1. Introduction
The soil is regarded as a major source of nutrients required for plant growth. Thus, ensuring nutrient supply and substrate quality, coupled with the recovery potential and resource recycling and use efficiency of the plants, determine the sustainability of a forest system [
1]. The loss of soil fertility from soil erosion and other management practices may increase the use of chemical fertilizers on soils to maintain productivity, which increases economic burdens on the local farmers [
2] and deteriorates soil health through acidification [
3,
4]. To avoid this loss of soil fertility, many soil revitalization practices are available for application to restore soil nutrients, improve soil fertility, and revitalize soil conditions to favor plant growth [
5,
6]. For example, intensive management practices such as fertilization and organic mulching are frequently applied in Lei bamboo (
Phyllostachys praecox) plantations to achieve higher production. Nevertheless, heavy winter mulching and high rates of fertilization could significantly enhance soil CO
2 efflux in Lei bamboo forests by increasing microbial respiration [
7]. In another instance, the oxygenation of subsurface drip-irrigation water was found to improve cotton yield and gross production water-use index by 10% and 7%, respectively [
6].
Cultivation and fertilization in Lei bamboo plantations are challenging and require special maneuvering skills to apply chemical fertilizer, land tilling, water management, and cultivation management practices. Hence, a much simpler, cost-effective, and long-term management practice remedy method of soil organic mulching has been adopted and employed in Lei bamboo plantations in recent years [
8]. Soil organic mulching can be defined as the application of non-living plant materials at the surface of the soil primarily to prevent the loss of water by evaporation, alter soil nutrients, and improve soil basic conditions. Soil properties and soil conditions that are affected directly or indirectly by soil organic mulching treatment include soil water content (SWC) through increased infiltration, water storage or retention, soil temperature through radiation shielding, soil organic matter (SOM), heat conduction and evaporation cooling, soil temperature moderation, soil nutrients mobility and percolation, soil biological organisms and microorganisms, etc. However, soil organic mulching could prevent proper gaseous exchange between the soil and the atmosphere, resulting in soil hypoxia [
8,
9] or the lack of oxygen to support and promote the proper growth of Lei bamboo and different plants. Soil organic mulching may also induce soil hypoxia during warming due to the decomposing organic material and fermentation. When there is soil hypoxia in Lei bamboo plantations, a problem of bamboo rhizome up-floating may occur [
8].
In plants, transient flooding events, waterlogging and microbial activity in the soil frequently and rapidly lead to hypoxia or oxygen deficiency [
10]. Soil hypoxia can also be caused by excessive soil compaction due to machinery, raindrops, and human traffic on soil surfaces. Soil compaction interferes with oxygen movement into the soil because of the loss of macrospore space. The large pores in well-structured soils are important routes for gaseous exchange that can be lost when soils are compacted or have high soil moisture, resulting from excessive irrigation or poor drainage. When soil pores are filled with water, oxygen cannot enter the profile, and the small amounts dissolved in water are rapidly depleted at sites of high metabolic activity [
11]. Coupled with other challenges, soil hypoxia is a huge challenge faced by the Lei bamboo farmers. Recent advances suggest that soil hypoxia can be prevented by employing a novel management practice of soil aeration irrigation, where the air is introduced into the soil using buried PVC pipes underneath the soil surface to supply additional air into the soil [
12]. Thus, aeration irrigation, a modified irrigation technique that involves injecting air into soils has been extensively proven to improve soil aeration and alleviate hypoxia [
12]. Other research conclusions suggest that soil oxygenation mitigates hypoxic conditions in soils by increasing dissolved oxygen content [
13] and increase access to oxygen by aeration has positive impacts on plant growth and physiology, even under saline stress [
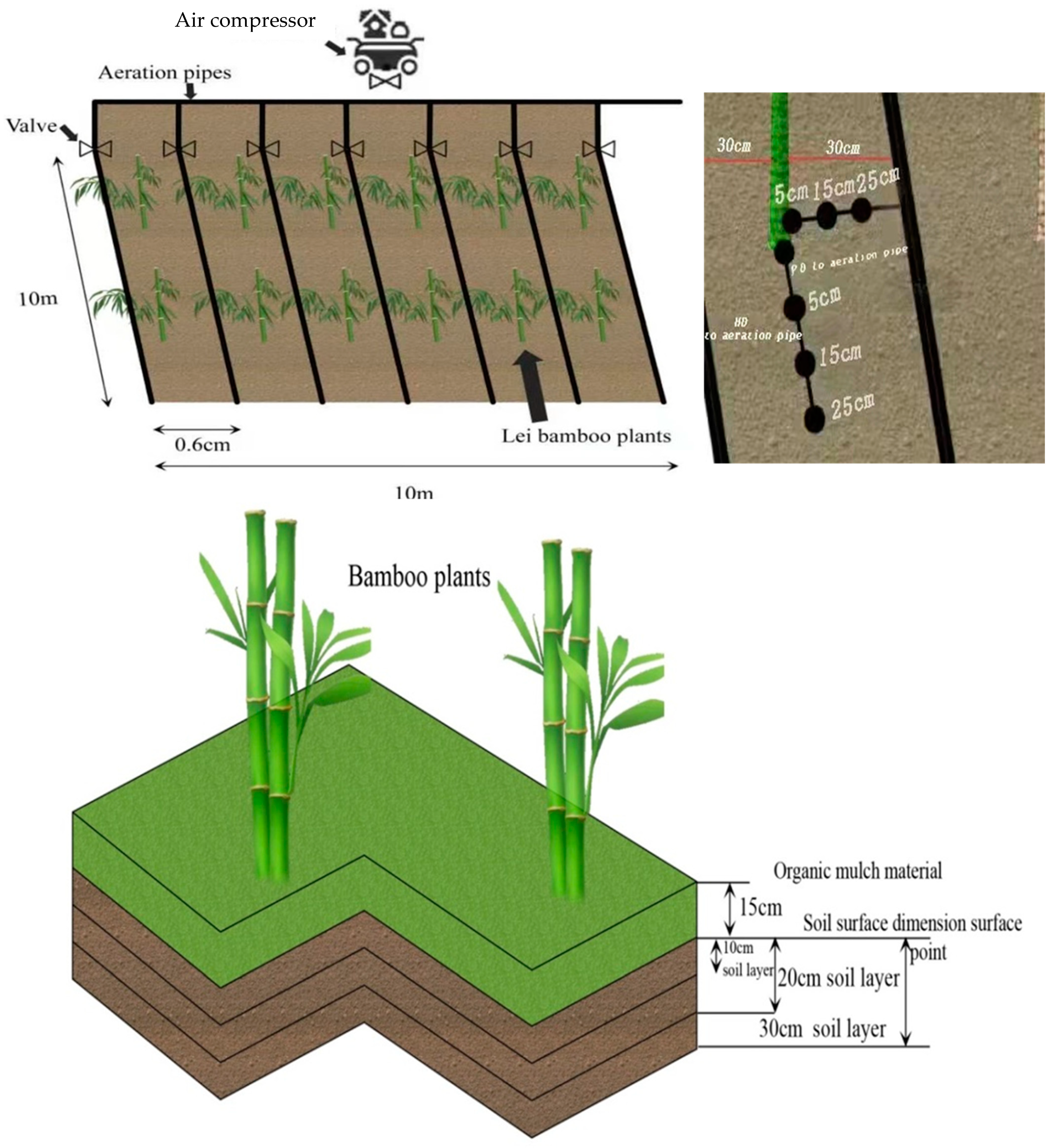
14]. Despite these research advancements, much still needs to be done to fully understand the strengths and weaknesses of aeration irrigation. For example, how well can aeration irrigation technology perform in conjunction with soil organic mulching or can it mitigate hypoxia in Lei bamboo plantation under soil organic mulching management? Thus, our main objective was to determine the effects of soil organic mulching combined with aeration irrigation on Lei bamboo shoot production, soil pH, soil oxygen content and temperature, soil water content (SWC), and soil physicochemical properties. To fully explore changes in these parameters, we considered variations around the plant rhizospheres at different radii distances (5, 15 and 25 cm) at different soil depths (10, 20 and 30 cm), and in two different aeration irrigation directions (perpendicular direction, PD and horizontal direction, HD).
3. Results
3.1. Variations in Soil Oxygen Content with Soil Depth and Distance from the Bamboo Trunk
Figure 2 shows that there were significant differences (
p < 0.05) in oxygen content between the soils of MNA at a soil depth of 10 cm with CK, MA and NMA, both in PD and HD directions, while no significant differences were observed between CK, MA and NMA. The average oxygen content in CK, MA, and NMA was 21.6%, 21.7%, and 20.7%, respectively, while there was a negative effect and reduction in soil oxygen content by 6.3% when the soil was mulched but not aerated (MNA). When the soil depth was increased to 20 cm in both PD and HD directions, the average oxygen content in CK, MA, NMA, and MNA was 18.9%, 20.62%, 20.5%, and 17.9%, respectively. There was a positive significant difference in oxygen contents between CK, MA, and NMA but not between CK and MNA in the PD direction. Further down to 30 cm, the average oxygen content in CK, MA, NMA, and MNA was 17.3%, 19.3%, 19.9%, and 16.7%, respectively. This shows that there was a positive significant difference in oxygen content between plots MA and NMA relative to CK but a negative significant difference between MNA and CK. Thus, irrespective of the treatment, soil oxygen content decreased with depth, suggesting that plant roots get exposed to a lower amount of oxygen as they grow further downwards.
At a distance of 5 cm from the Lei bamboo trunk in both PD and HD directions and symmetrical to the aeration pipes, the average soil oxygen content in CK, MA, NMA, and MNA was 19.3%, 20.4%, 20.4%, and 18.3%, respectively. At this distance, there was a positive significant difference between plots MA and NMA relative to CK but a negative significant difference between MNA and CK. Compared to CK, treatments that received mulching and aeration (MA) or only aeration (NMA) experienced an increase in oxygen content of 5.7% and 5.2%, respectively. At a distance of 15 cm, the oxygen content in CK, MA, NMA, and MNA was 19.3%, 20.4%, 20.6%, and 18.3%. Accordingly, there was an increase in the oxygen content by 5.8% in MA and 7.1% in NMA, but a 4.9% decrease in MNA. Unlike at different depths where oxygen content decreased with depth, there was little variation with distance from the tree trunk. This was evident as at a distance of 25 cm, the oxygen content in CK, MA, NMA, and MNA was 19.3%, 20.5%, 21.2%, and 18.4%, respectively. There were positive significant differences in plots MA and NMA and a negative significant difference in MNA. In both MA (6.2%) and NMA (9.7%), there was a positive increase in the oxygen content in the soil and this was more than the oxygen concentration in ambient air. This observation was probably induced by the artificial aeration technique. Nevertheless, there was a negative effect and a 4.6% reduction in soil oxygen content in MNA treatment.
3.2. Variations in Soil Temperature with Soil Depth and Distance from the Bamboo Trunk
Figure 3 shows that at a soil depth of 10 cm, there was a significant difference in temperature between plot MNA and the other treatments. The average soil temperature was 13.4 °C in CK, 13.4 °C in MA, 13.1 °C in NMA, and 15.3 °C in MNA, with an average percentage increase of 14.0% in MNA compared to CK. When soil depth was 20 cm, there were significant differences between CK, MA, and MNA but not with NMA. CK had an average soil temperature of 7.2 °C, 11.5 °C for MA, 6.1 °C for NMA, and 13.7 °C for MNA. At this depth, there was a 59.4% increase in soil temperature in MA and 89.5% in MNA compared to CK. The soil average temperature continued to decrease as the sampling depth was increased to 30 cm. Specifically, the soil average temperature was decreased to 4.3 °C in CK, 10.4 °C in MA, 4.2 °C in NMA, and 11.0 °C in MNA. Comparatively, there was an average percentage increase in soil temperature of 140.6% in MA and 154.1% in MNA relative to CK at the 30 cm depth.
The average soil temperature in plot CK was 8.3 °C, 11.8 °C in MA, 7.8 °C in NMA, and 13.4 °C in MNA at a distance of 5 cm from the bamboo plant trunk in both PD and HD directions. Relative to CK, soil temperature was increased by 43.1% in MA and 61.4% in MNA. At 15 cm from the bamboo trunk, MA and MNA showed significant differences in soil temperature compared to CK while NMA did not. There was an average increase in soil temperature of 42.4% in MA and 60.1% in MNA relative to CK. The changing trend observed in soil temperature at 15 cm was also observed at 25 cm. Specifically, a 37.7% and 56.3% increase in soil temperature was recorded in MA and MNA relative to CK, respectively. These results show that soil mulching increased soil temperature both vertically and horizontally and aerating the soil mitigated this increase. Nevertheless, for the same treatment, soil temperature significantly decreased with depth but remained almost similar at the horizontal distance from the bamboo trunk.
3.3. Variations in Soil pH with Soil Depth, Distance from the Bamboo Trunk, and Treatment
Figure 4 shows variations in soil pH with depth and distance from the bamboo trunk. At a soil depth of 10 cm, there were no significant differences in soil pH between CK, MA, and NMA in the PD direction unlike in the HD direction. Compared to CK, MA experienced a slight increase of 0.17 units while NMA and MNA both recorded a decrease of 0.32 and 0.74 units, respectively. When the soil depth was increased to 20 cm, the soil pH was significantly lower compared to that at 10 cm depth. At this depth, the average soil pH values were 5.57, 5.78, 5.83, and 4.96 for CK, MA, NMA, and MNA. Furthermore, the soil pH was further decreased to 4.75, 5.30, 4.96, and 4.28 for CK, MA, NMA, and MNA at a soil depth of 30 cm.
In both PD and HD aeration directions and 5 cm away from the tree trunk, soil pH in CK was 5.85, which was 0.46 and 0.23 units lower than in MA and NMA but 0.70 units higher than in MNA. At 15 cm away from the bamboo trunk in both PD and HD directions, the average soil pH in CK was decreased to 5.54 and was still lower relative to MA and NMA but higher than in MNA. At 25 cm, soil pH in CK was slightly recovered compared to that at 15 cm while that in MA, NMA, and MNA maintained their decreasing trend. Generally, the average soil pH was 5.73, 5.85, 5.58, and 4.90 for CK, MA, NMA, and MNA, respectively (
Figure 4). As evident, soil mulching without aeration (MNA) negatively impacted soil pH while mulching in combination with aeration (MA) retarded pH decline at different depth and distance from the bamboo trunk.
For the respective treatments at different depths and directions (PD and HD), soil pH generally showed a significant decreasing trend (
Figure S2). This decrease was even more significant for treatments that received mulching without aeration (MNA) and for CK. For CK in the PD direction, the pH at 10 cm was 6.80, which decreased by 1.12 and 2.06 units at 20 and 30 cm, respectively. Similarly, in the HD direction, soil pH at 20 and 30 cm was 1.33 and 2.03 units lower than for CK. When the soil was mulched and aerated (MA), the highest pH of all the treatments was recorded to be pH 7.01 at 10 cm in the PD direction. This was decreased to 5.91 and 5.41 at depths of 20 and 30 cm, respectively. The changing trend of pH in the HD direction followed a similar trend of 10 cm (pH 6.93) > 20 cm (pH 5.65) > 30 cm (pH 5.20). Relative to CK, MA, and NMA, the treatment that received mulching with no aeration (MNA) had the lowest pH in both the PD and HD directions. For instance, at 10, 20, and 30 cm in the PD direction for MNA, soil pH was 6.21, 5.13, and 4.36, with corresponding values of 5.90, 4.78, and 4.20 in the HD direction, respectively. As the soil pH decreased with sampling depth (to pH < 5.0), the bamboo plant growth could be inhibited by aluminum toxicity [
16] and the negative effect would be most significant for CK, NMA, and MNA.
3.4. Variations in Soil Water (SWC) and Organic Matter Contents with Soil Depth, Distance from the Bamboo Trunk, and Treatment
There was little variation in the SWC at the same depth for all treatments (
Figure 5). Nevertheless, for the same treatment and either in the HD or PD direction, SWC decreased with depth from 10 to 30 cm. According to the results, only the soil sample from CK at point HD30 cm showed a significant difference in SWC compared with the other treatments at all points. The average SWC for all treatments (i.e., CK, MA, NMA and MNA) at different soil depths was 3.5% at 10 cm, 3.0% at 20 cm, and 2.9% at 30 cm. Moving away from the bamboo trunk at a radius of 5 cm, the average SWC was 2.8%, 3.7%, 3.3%, and 2.99%, at 15 cm, the average SWC was 2. 9%, 3.5%, 3.1%, and 3.1% while at 25 cm it was 2.9%, 3.4%, 3.0%, and 3.0% for CK, MA, NMA, and MNA, respectively. As shown, MA and NMA treatments had a negative impact on SWC as the radius from the bamboo trunk was increased.
The content of soil organic matter showed significant variations between CK and MA at all the sampling depths and distances from the bamboo trunk (
Figure 6). For the different treatments, the average SOM content in CK was 3.7%, 5.9% in MA, 4.3% in NMA, and 4.9% in MNA at 10 cm. Relative to CK, the SOM at 10 cm depth was increased by 60.4%, 16.3%, and 32.8% for MA, NMA, and MNA, respectively. This trend was similar at 20 and 30 cm depths but with decreased SOM under each treatment. For example, at 20 cm, the average SOM content was 3.0% in CK, 5.0% in MA, 3.8% in NMA, and 3.9% in MNA. Nevertheless, compared to the other treatments, MA induced the most significant increase of 65.6% relative to CK while at 30 cm, this increase was estimated to be 80.9% in the PD direction. When sampling was done at increasing radii away from the bamboo trunk, there were no significant differences between different depths of the same treatment. However, between the different treatments, MA induced the most significant increment in SOM content at the respective radius. At 5 cm from the bamboo trunk, MNA HD also induced a significant difference relative to CK, suggesting that with or without aeration, mulching had a significant impact on SOM content. The average SOM content for the different treatments at this radius was 3.0%, 5.2%, 3.2%, and 3.7% for CK, MA, NMA, and MNA, respectively. Overall, there was a 73.9% and 34.0% increment in SOM induced by MA and MNA (HD 5 cm) compared to CK. At a radius of 15 cm, MA exhibited the most significant increment in SOM of 67.9%, while at 25 cm, both MA and MNA (HD) induced an increase of 53.1% and 55.3%, respectively.
3.5. Variations in Soil Nutrients (NPK) with Soil Depth, Distance from the Bamboo Trunk, and Treatment
The content of soil total nitrogen (TN) decreased with sampling depth for all treatments (
Figure 7). At 10 cm, there were no significant differences in TN content in either the HD and PD directions. At 10 cm, the average soil TN was 1930, 2519.3, 2463.1, and 2700.2 mg kg
−1 for CK, MA, NMA, and MNA, respectively. In the HD direction, MNA and MA induced a 39.9% and 34.0% increment in TN compared to CK. Even at a soil depth of 20 cm, CK still had the least content of TN, with an average value of 1473.5 mg kg
−1 compared to 1971.5, 1814.0, and 1768.9 mg kg
−1 for MA, NMA, and MNA, respectively. In the PD direction, the average increase in TN induced by MA at 20 cm was 41.0% while it was 36.8% for NMA PD at 20 cm and 36.3% for MNA. At a depth of 30 cm, the average content of TN followed the sequence MA (1336.4 mg kg
−1) > CK (1143.4 mg kg
−1) > NMA (1015.6 mg kg
−1) > MNA (827.7 mg kg
−1). In addition, sampling at a radius of 5 cm, MA and NMA showed positive significant differences in TN relative to CK (PD direction). Here, the average TN contents followed the sequence CK (1574.5 mg kg
−1) < MNA (1790.0 mg kg
−1) < NMA (1891.4 mg kg
−1) < MA (2056.5 mg kg
−1), while at 15 cm the trend was CK (1533.6 mg kg
−1) < NMA (1660.8 mg kg
−1) < MNA (1790.5 mg kg
−1) < MA (1946.08 mg kg
−1) and at 25 cm it was CK (1438.9 mg kg
−1) < MNA (1719.5 mg kg
−1) < NMA (1742.1 mg kg
−1) < MA (1824.5 mg kg
−1). As shown, only the CK and MA treatments showed decreasing trends in TN as sampling radii were increased while NMA treatments recovered after an initial decrease from 10–15 cm. Nevertheless, all the treatments showed the potential of improving soil TN content.
Figure 8 shows variations in soil phosphorus with treatments, depth, and distance away from the plant. The average soil total phosphorus in the respective treatments was 0.58%, 0.34%, 0.59%, and 0.61% at 10 cm for CK, MA, NMA, and MNA, respectively. The corresponding phosphorus content was 0.69%, 0.41%, 0.68%, and 0.52% at 20 cm and 0.51%, 0.30%, 0.33%, and 0.27% at 30 cm for CK, MA, NMA, and MNA, respectively. The resulting trend shows that soil phosphorus in CK, MA, and NMA increased from 10 to 20 cm depth and then decreased thereafter while MNA showed a continuous decrease with depth. According to the results, the observed variations in phosphorus content with depth were also observed at an increasing distance away from the bamboo trunk. At 5 cm from the bamboo trunk and 10 cm depth, the average value of total phosphorus was 0.64%, 0.28%, 0.62%, and 0.50%. The MA treatment induced the most significant reduction of 58.1% compared to CK (HD direction). At 25 cm and 30 cm depth, MA induced a 65.5% reduction in the average value of total phosphorus. This result shows the mulching treatments generally had a negative impact on soil phosphorus and together with aeration, the impact became more significant.
At 10 cm, soil total potassium did not exhibit any significant differences between the different treatments (
Figure S3). Nevertheless, at a soil depth of 20 cm, there was a significant difference between CK (PD direction) and NMA, with a total potassium increment of 27.6%. At a soil depth of 30 cm, similar results were obtained as the latter, with a total potassium increment of 21.1% in NMA. For this treatment, the total potassium was also increased by 38.1% and 18.9% at 5 and 15 cm in the PD aeration direction away from the bamboo trunk, respectively. Generally, there was less variation in the content of total potassium induced by the treatments relative to CK compared to those observed for nitrogen and phosphorus.
Figure S4 shows that there is a significant (R
2 < 0.999) polynomic relationship between the average values of SOM, pH, and nutrients (N, P, and K) at 10 cm. This relationship was observed even at 20 and 30 cm (result not shown) depths and at different distances from the bamboo trunk. Thus, it can be inferred that the variations (decrease or increase) in soil pH, SOM, and nutrients were a result of the different treatments.
3.6. Bamboo Rhizome Yield and Growth Parameters
Figure S1 and
Table 1 show that after one month of sprouting, there was a significant (
p < 0.05) difference in the number of fully grown bamboo shoots ready for harvest between CK and the other treatments. The average number of shoots per treatment followed the sequence MA (591.0) > MNA (554.3) > NMA (305.7) > CK (53.3). In addition, there were positive significant differences in the shoot height, diameter, and fresh weight. Bamboo shoots from plots MA, NMA, and MNA exhibited larger dimensions and quality than bamboo shoots from CK (
Figure 9). This result shows that organic mulching with/without aeration provided optimum conditions required for bamboo growth. Nevertheless, organic mulching with aeration should be adopted to achieve maximum economic benefits and preserve soil health. This observation agrees with the improvement magnitude of soil quality parameters induced by the different treatments.
4. Discussion
The roots of most plant species require a good supply of oxygen to satisfy their water and nutrient needs [
10,
17]. This is because roots need adequate oxygen for root respiration as well as for a healthy metabolic function. Thus, amelioration of an anoxic/hypoxic root zone to improve effective soil aeration is crucial for improving plant performance. Our experimental results show that treatments (CK and MNA) which did not receive aeration generally contained lower oxygen contents relative to those (MA and NMA) that were aerated (
Figure 2). In the absence of aeration, the application of organic mulching materials in MNA demonstrated significant adverse effects on soil oxygen content and this effect increased with soil depth. According to previous studies, the large pores in a well-structured soil are important avenues for gas exchange with the atmosphere and plants grown on soils that are poorly aerated suffer from stunted growth due to water and nutrient deficiencies [
11,
13,
18]. The application of organic mulching material probably inhibited gaseous exchange between the soil and the atmosphere by sealing the soil’s top surface and causing soil oxygen deficiency, which may become hazardous to plants in severe cases. For all the treatments, soil depth had an adverse effect on soil oxygen content, suggesting a slower diffusion of oxygen from the atmosphere to deeper soil layers as the sampling depth was increased from 10 to 30 cm.
Long-term (3–4 year) field studies have demonstrated that continuous organic mulching without adequate aeration could cause Lei bamboo rhizome up-floating, where the rhizomes floated to the surface of 0–10 cm soil layer to cause bamboo growth to recede [
8]. Plant roots cannot obtain enough energy to maintain health and normal physiological activity under hypoxic conditions. Thus, soil management practices that improve aeration are important for plant growth and ensuring food security. In their study, Li et al. [
14] suggested that applying artificial aeration to soils has the advantage of increasing the content of dissolved oxygen which overturns the negative effects of hypoxia stress on plants. As shown in
Figure 2, aeration-treated plots MA and NMA at distances 5, 15, and 25 cm from the bamboo trunk, showed elevated oxygen levels that were significantly higher than those of non-aerated plots (MNA and CK) and the ambient air oxygen concentration. Thus, combining aeration treatment with organic mulching can alleviate the negative impact of organic mulching on soil oxygen content.
The uptake of water and nutrients and consequently plant growth is highly affected by the root-zone temperature. It has been observed that the growth of
Cucumis sativus L. under different root-zone temperatures showed different response rates to nutrient uptake and consequently, the plant’s fresh and dry weights were different [
19]. Our results (
Figure 3) show that at soil depths of 10 to 30 cm, soil temperature was significantly higher in the mulched non-aerated plot (MNA) than in any other plot. This was probably due to the reduced gaseous exchange between the mulched soil and the atmosphere. Nevertheless, combining organic mulching and aeration (MA) induced a cooling effect that decreased the soil temperature by 14.2%, 19.1%, and 5.8% at depths of 10, 20, and 30 cm, respectively, compared to the non-aerated treatment (MNA). In their study, Liu et al. [
7] observed that organic mulching altered soil temperature by increasing microbial respiration rate. This means that organic mulching materials promote microbial life and population growth by providing the nutrients required for growth. Therefore, an increase in the microbial population implies an equal increase in heat production and enzymatic activities [
20] associated with microbial respiration. Other avenues for heat production during mulching could be linked to chemical processes such as decomposition and fermentation of the added organic materials.
Soil pH is an important soil parameter that regulates nutrient availability and other important functions of the soil. Continuous planting of Lei bamboo under intensive management practices for extended periods tends to enhance nutrient accumulation within the soil while significantly decreasing soil pH [
21]. This condition may be aggravated during high inputs of chemical fertilizer and the excretion of organic acids by bamboo roots [
22]. Under such practices, the content of soil exchangeable aluminum increases as the soil pH decreases, which becomes detrimental to plant growth due to inhibited root elongation [
16,
23]. In this study, soil pH (
Figure 4) was significantly lower for the mulched and non-aerated treatment (MNA), and the magnitude of pH decrease was increased with sampling depth. Combined with the high temperature of this treatment (
Figure 3), we infer that organic material decomposition in the MNA treatment produced organic molecules with a pH-decreasing effect compared to when mulching was applied simultaneously with aeration. This inference is supported by the significant relationship that exists between SOM and soil pH (
Figure S4). Therefore, to preserve Lei bamboo plantations and ensure economic sustainability, organic mulching combined with aeration is a good management practice to alleviate severe soil acidification of Lei bamboo soils.
The movement of water in unsaturated soils is an important concern for the rational use of water and soil resources to ensure food security without degrading environmental health. Soil hydraulic properties such as the retention and hydraulic capacities as well as gas conductivity have agronomical and ecological implications [
24]. Thus, an increase in soil bulk density induces changes to soil pore-size distribution that affect the ability of the soil to shrink and conduct water under unsaturated conditions. Simply put, the relative proportion of the three phases of soil (water, gas, and solid) is influenced by soil texture and structure, biological activity, weather, and management practices [
25]. Our results (
Figure 5) show that only CK in the HD direction and at a depth of 30 cm exhibited a significant reduction in SWC compared with samples from the other treatments at all different soil depths. Even though the differences in SWC were not significant, the MA and NMA treatments slightly increased the SWC compared to CK. These findings suggest that even though mulching treatments tend to increase soil temperature, they can do so without compromising the water needed for plant growth.
SOM is an important component in ecosystems and forms an essential part of a set of relevant processes that contribute to climate change [
26]. Beyond its role in climate change, SOM plays an important part in improving soil fertility and sustaining soil productivity [
27]. Our results (
Figure 6) show that applying mulching and aeration (MA) maintained a healthy pool of SOM even at a depth of 30 cm and a distance of 25 cm from the bamboo trunk, suggesting that top soils in the MA plot were richer in humus than in other plots. Even though the mulching and non-aerated (MNA) treatment showed a larger SOM relative to CK, its effect on SOM decreased with depth. These findings are consistent with other studies which earlier reported significant correlations between mulching treatment, organic carbon, and available nutrients [
7,
28], with the contents of organic carbon and nutrients being higher on the surface than in subsurface soils [
28].
During the decomposition of labile SOM, soil respiration and temperature increase [
7,
29]. As a result, nutrient elements that were bound to different functional moieties are released and made available to plants as well as leaching agents. Thus, as erosion forces set in, they may erode both the content of SOM [
30] and nutrients. Nitrogen is an important nutrient required for plant growth. The application of exogenous N to soils can alter soil N availability and/or induce soil acidification [
31,
32]. Soil organic N can be converted by microorganisms through ammonification, nitrification, and denitrification to forms (NH
4+-N and NO
3−-N) that can be easily absorbed by plants [
33,
34]. Our results show (
Figure 7) that at soil depth 10–20 cm, all the treatments demonstrated positive effects on the content of total N, with the effect being most significant in the PD direction relative to CK. Nevertheless, at 30 cm, MNA and NMA induced negative effects on N content in the HD direction relative to CK. Even when sampling was done away from the bamboo trunk, all the treatments demonstrated similar effects on total N. Contrary to total N, the content of phosphorus was negatively impacted by most of the treatments relative to CK (
Figure 8) while the content of potassium was increased in most instances (
Figure S3) even though not significantly in some. Compared to CK, all other treatments had reduced average phosphorus content except at 10 cm for MNA and 20 cm for NMA. For instance, the average phosphorus content in MA and MNA (HD direction) was reduced by 53.2% and 47.7% at 20 cm relative to CK, respectively, while at 30 cm, the average reduction rate reached 67% for MNA compared to CK. The overall negative effect of mulching and aeration (MA) was significant compared to the other treatments.
The overall effect of soil management practices is often quantified based on soil quality parameters and plant growth. When a soil management practice improves soil nutrient content but enhances soil acidification, it becomes detrimental to plant growth in the long term. In such instances, other soil amendments are required to lime the soil and increase soil pH, which increases the general cost of production. In this study, the application of organic mulching (MNA) decreased soil oxygen content and pH (
Figure 2 and
Figure 4) but maintained a favorable temperature and water environment (
Figure 3 and
Figure 5) compared to CK. When applied with aeration (MA), all these parameters, including the contents of SOM and nitrogen (
Figure 6 and
Figure 7) were significantly improved. This improvement in the determined parameters translated to a significant increase in the number of bamboo shoots and recorded growth parameters (
Figure 9,
Table 1 and
Figure S1). For example, the number of bamboo shoots increased by 968%, 452%, and 902% in MA, NMA, and MNA relative to CK (
Table 1 and
Figure S1). Despite the improvement of bamboo growth in NMA and MNA treatments, we recommend the use of mulching and aeration (MA) for long-term cultivation given that this combination has the potential to retard soil pH decrease better than the other treatments.