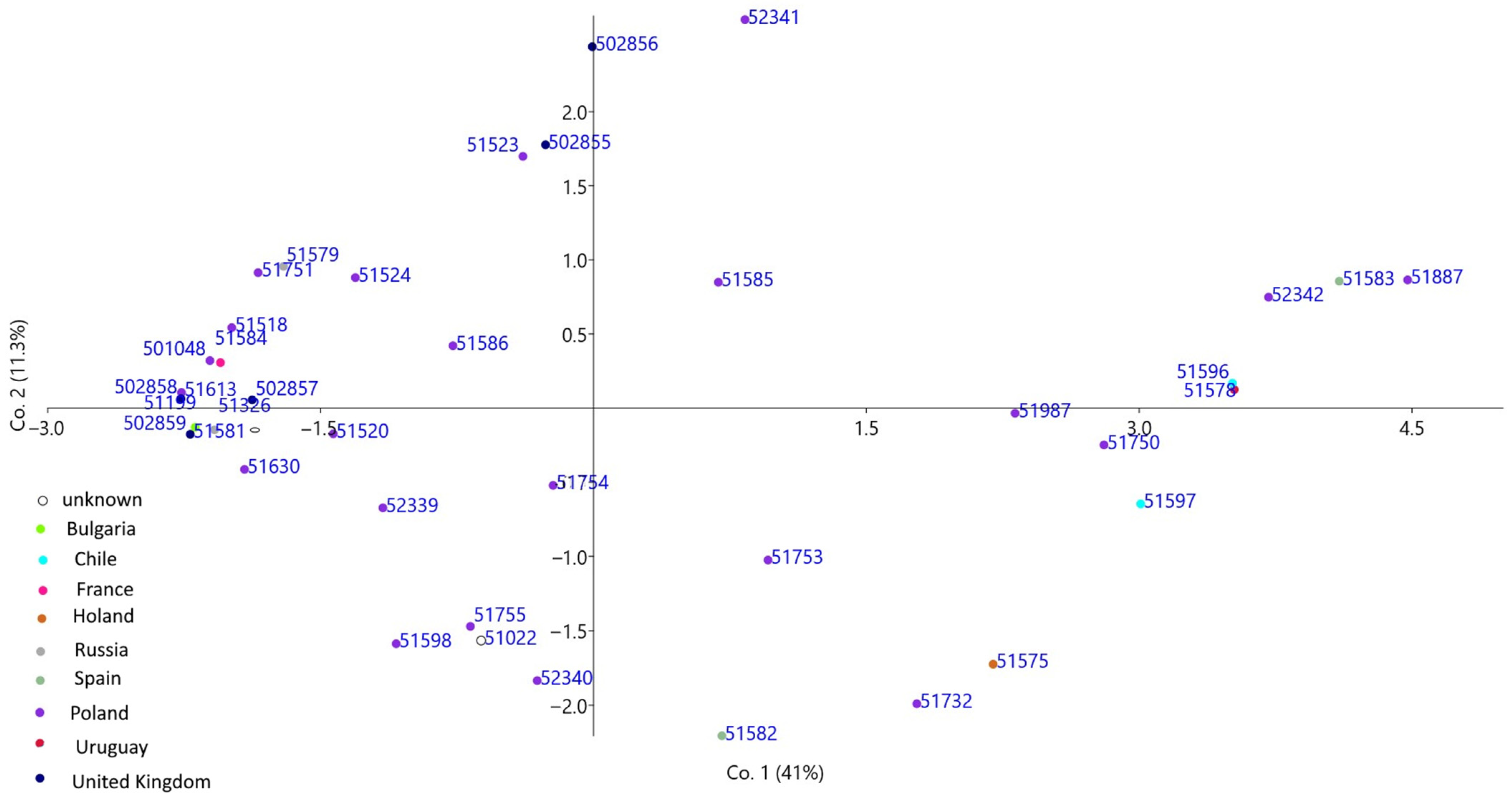
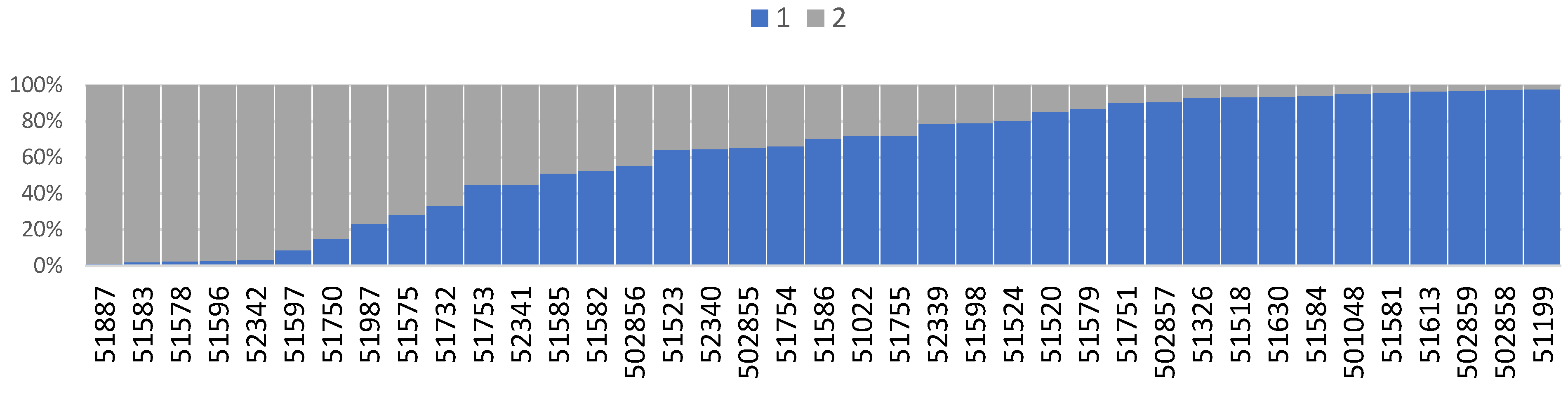
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boczkowska, M.; Podyma, W.; Łapiński, B. Oat. In Genetic and Genomic Resources for Grain Cereals Improvement; Singh, M., Upadhyaya, H.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 159–225. ISBN 9780128020005. [Google Scholar]
- Chaves, M.S.; Martinelli, J.A.; de Wesp, C.L.; Graichen, F.A.S. The cereal rusts: An overview. Pest Technol. 2008, 2, 38–55. [Google Scholar]
- Paczos-Grzȩda, E.; Sowa, S. Virulence structure and diversity of Puccinia coronata f. sp. avenae P. syd. & syd. in Poland during 2013 to 2015. Plant Dis. 2019, 103, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Sowa, S.; Paczos-Grzeda, E.; Paczos-Grzȩda, E. Virulence structure of Puccinia coronata f. sp. avenae and effectiveness of Pc resistance genes in Poland during 2017–2019. Phytopathology 2021, 111, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- CDL Cereal Disease Laboratory. Resistance Genes. Oat. Pc (Crown Rust). Available online: https://www.ars.usda.gov/midwest-area/stpaul/cereal-disease-lab/docs/resistance-genes/resistance-genes/ (accessed on 16 July 2021).
- Aung, T.; Chong, J.; Leggett, M. The transfer of crown rust resistance Pc94 from a wild diploid to cultivated hexaploid oat. In Proceedings of the 9th International European and Mediterranean Cereal Rust and Powdery Mildews Conference, Lunteren, The Netherlands, 2–6 September 1996; Kema, G.H.J., Niks, R.E., Daamen, R.A., Eds.; Wageningen, European and Mediterranean Cereal Rust Foundation: Lunteren, The Netherlands, 1996; pp. 167–171. [Google Scholar]
- Jellen, E.; Leggett, J.M. Cytogenetic Manipulation in Oat Improvement. In Genetic Resources, Chromosome Engineering, and Crop Improvement; Singh, R.J., Jauhar, P.P., Eds.; Genetic Resources Chromosome Engineering & Crop Improvement; CRC Press: Boca Raton, FL, USA, 2006; pp. 199–231. ISBN 978-0-8493-1432-2. [Google Scholar]
- Rothman, P.G. Registration of four stem rust and crown rust resistant oat germplasm lines. Crop Sci. 1984, 24, 1217. [Google Scholar] [CrossRef]
- Chong, J.; Gruenke, J.; Dueck, R.; Mayert, W.; Woods, S. Virulence of oat crown rust Puccinia coronata f. sp. avenae in Canada during 2002–2006. Can. J. Plant Pathol. 2008, 30, 115–123. [Google Scholar] [CrossRef]
- McCartney, C.A.; Stonehouse, R.G.; Rossnagel, B.G.; Eckstein, P.E.; Scoles, G.J.; Zatorski, T.; Beattie, A.D.; Chong, J. Mapping of the oat crown rust resistance gene Pc91. Theor. Appl. Genet. 2011, 122, 317–325. [Google Scholar] [CrossRef]
- Menzies, J.G.; Xue, A.; Dueck, R.; Greunke, J. Virulence of Puccinia coronata f. sp. avenae in Canada; 2010 to 2014. In Proceedings of the 14th International Cereal Rust and Powdery Mildew Conference, Denmark, Copenhagen, 5–8 July 2015; p. 95. [Google Scholar]
- Park, R. New oat crown rust pathotype with virulence for Pc91. Cereal Rust Rep. 2013, 11, 8–10. [Google Scholar]
- Carson, M.L. Crown rust development and selection for virulence in Puccinia coronata f. sp. avenae in an oat multiline cultivar. Plant Dis. 2009, 93, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Dyck, P.L.; Zillinsky, F.J. Inheritance of crown rust resistance transfrred from diploid to hexaploid oats. Can. J. Genet. Cytol. 1963, 5, 398–407. [Google Scholar] [CrossRef]
- Mitchell Fetch, J.W.; Duguid, S.D.; Brown, P.D.; Chong, J.; Fetch, T.G.; Haber, S.M.; Menzies, J.G.; Ames, N.; Noll, J.; Aung, T.; et al. Leggett oat. Can. J. Plant Sci. 2007, 87, 509–512. [Google Scholar] [CrossRef]
- Mitchell Fetch, J.W.; Tekauz, A.; Brown, P.D.; Ames, N.; Chong, J.; Fetch, T.G.; Haber, S.M.; Menzies, J.G.; Townley-Smith, T.F.; Stadnyk, K.D.; et al. Stride oat. Can. J. Plant Sci. 2013, 93, 749–753. [Google Scholar] [CrossRef]
- Rines, H.W.; Miller, M.E.; Carson, M.; Chao, S.; Tiede, T.; Wiersma, J.; Kianian, S.F. Identification, introgression, and molecular marker genetic analysis and selection of a highly effective novel oat crown rust resistance from diploid oat, Avena strigosa. Theor. Appl. Genet. 2018, 131, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Vavilov, N.I. Studies on the Origin of Cultivated Plants; FAO: Leningrad, Russia, 1926. [Google Scholar] [CrossRef]
- Vavilov, N.I. World Resources of Varieties of Small Grains, Grain Legumes and Flax, and Their Use in Breeding; Leningrad Izdvo AN SSSR: Moscow, Russia, 1957. (In Russian) [Google Scholar]
- Kubiak, K. Genetic diversity of Avena strigosa Schreb. ecotypes on the basis of isoenzyme markers. Biodivers. Res. Conserv. 2009, 15, 23–28. [Google Scholar] [CrossRef]
- Scholten, M.; Spoor, B.; Green, N. Machair corn: Management and conservation of a historical machair component. Glas. Nat. 2009, 25, 63–71. [Google Scholar]
- Weibull, J.; Bojesen, L.; Rasomavieius, V. Avena strigosa in Denmark and Lithuania: Prospects for in situ conservation. Plant Genet. Resour. Newsl. 2002, 131, 1–6. [Google Scholar]
- Smitterberg, M. Differences among Variety Samples of Avena strigosa Regarding β-Glucan, Tocopherols, Tocotrienols and Avenanthramides; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- Harder, D.; Chong, J.; Brown, P.; Sebesta, J.; Fox, S. Wild oat as a source of disease resistance: History, utilization, and prospects. In Proceedings of the Fourth International Oat Conference, Wild Oats in World Agriculture, Adelaide, Australia, 19–23 October 1992. [Google Scholar]
- Leggett, J. The conservation and exploration of wild oat species. In Proceedings of the Fourth International Oat Conference, Wild Oats in World Agriculture, Adelaide, Australia, 19–23 October 1992; pp. 57–60. [Google Scholar]
- Gold Steinberg, J.; Mitchell Fetch, J.; Fetch, T.G. Evaluation of Avena spp. accessions for resistance to oat stem rust. Plant Dis. 2005, 89, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Andolfo, G.; Iovieno, P.; Frusciante, L.; Ercolano, M.R. Genome-Editing Technologies for Enhancing Plant Disease Resistance. Front. Plant Sci. 2016, 7, 1813. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.L.; Park, R.F. Genetic analysis of seedling resistance to crown rust in five diploid oat (Avena strigosa) accessions. J. Appl. Genet. 2016, 57, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.L.; Park, R.F. Seedling resistance to Puccinia coronata f. sp. avenae in Avena strigosa, A. barbata and A. sativa. Euphytica 2014, 196, 385–395. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Rubiales, D.; Sillero, J.C.; Prats, E. Identification and characterization of sources of resistance in Avena sativa, A. byzantina and A. strigosa germplasm against a pathotype of Puccinia coronata f.sp. avenae with virulence against the Pc94 resistance g. Plant Pathol. 2012, 61, 315–322. [Google Scholar] [CrossRef]
- Paczos-Grzęda, E.; Sowa, S.; Koroluk, A.; Langdon, T. Characteristics of resistance to Puccinia coronata f. sp. avenae in Avena fatua. Plant Dis. 2018, 102, 2616–2624. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Leonard, K.J.; Salmeron, J.J. A North American System of Nomenclature for Puccinia coronata f. sp. avenae. Plant Dis. 2007, 84, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paczos-Grzęda, E.; Boczkowska, M.; Sowa, S.; Koroluk, A.; Toporowska, J. Hidden diversity of crown rust resistance within genebank resources of Avena sterilis L. Agronomy 2021, 11, 315. [Google Scholar] [CrossRef]
- Sowa, S.; Paczos-Grzęda, E.; Koroluk, A.; Okoń, S.; Ostrowska, A.; Ociepa, T.; Chrząstek, M.; Kowalczyk, K. Resistance to Puccinia coronata f. sp. avenae in Avena magna, A. murphyi, and A. insularis. Plant Dis. 2016, 100, 1184–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paczos-Grzęda, E.; Sowa, S.; Boczkowska, M.; Langdon, T. Detached leaf assays for resistance to crown rust reveal diversity within populations of Avena sterilis L. Plant Dis. 2019, 103, 832–840. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Peters, N.; Paderina, E.V.; Felsenstein, F.; Oppitz, K.; Zeller, F.J. Genetic studies of powdery mildew resistance in common oat (Avena sativa L.) I. Cultivars and breeding lines grown in Western Europe and North America. Euphytica 1997, 96, 421–427. [Google Scholar] [CrossRef]
- Sowa, S.; Paczos-Grzęda, E. A study of crown rust resistance in historical and modern oat cultivars representing 120 years of Polish oat breeding. Euphytica 2020, 216, 12. [Google Scholar] [CrossRef]
- Nazareno, E.S.; Li, F.; Smith, M.; Park, R.F.; Kianian, S.F.; Figueroa, M. Puccinia coronata f. sp. avenae: A threat to global oat production. Mol. Plant Pathol. 2018, 19, 1047–1060. [Google Scholar] [CrossRef] [Green Version]
- Murphy, H.C. Physiologic specialisation in Puccinia coronata f. sp. avenae. Bull. U.S. Dep. Agric. 1935, 433, 1–48. [Google Scholar]
- Sowa, S.; Paczos-Grzęda, E. Identification of molecular markers for the Pc39 gene conferring resistance to crown rust in oat. Theor. Appl. Genet. 2020, 133, 1081–1094. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosman, E. Nei’s gene diversity and the index of average differences are identical measures of diversity within populations. Plant Pathol. 2003, 52, 533–535. [Google Scholar] [CrossRef]
- Kosman, E.; Dinoor, A.; Herrmann, A.; Schachtel, G.A. Virulence Analysis Tool (VAT) User Manual. Available online: Ttp://www.tau.ac.il/lifesci/departments/plant_s/members/kosman/VAT.html (accessed on 15 September 2022).
- Schachtel, G.A.; Dinoor, A.; Herrmann, A.; Kosman, E. Comprehensive Evaluation of Virulence and Resistance Data: A New Analysis Tool. Plant Dis. 2012, 96, 1060–1063. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistocs Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, A.; Lareu, M.V. An overview of STRUCTURE: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Okoń, S.M.; Ociepa, T.; Paczos-Grzęda, E.; Ladizinsky, G. Evaluation of resistance to Blumeria graminis (DC.) f. sp. avenae, in Avena murphyi and A. magna genotypes. Crop Prot. 2018, 106, 177–181. [Google Scholar] [CrossRef]
- Tan, M.Y.A.; Carson, M.L. Screening wild oat accessions from Morocco for resistance to Puccinia coronata. Plant Dis. 2013, 97, 1544–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, M.L. Broad-spectrum resistance to crown rust, Puccinia coronata f. sp. avenae, in accessions of the tetraploid slender oat, Avena barbata. Plant Dis. 2009, 93, 363–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podyma, W.; Boczkowska, M.; Wolko, B.; Dostatny, D.F. Morphological, isoenzymatic and ISSRs-based description of diversity of eight sand oat (Avena strigosa Schreb.) landraces. Genet. Resour. Crop Evol. 2017, 64, 1661–1674. [Google Scholar] [CrossRef]
- Podyma, W.; Bolc, P.; Nocen, J.; Puchta, M.; Wlodarczyk, S.; Lapinski, B.; Boczkowska, M. A multilevel exploration of Avena strigosa diversity as a prelude to promote alternative crop. BMC Plant Biol. 2019, 19, 291. [Google Scholar] [CrossRef]
- Rodionova, N.; Soldatov, V.; Merezhko, V.; Jarosh, N.; Kobyljanskij, V. Flora of Cultivated Plants; Kolos: Moscow, Russia, 1994; Volume 2. [Google Scholar]
- Jackson, E.W.; Obert, D.E.; States, U.; Agricultural, A.; Chong, J.; Avant, J.B.; Bonman, J.M. Detached-leaf method for propagating Puccinia coronata and assessing crown rust resistance in oat. Plant Dis. 2008, 92, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Admassu-Yimer, B.; Gordon, T.; Harrison, S.; Kianian, S.; Bockelman, H.; Bonman, J.M.; Esvelt Klos, K. New Sources of Adult Plant and Seedling Resistance to Puccinia coronata f. sp. avenae Identified among Avena sativa Accessions from the National Small Grains Collection. Plant Dis. 2018, 102, 2180–2186. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, S.; Mohler, V.; Paczos-Grzęda, E. Searching for Novel Oat Crown Rust Resistance in Diploid Oat Avena strigosa Schreb. Reveals the Complexity and Heterogeneity of the Analyzed Genebank Accessions. Agriculture 2023, 13, 296. https://doi.org/10.3390/agriculture13020296
Sowa S, Mohler V, Paczos-Grzęda E. Searching for Novel Oat Crown Rust Resistance in Diploid Oat Avena strigosa Schreb. Reveals the Complexity and Heterogeneity of the Analyzed Genebank Accessions. Agriculture. 2023; 13(2):296. https://doi.org/10.3390/agriculture13020296
Chicago/Turabian StyleSowa, Sylwia, Volker Mohler, and Edyta Paczos-Grzęda. 2023. "Searching for Novel Oat Crown Rust Resistance in Diploid Oat Avena strigosa Schreb. Reveals the Complexity and Heterogeneity of the Analyzed Genebank Accessions" Agriculture 13, no. 2: 296. https://doi.org/10.3390/agriculture13020296





