Development of Synbiotic Preparations That Restore the Properties of Cattle Feed Affected by Toxin-Forming Micromycetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Strains
2.2. Selection of the Optimal Substrate for Cultivation
2.3. Production of Preparation
2.4. Animal Experiments
- Control.
- Control with mycotoxins, 4500 g of bran.
- Positive control. “Cellobacterin” 600 g, 3900 g bran.
- Synbiotic, lower dose. 100 g bacteria preparation, 350 g biochar, 4050 g bran.
- Synbiotic + mycotoxins, lower dose. 100 g bacteria preparation, 350 g biochar, 4050 g bran.
- Synbiotic, high dose. 450 g of the preparation, 350 g of biochar, 3700 g of bran.
- Synbiotic, high dose + mycotoxins 450 g of the preparation, 350 g of biochar, 3700 g of bran.
2.5. Statistical Analysis
3. Results
3.1. Genetic Characteristics of Strains
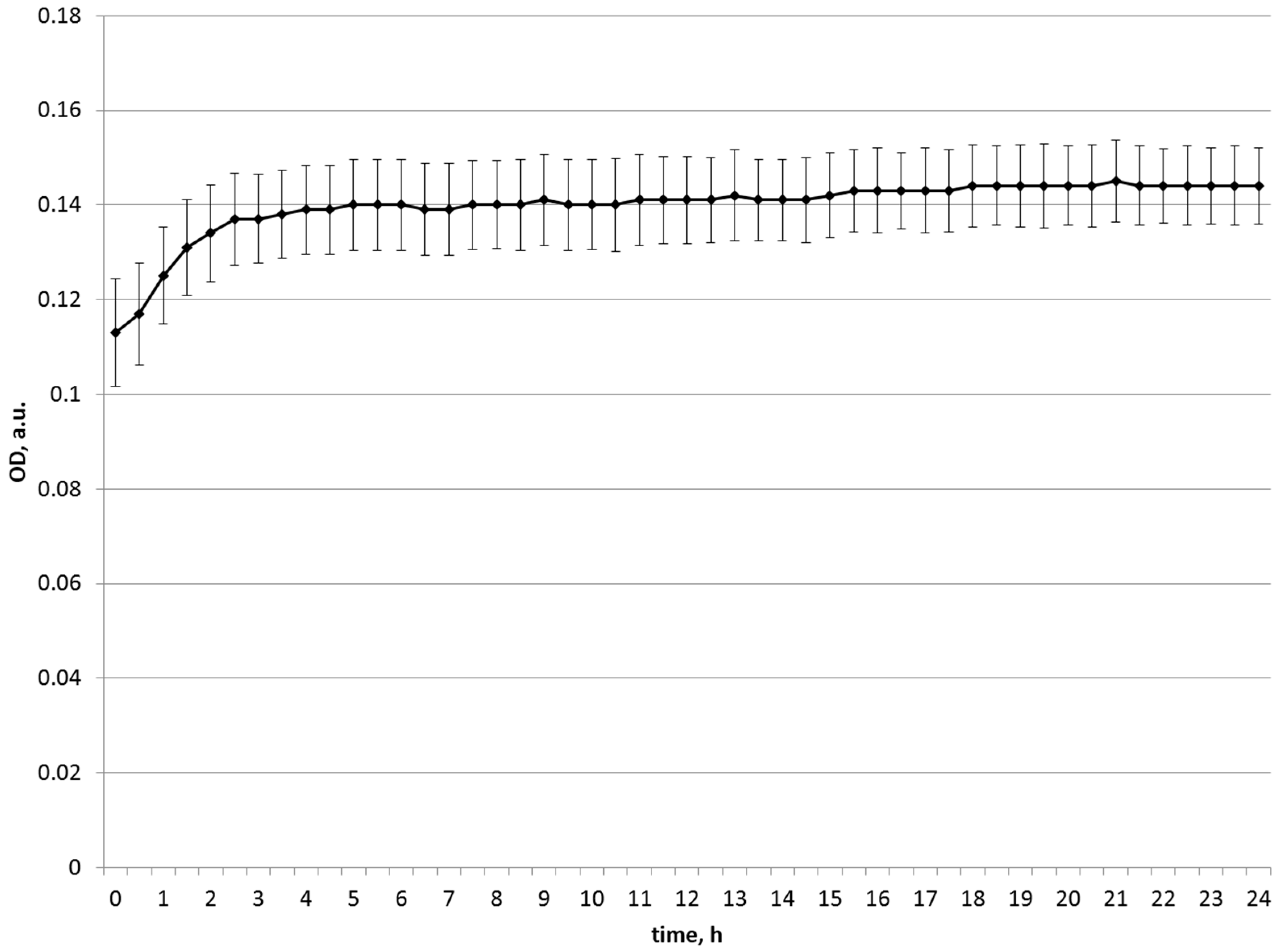
3.2. Selection of the Optimal Substrate
3.3. Influence of the Synbiotic Preparation on the Physiological Parameters of Cattle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10. [Google Scholar] [CrossRef]
- Connolly, L.; Ropstad, E.; Verhaegen, S. In vitro bioassays for the study of endocrine-disrupting food additives and contaminants. TrAC Trends Anal. Chem. 2011, 30, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Kępińska-Pacelik, J.; Biel, W. Alimentary Risk of Mycotoxins for Humans and Animals. Toxins 2021, 13, 822. [Google Scholar] [CrossRef]
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals-A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef]
- Mihalache, O.A.; Dellafiora, L.; Dall’Asta, C. Assessing the Mycotoxin-related Health Impact of Shifting from Meat-based Diets to Soy-based Meat Analogues in a Model Scenario Based on Italian Consumption Data. Expo. Health 2022, 1–15. [Google Scholar] [CrossRef]
- Enguita, F.J.; Martins, L.O.; Henriques, A.O.; Carrondo, M.A. Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J. Biol. Chem. 2003, 278, 19416–19425. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Momb, J.; Thomas, P.W.; Moulin, A.; Petsko, G.A.; Fast, W.; Ringe, D. Mechanism of the Quorum-Quenching Lactonase (AiiA) from Bacillus thuringiensis. Product-Bound Structures. Biochemistry 2008, 47, 7706–7714. [Google Scholar] [CrossRef] [Green Version]
- Rasch, M.; Buch, C.; Austin, B.; Slierendrecht, W.J.; Ekmann, K.S.; Larsen, J.L.; Johansen, C.; Riedel, K.; Eberl, L.; Givskov, M.; et al. An inhibitor of bacterial quorum sensing reduces mortalities caused by Vibriosis in Rainbow Trout (Oncorhynchus mykiss, Walbaum). Syst. Appl. Microbiol. 2004, 27, 350–359. [Google Scholar] [CrossRef]
- Pan, J.; Huang, T.; Yao, F.; Huang, Z.; Powell, C.A.; Qiu, S.; Guan, X. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 2008, 163, 711–716. [Google Scholar] [CrossRef]
- Ming, L.-J.; Epperson, J.D. Metal binding and structure-activity relationship of the metalloantibiotic peptide bacitracin. J. Inorg. Biochem. 2002, 91, 46–58. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli inhibit Salmonella biofilm formation without killing planktonic cells. Front. Microbiol. 2021, 12, 242. [Google Scholar] [CrossRef]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Liu, F.; Wu, J.; Zou, J.; Xiao, X.; Zhao, F.; Zhu, B. HTQC: A fast quality control toolkit for Illumina sequencing data. BMC Bioinform. 2013, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.; Nikolenko, S.; Pham, S.; Prjibelski, A.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Fedorenko, G.M.; Fedorenko, A.G.; Chistyakov, V.A.; Prazdnova, E.V.; Usatov, A.V.; Chikindas, M.L.; Mazanko, M.S.; Weeks, R. Method of preparation, visualization and ultrastructural analysis of a formulation of probiotic Bacillus subtilis KATMIRA1933 produced by solid-phase fermentation. MethodsX 2019, 6, 2515–2520. [Google Scholar] [CrossRef]
- GOST 31653-2012; Feed. Method of Enzyme Immunoassay of Mycotoxins. Standardinform: Moscow, Russia, 2012.
- Wochner, K.F.; Becker-Algeri, T.A.; Colla, E.; Badiale-Furlong, E.; Drunkler, D.A. The action of probiotic microorganisms on chemical contaminants in milk. Crit. Rev. Microbiol. 2018, 44, 112–123. [Google Scholar] [CrossRef]
- Jeżewska-Frąckowiak, J.; Seroczyńska, K.; Banaszczyk, J.; Jedrzejczak, G.; Żylicz-Stachula, A.; Skowron, P.M. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Chen, S.; Zhang, J.; Sun, M.; Liu, Z.; Yu, Z. Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresour. Technol. 2008, 99, 3318–3323. [Google Scholar] [CrossRef]
- Rangarajan, V.; Clarke, K.G. Process development and intensification for enhanced production of Bacillus lipopeptides. Biotechnol. Genet. Eng. Rev. 2015, 31, 46–68. [Google Scholar] [CrossRef]
- Umar, A.; Zafar, A.; Wali, H.; Siddique, M.P.; Qazi, M.A.; Naeem, A.H.; Malik, Z.A.; Ahmed, S. Low-cost production and application of lipopeptide for bioremediation and plant growth by Bacillus subtilis SNW3. AMB Express 2021, 11, 165. [Google Scholar] [CrossRef]
- Subsanguan, T.; Khondee, N.; Rongsayamanont, W.; Luepromchai, E. Formulation of a glycolipid: Lipopeptide mixture as biosurfactant-based dispersant and development of a low-cost glycolipid production process. Sci. Rep. 2022, 12, 16353. [Google Scholar] [CrossRef]
- Ndlovu, T.; Rautenbach, M.; Khan, S.; Khan, W. Variants of lipopeptides and glycolipids produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa cultured in different carbon substrates. AMB Express 2017, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Guan, L.L. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol. 2017, 83, e00061-17. [Google Scholar] [CrossRef] [Green Version]
- Kittelmann, S.; Pinares-Patiño, C.S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; McEwan, J.C.; Janssen, P.H. Two different bacterial community types are linked with the low-methane emission trait in sheep. PLoS ONE 2014, 9, e103171. [Google Scholar] [CrossRef]
- Silberberg, M.; Chaucheyras-Durand, F.; Commun, L.; Mialon, M.M.; Monteils, V.; Mosoni, P.; Morgavi, D.P.; Martin, C. Repeated acidosis challenges and live yeast supplementation shape rumen microbiota and fermentations and modulate inflammatory status in sheep. Animal 2013, 7, 1910–1920. [Google Scholar] [CrossRef] [Green Version]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Berry, D.P.; Crowley, J.J. Cell biology symposium: Genetics of feed efficiency in dairy and beef cattle. J. Anim. Sci. 2013, 91, 1594–1613. [Google Scholar] [CrossRef] [Green Version]
- Veras, F.F.; Correa AP, F.; Welke, J.E.; Brandelli, A. Inhibition of mycotoxin-producing fungi by Bacillus strains isolated from fish intestines. Int. J. Food Microbiol. 2016, 238, 23–32. [Google Scholar] [CrossRef]
- Qin, X.; Su, X.; Tu, T.; Zhang, J.; Wang, X.; Wang, Y.; Wang, Y.; Bai, Y.; Yao, B.; Luo, H.; et al. Enzymatic degradation of multiple major mycotoxins by dye-decolorizing peroxidase from Bacillus subtilis. Toxins 2021, 13, 429. [Google Scholar] [CrossRef]
- Hanif, A.; Zhang, F.; Li, P.; Li, C.; Xu, Y.; Zubair, M.; Zhang, M.; Jia, D.; Zhao, X.; Liang, J.; et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins 2019, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Hassan, Z.U.; Al Thani, R.; Alsafran, M.; Migheli, Q.; Jaoua, S. Selection of Bacillus spp. with decontamination potential on multiple Fusarium mycotoxins. Food Control 2021, 127, 108119. [Google Scholar] [CrossRef]
- Abdallah, Y.; Ul Hassan, Z.; Al-Thani, R.; Al-Shamary, N.; Al-Yafei, T.; Alnaimi, H.; Jaoua, S. Prevalence of toxigenic mycobiota and mycotoxins in date palm fruits and investigation on Bacillus cereus 342-2 as biocontrol agent. Biocontrol Sci. Technol. 2022, 32, 1372–1388. [Google Scholar] [CrossRef]
- Guimarães, R.A.; Pherez-Perrony, P.E.; Müller, H.; Berg, G.; Medeiros FH, V.; Cernava, T. Microbiome-guided evaluation of Bacillus subtilis BIOUFLA2 application to reduce mycotoxins in maize kernels. Biol. Control 2020, 150, 104370. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2012, 11, 33–44. [Google Scholar] [CrossRef]
- Ghazali, M.F.; Koh-Tan, H.H.; McLaughlin, M.; Montague, P.; Jonsson, N.N.; Eckersall, P.D. Alkaline phosphatase in nasal secretion of cattle: Biochemical and molecular characterisation. BMC Vet. Res. 2014, 10, 204. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, T.; Dulani, S.; Nimali, S.S.; De Silva, A.P.; Fernando, J.; De Silva, H.J.; Wickramasinghe, V.P. Effects of probiotics combined with dietary and lifestyle modification on clinical, biochemical, and radiological parameters in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: A randomized clinical trial. Clin. Exp. Pediatr. 2022, 65, 304. [Google Scholar] [CrossRef]
- Baralić, K.; Živančević, K.; Bozic, D.; Đukić-Ćosić, D. Probiotic cultures as a potential protective strategy against the toxicity of environmentally relevant chemicals: State-of-the-art knowledge. Food Chem. Toxicol. 2022, 172, 113582. [Google Scholar] [CrossRef]
- Hsu, T.C.; Yi, P.J.; Lee, T.Y.; Liu, J.R. Probiotic characteristics and zearalenone removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Ju, J.; Tinyiro, S.E.; Yao, W.; Yu, H.; Guo, Y.; Qian, H.; Xie, Y. The ability of Bacillus subtilis and Bacillus natto to degrade zearalenone and its application in food. J. Food Process Preserv. 2019, 43, e14122. [Google Scholar] [CrossRef]
- Lee, A.; Cheng, K.C.; Liu, J.R. Isolation and characterization of a Bacillus amyloliquefaciens strain with zearalenone removal ability and its probiotic potential. PLoS ONE 2017, 12, e0182220. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Chang, J.; Wang, P.; Liu, C.; Yin, Q.; Zhu, Q.; Lu, F.; Gao, T. Effect of the combined compound probiotics with mycotoxin–degradation enzyme on detoxifying aflatoxin B1 and zearalenone. J. Toxicol. Sci. 2018, 43, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ma, Q.; Ma, S.; Zhang, J.; Jia, R.; Ji, C.; Zhao, L. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins 2016, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.-Y.; Qi, M.; Zhao, L.; Zhu, M.-K.; Guo, J.; Liu, J. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins 2016, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.T.; Bang, N.N.; Dart, P.; Callaghan, M.; Klieve, A.; Hayes, B.; McNeill, D. Feed preference response of weaner bull calves to Bacillus amyloliquefaciens H57 probiotic and associated volatile organic compounds in high concentrate feed pellets. Animals 2020, 11, 51. [Google Scholar] [CrossRef]
- Begley, M.; Cotter, P.D.; Hill, C.; Ross, R.P. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microbiol. 2009, 75, 5451–5460. [Google Scholar] [CrossRef] [Green Version]
- Caetano, T.; Krawczyk, J.M.; Mösker, E.; Süssmuth, R.D.; Mendo, S. Heterologous expression, biosynthesis, and mutagenesis of type II lantibiotics from Bacillus licheniformis in Escherichia coli. Chem. Biol. 2011, 18, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.C.; Silva, Í.C.; Caetano, T.; Mösker, E.; Seidel, M.; Lourenço, J.; Süssmuth, R.D.; Santos, N.C.; Gonçalves, S.; Mendo, S. Assessing the potential of the two-peptide lantibiotic lichenicidin as a new generation antimicrobial. World J. Microbiol. Biotechnol. 2022, 38, 18. [Google Scholar] [CrossRef]
- Panina, I.S.; Balandin, S.V.; Tsarev, A.V.; Chugunov, A.O.; Tagaev, A.A.; Finkina, E.I.; Antoshina, D.V.; Sheremeteva, E.V.; Paramonov, A.S.; Rickmeyer, J.; et al. Specific Binding of the α-Component of the Lantibiotic Lichenicidin to the Peptidoglycan Precursor Lipid II Predetermines Its Antimicrobial Activity. Int. J. Mol. Sci. 2023, 24, 1332. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, S.; Makowski, M.; Silva, Í.C.; Caetano, T.; Schneider, T.; Mendo, S. Insights into the mode of action of the two-peptide lantibiotic lichenicidin. Colloids Surf. B Biointerfaces 2022, 211, 112308. [Google Scholar] [CrossRef]
- Coronel-León, J.; de Grau, G.; Grau-Campistany, A.; Farfan, M.; Rabanal, F.; Manresa, A.; Marqués, A.M. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015, 65, 2065–2078. [Google Scholar] [CrossRef]
- Anuradha, S.N. Structural and molecular characteristics of lichenysin and its relationship with surface activity. Biosurfactants 2010, 672, 304–315. [Google Scholar] [CrossRef]
- Medeot, D.B.; Fernandez, M.; Morales, G.M.; Jofré, E. Fengycins from Bacillus amyloliquefaciens MEP218 exhibit antibacterial activity by producing alterations on the cell surface of the pathogens Xanthomonas axonopodis pv. vesicatoria and Pseudomonas aeruginosa PA01. Front. Microbiol. 2020, 10, 3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Tscheka, C.; Edwards, K.; Karlsson, G.; Heerklotz, H. All-or-none membrane permeabilization by fengycin-type lipopeptides from Bacillus subtilis QST713. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sun, C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018, 84, e00445-18. [Google Scholar] [CrossRef] [Green Version]
- González-Jaramillo, L.M.; Aranda, F.J.; Teruel, J.A.; Villegas-Escobar, V.; Ortiz, A. Antimycotic activity of fengycin C biosurfactant and its interaction with phosphatidylcholine model membranes. Colloids Surf. B Biointerfaces 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Su, Z.; Chen, X.; Liu, X.; Guo, Q.; Li, S.; Lu, X.; Zhang, X.; Wang, P.; Dong, L.; Zhao, W.; et al. Genome mining and UHPLC–QTOF–MS/MS to identify the potential antimicrobial compounds and determine the specificity of biosynthetic gene clusters in Bacillus subtilis NCD-2. BMC Genom. 2020, 21, 767. [Google Scholar] [CrossRef]
- Hertlein, G.; Müller, S.; Garcia-Gonzalez, E.; Poppinga, L.; Süssmuth, R.D.; Genersch, E. Production of the catechol type siderophore bacillibactin by the honey bee pathogen Paenibacillus larvae. PLoS ONE 2014, 9, e108272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Heng, J.; Qin, S.; Bian, K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Liu, F.; Yang, X.; Jin, J.; Dong, X.; Zeng, K.W.; Liu, D.; Zhang, Y.; Ma, M.; Yang, D. Bacillibactin and bacillomycin analogues with cytotoxicities against human cancer cell lines from marine Bacillus sp. PKU-MA00093 and PKU-MA00092. Mar. Drugs 2018, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Vasilchenko, A.S.; Rogozhin, E.A. Subinhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef] [Green Version]

| Strain | Metabolite | Percentage of Similarity with a Known Analogue, % |
|---|---|---|
| B. licheniformis CL 33 | Lichenicidin | 100% |
| Lichenisin | 100% | |
| Fengycin-like peptide | 53% | |
| Bacillibactin-like peptide | 53% | |
| Butyrosine-like metabolite | 7% | |
| B. licheniformis CL 56 | Lichenicidin | 100% |
| Lichenisin | 100% | |
| Bacillibactin-like peptide | 52% | |
| Fengycin-like peptide | 53% |
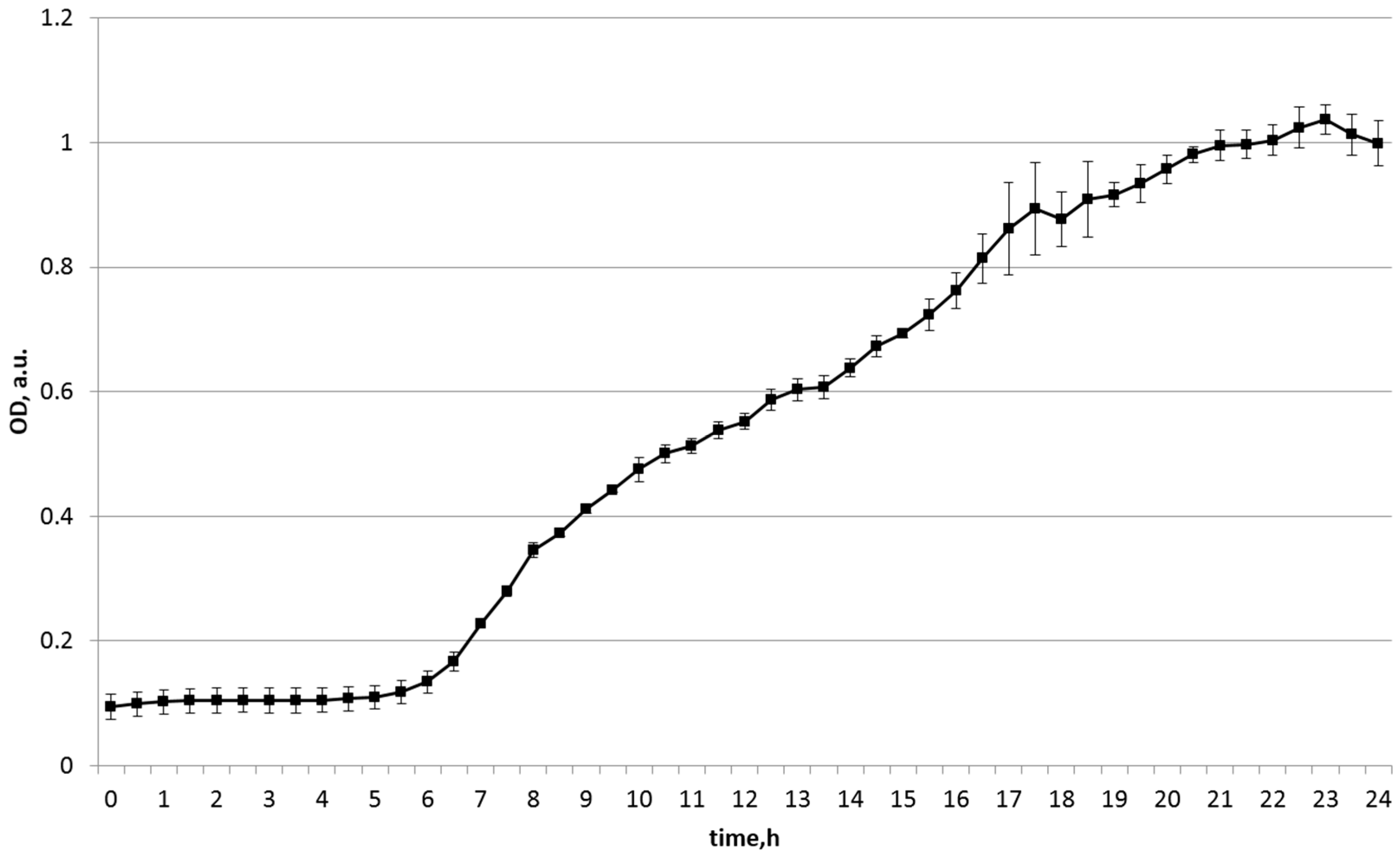
| Substrate Source | Start OD | Maximum OD | Final OD (24 h) |
|---|---|---|---|
| Soy | 0.099 | 1.037 | 0.999 |
| Chickpea | 0.145 | 0.902 | 0.531 |
| Bell pepper | 0.738 | 0.856 | 0.856 |
| Zucchini | 0.158 | 0.254 | 0.254 |
| Green pea | 0.119 | 0.136 | 0.136 |
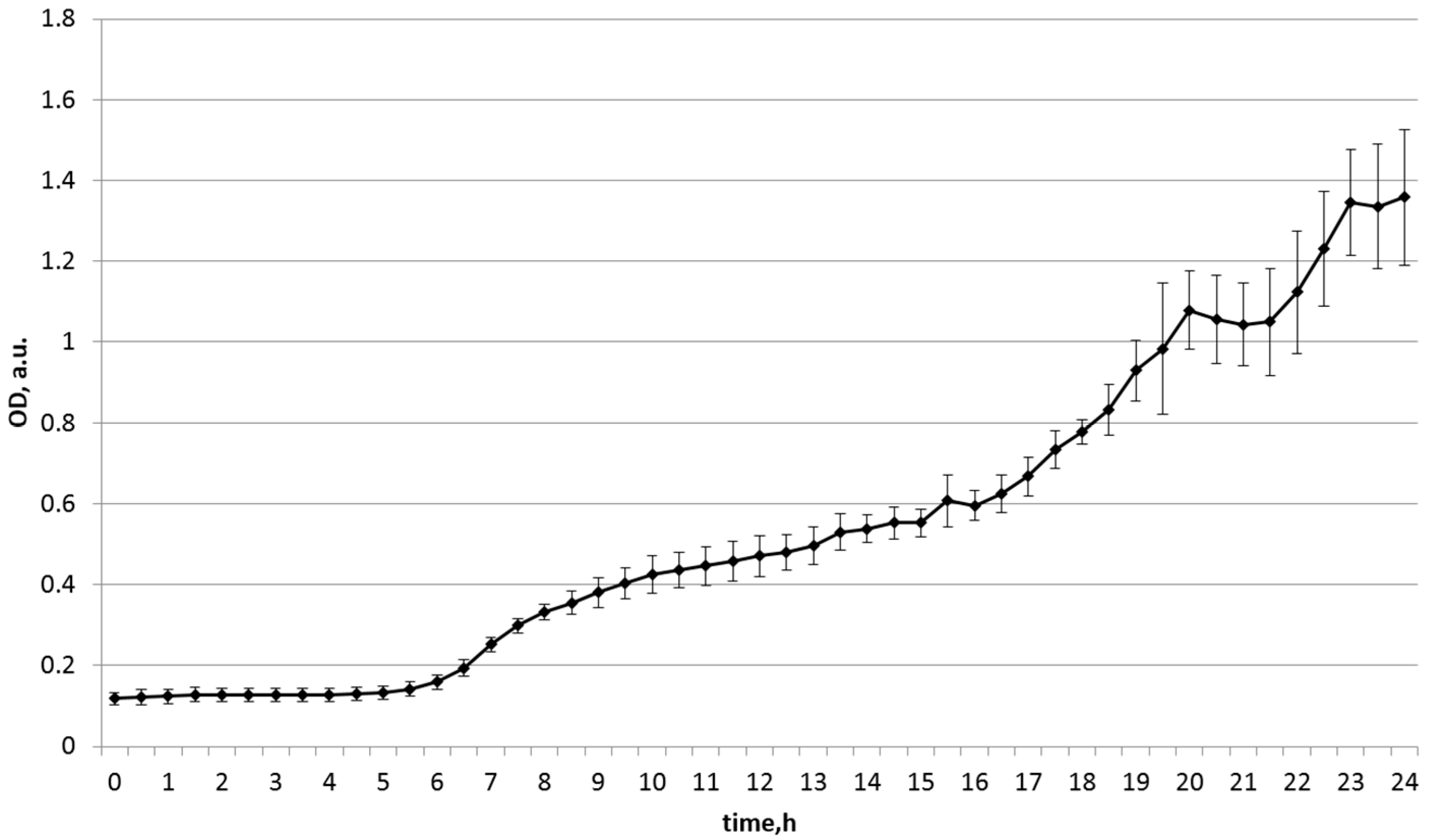
| Substrate Source | Start OD | Maximum OD | Final OD (24 h) |
|---|---|---|---|
| soy | 0.118 | 1.358 | 1.358 |
| chickpea | 0.116 | 0.733 | 0.567 |
| bell pepper | 0.732 | 0.846 | 0.846 |
| zucchini | 0.125 | 0.386 | 0.386 |
| green pea | 0.113 | 0.144 | 0.144 |
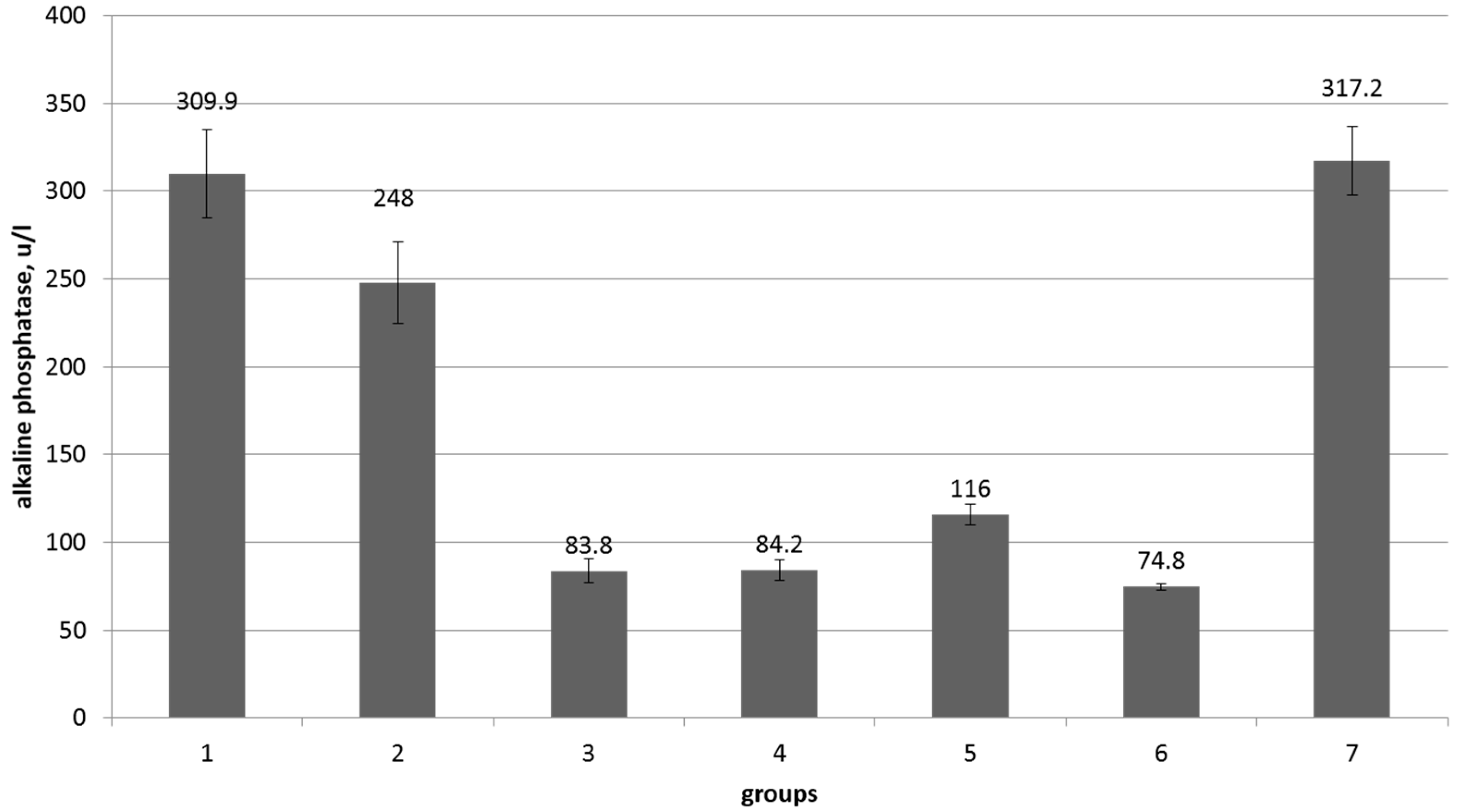
| Indicators, Units | Reference Values | Control | Control + Myco-Toxins | Cello-Bacterin | Synbiotic Low Dose | Synbiotic Low Dose + Mycotoxins | Synbiotic High Dose | Synbiotic High Dose + Mycotoxins |
|---|---|---|---|---|---|---|---|---|
| Weight gain, kg | - | 12.5 ± 1.2 | 9.6 ± 2.2 | 12.5 ± 1.4 | 13.2 ± 1.3 ** | 11.9 ± 1.0 | 13.3 ± 1.3 * | 12.4 ± 1.2 * |
| Weight, kg | 280–330 | 316.7 ± 2.5 | 317.6 ± 3.8 | 316.1 ± 2.0 | 306.4 ± 26.4 | 309.7 ± 3.6 | 312.9 ± 3.2 | 319.6 ± 2.8 |
| Temperature, °C | 37.5–39.0 | 38.3 ± 0.08 | 38.14 ± 0.09 | 38.2 ± 0.06 | 38.1 ± 0.12 | 38.0 ± 0.09 | 38.2 ± 0.07 | 38.1 ± 0.04 |
| Pulse beats/min. | 50–80 | 78.8 ± 2.6 | 63.8 ± 2.1 | 75.7 ± 3.66 | 70.1 ± 2.1 | 66 ± 1.6 | 73.7 ± 2.9 | 76.6 ± 2.8 |
| Breathing in/min | 15–30 | 21.5 ± 0.6 | 21.9 ± 1.2 | 20.6 ± 0.5 | 21.9 ± 1.1 | 22 ± 0.8 | 21.1 ± 1.3 | 23 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bren, A.; Denisenko, Y.; Prazdnova, E.; Mazanko, M.; Gorovtsov, A.; Chistyakov, V.; Pakhomov, V.; Rudoy, D.; Olshevskaya, A. Development of Synbiotic Preparations That Restore the Properties of Cattle Feed Affected by Toxin-Forming Micromycetes. Agriculture 2023, 13, 523. https://doi.org/10.3390/agriculture13030523
Bren A, Denisenko Y, Prazdnova E, Mazanko M, Gorovtsov A, Chistyakov V, Pakhomov V, Rudoy D, Olshevskaya A. Development of Synbiotic Preparations That Restore the Properties of Cattle Feed Affected by Toxin-Forming Micromycetes. Agriculture. 2023; 13(3):523. https://doi.org/10.3390/agriculture13030523
Chicago/Turabian StyleBren, Angelica, Yury Denisenko, Evgeniya Prazdnova, Mariya Mazanko, Andrey Gorovtsov, Vladimir Chistyakov, Viktor Pakhomov, Dmitry Rudoy, and Anastasiya Olshevskaya. 2023. "Development of Synbiotic Preparations That Restore the Properties of Cattle Feed Affected by Toxin-Forming Micromycetes" Agriculture 13, no. 3: 523. https://doi.org/10.3390/agriculture13030523
APA StyleBren, A., Denisenko, Y., Prazdnova, E., Mazanko, M., Gorovtsov, A., Chistyakov, V., Pakhomov, V., Rudoy, D., & Olshevskaya, A. (2023). Development of Synbiotic Preparations That Restore the Properties of Cattle Feed Affected by Toxin-Forming Micromycetes. Agriculture, 13(3), 523. https://doi.org/10.3390/agriculture13030523









