Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Soil Characteristics Used in the Experiment
2.3. AMF Soil Inoculation
2.4. Wheat Seed Sterilization and Germination
2.5. Water Stress Application
2.6. Measurements
2.6.1. Plant Growth
2.6.2. Chlorophyll Content Determination
2.6.3. Proline Estimation
2.6.4. Membrane Stability Index Measurement
2.6.5. Soluble Protein Concentrations Determination
2.6.6. Determination of Antioxidants Enzymes
2.6.7. AMF Root Colonization Measurement
2.7. Statiscal Analysis
3. Results
3.1. Effect of the Tested Treatments on the Morphological Parameters
3.2. Effect of the Tested Treatments on the Chlorophyll and Proline Contents
3.3. Effect of the Tested Treatments on the Soluble Protein Content and Membrane Stability Index
3.4. Effect of the AMF Inoculation on the Enzymatic Activity of Wheat
3.5. Wheat Root Colonization Rate by AMF According to the Tested Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferret, M. Le progrès technique au service de la qualité. Perspect. Agric. 1996, 218, 36–38. [Google Scholar]
- FAO. Crops and Livestock Products. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 February 2023).
- Zargar, M.; Rebouh, N.; Pakina, E.; Gadzhikurbanov, A.; Lyashko, M.; Ortskhanov, B. Impact of climate change on cereal production in the highlands of eastern Algeria. Res. Crops 2017, 18, 575–582. [Google Scholar] [CrossRef]
- Benbelkacem, A.; Kellou, K. Evaluation du progrès génétique chez quelques variétés de blé dur (Triticum turgidum L. var. durum) cultivées en Algérie. In Durum Wheat Improvement in the Mediterranean Region: New Challenges; Royo, C., Nachit, M., Di Fonzo, N., Araus, J.L., Eds.; Options Méditerranéennes: Série A. Séminaires Méditerranéens; CIHEAM: Zaragoza, Spain, 2000; Volume 40, pp. 105–110. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Ayed, S.; Othmani, A.; Bouhaouel, I.; Teixeira da Silva, J.A. Multi-Environment Screening of Durum Wheat Genotypes for Drought Tolerance in Changing Climatic Events. Agronomy 2021, 11, 875. [Google Scholar] [CrossRef]
- Al-Selhab, O.A.; El-Tabbakh, S.S.; Nawar, A.I.; EL-Genbeehy, M.M.; Shaalan, A.M. Tolerance of Durum wheat cultivars to postanthesis drought conditions as affected by seed priming, bio-Fertilizer and potassium application. Alex. J. Agric. Sci. 2016, 61, 51–59. [Google Scholar]
- Aberkane, H.; Amri, A.; Belkadi, B.; Filali-Maltouf, A.; Kehel, Z.; Tahir, I.S.A.; Meheesi, S.; Tsivelikas, A. Evaluation of durum wheat lines derived from interspecific crosses under drought and heat stress. Crop Sci. 2020, 61, 119–136. [Google Scholar] [CrossRef]
- Temirbekova, S.K.; Kulikov, I.M.; Afanasyeva, Y.V.; Beloshapkina, O.O.; Kalashnikova, E.A.; Kirakosyan, R.N.; Dokukin, P.A.; Kucher, D.E.; Latati, M.; Rebouh, N.Y. The Evaluation of Winter Wheat Adaptation to Climate Change in the Central Non-Black Region of Russia: Study of the Gene Pool Resistance of Wheat from the N.I. Vavilov Institute of Plant Industry (VIR) World Collection to Abiotic Stress Factors. Plants 2021, 10, 2337. [Google Scholar] [CrossRef]
- Kherif, O.; Keskes, M.I.; Pansu, M.; Ouaret, W.; Rebouh, Y.N.; Dokukin, P.; Kucher, D.; Latati, M. Agroecological modeling of nitrogen and carbon transfers between decomposer micro-organisms, plant symbionts, soil and atmosphere in an intercropping system. Ecol. Model. 2021, 440, 109–390. [Google Scholar] [CrossRef]
- Latati, M.; Rebouh, N.Y.; Aouiche, A.; Laouar, M. Modeling the functional role of the microorganisms in the daily exchanges of carbon and nitrogen in intercropping system under mediterranean conditions. Agron. Res. 2019, 17, 559–573. [Google Scholar]
- Sheteiwy, M.S.; Abd Elgawad, H.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Alhaj Hamoud, Y.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.S.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; El-Tahan, A.M.; El-Tarabily, K.; Desoky, E.M.; Rady, M.M. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobiumunder drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Simon, L.; Bousquet, J.; Levesque, C.; Lalonde, M. Origin and diversification of endomycorrizal fungi and coincidence with vascular land plants. Nature 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, 99–127. [Google Scholar] [CrossRef]
- Morse, L.J.; Day, T.A.; Faeth, S.H. Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of Arizona fescue under contrasting water availability regimes. Environ. Exp. Bot. 2002, 48, 257–268. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; El-Sawah, A.M.; Korany, S.M.; Alsherif, E.A.; Mowafy, A.M.; Chen, J.; Jośko, I.; Selim, S.; AbdElgawad, H. Arbuscular Mycorrhizal Fungus “Rhizophagus irregularis” impacts on physiological and biochemical responses of ryegrass and chickpea plants under beryllium stress. Environ. Pollut. 2022, 315, 120356. [Google Scholar] [CrossRef] [PubMed]
- El-Sawah, A.; Abdel-Fattah, G.; Holford, P.; Korany, S.; Alsherif, E.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Basharat, A.; Sheteiwy, M. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef]
- Labidi, S.; Ben Jeddi, F.; Tisserant, B.; Debiane, D.; Rezgui, S.; Grandmougin-Ferjani, A.; Lounès-Hadj Sahraoui, A. Role of arbuscular mycorrhizal symbiosis in root mineral uptake under CaCO3 stress. Mycorrhiza 2012, 22, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Khennaoui, A.; Djekoun, A.; Benbelkacem, A.; Ykhlef, N. Agromorphological characterization and study of grain yield of Algerian durum wheat (Triticum durum Desf.). Int. J. Adv. Res. 2016, 4, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudersa, N.; Ghania, C.; Aicha, A.; Cherfia, R. Assessment of biological and agronomic diversity ofseven durum wheat varieties cultivated in the North easte rnregion of Algeria. Biodivers. J. Biol. Divers. 2021, 22, 220–259. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Zargar, M.; Pakina, E.; Aabdelaziz, N.; Astrakhanova, T.; Chuburkova, S. Analysis of various cultivars of durumwheat cultivated in the highlands of Algeria condition. Res. Crops 2018, 19, 200–204. [Google Scholar] [CrossRef]
- Hurley, M.A.; Roscoe, M.E. Automated statistical analysis of microbial enumeration by dilution series. J. Bacteriol. 1983, 55, 159–164. [Google Scholar] [CrossRef]
- Tarraf, W.; Ruta, C.; Tagarelli, A.; De Cillis, F.; De Mastro, G. Influence of arbuscular mycorrhizae on plant growth, essential oil production and phosphorus uptake of Salvia officinalis L. Ind. Crops Prod. 2017, 102, 144–153. [Google Scholar] [CrossRef]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual of Seed Handling in Genebanks; Handbooks for Genebanks No. 8; Bioversity International: Rome, Italy, 2006. [Google Scholar]
- Torrecillas, A.; León, A.; Del Amor, F.; Martinez-Mompeán, M.C. Determinación rápida de clorofila en discos foliares delimonero. Fruits 1984, 39, 617–622. [Google Scholar]
- Carillo, P.; Gibon, Y. Protocol: Extraction and determination of proline. J. Biol. Chem. 2011, 215, 655–660. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgramquantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Popov, T.; Neykovskaya, L. Method for determining blood peroxidase activity. Hyg. Sanit. 1971, 10, 89–91. (In Russian) [Google Scholar]
- Phillips, M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Gui, Y.W.; Sheteiwy, M.S.; Zhu, S.G.; Batool, A.; Xiong, Y.C. Differentiate effects of non-hydraulic and hydraulic root signaling on yield and water use efficiency in diploid and tetraploid wheat under drought stress. Environ. Exp. Bot. 2021, 181, 104287. [Google Scholar] [CrossRef]
- Giunta, F.; Motzo, R.; Deidda, M. Effect of drought on yield and yield components of durum wheat and triticale in a Mediterranean environment. Field Crops Res. 1993, 4, 399–409. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Cao, W.; Wu, L.; Geng, M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.S.; Saleem, M.F.; Anjum, S.A.; Khaliq, T.; Wahid, M.A. Foliar application of potassium under water deficit conditions improved the growth and yield of wheat (Triticum aestivum L.). J. Anim. Plant Sci. 2012, 22, 431–437. [Google Scholar]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.; Alhejeli, A. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.S.; Xu, J.; Guo, Y.; Koide, R.T.; Dai, Y.; Xu, M.; Bian, L.; Bian, X.; Zhang, Q. Predicting plant response to arbuscular mycorrhizas: The role of host functional traits. Fungal Ecol. 2016, 20, 79–83. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chan, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Armada, E.; Azcon, R.; Lopez-Castillo, O.M.; Calvo-Polanco, M.; Ruiz-Lozano, J.M. Autochthonous arbuscular mycorrhizal fungi and Bacillus thuringiensis from a degraded Mediterranean area can be used to improve physiological traits and performance of a plant of agronomic interest under drought conditions. Plant Physiol. Biochem. 2015, 90, 64–74. [Google Scholar] [CrossRef]
- Xiao, D.; Che, R.; Liu, X.; Tan, Y.; Yang, R.; Zhang, W.; He, X.; Xu, Z.; Wang, K. Arbuscular mycorrhizal fungi abundance was sensitive to nitrogen addition but diversity was sensitive to phosphorus addition in karst ecosystems. Biol. Fertil. Soils 2019, 55, 457–469. [Google Scholar] [CrossRef]
- Rani, B.; Madan, S.; Sharma, K.D.; Pooja, P.; Kumar, A. Influence ofarbuscular mycorrhiza on antioxidative system of wheat (Triticum aestivum) under drought stress. Ind. J. Agric. Sci. 2018, 88, 289–295. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The relationship between chlorophyll content and rate of photosynthesis in soybeans. Can. J. Plant Sci. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Mafakheri, A.B.; Siosemardeh, P.C.; Bahramnejad, Y.; Struik, T.; Sohrabi, S. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Bhosale, K.S.; Shinde, B.P. Influence of arbuscular mycorrhizal fungi on proline and chlorophyll content in Zingiber officinale Rosc grown under water stress. Indian J. Fundam. Appl. Life Sci. 2011, 1, 172–176. [Google Scholar]
- Sebbane, M.; Hafsi, M. Effect of arbuscular mycorrhizal fungi on drought tolerance in durum wheat. Int. J. Biosci. 2020, 17, 99–112. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016. [Google Scholar]
- Cechin, I.; Rossi, C.; Oliveira, C.; Fumis, F. Photosynthetic responses and proline content of mature and young leaves of sunflower plants under water deficit. Photosynthetica 2006, 44, 143–146. [Google Scholar] [CrossRef]
- Handa, S.; Handa, A.K.; Hasegawa, P.M.; Bressan, R.A. Proline Accumulation and the Adaptation of Cultured Plant Cells to Water Stress. Plant Physiol. 1986, 80, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, Y. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. Eur. J. Agron. 2021, 122, 126196. [Google Scholar] [CrossRef]
- Irani, S.; Majidi, M.M.; Mirlohi, A.; Zargar, M.; Karami, M. Assessment of Drought Tolerance in Sainfoin: Physiological and Drought Tolerance Indices. Agron. J. 2015, 107, 1771–1781. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sanjeeta, K.; Parkash, J.; Kalita, P.; Devi, M.; Pathania, J.; Joshi, R.; Dutt, S. Comparative proteome analysis of Picrorhiza kurrooa Royle ex Benth. in response to drought. J. Proteome Sci. Comput. Biol. 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.H.U.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Shah, S.; Tian, H. The Roles of Arbuscular Mycorrhizal Fungi in Influencing Plant Nutrients, Photosynthesis, and Metabolites of Cereal Crops—A Review. Agronomy 2022, 12, 2191. [Google Scholar] [CrossRef]
- Caravaca, F.; Alguacil, M.M.; Hernández, J.A.; Roldán, A. Involvement of antioxidant enzyme and nitrate reductase activities during water stress and recovery of mycorrhizal Myrtuscommunis and Phillyrea angustifolia plants. Plant Sci. 2005, 169, 191–197. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Wu, Q.-S.; Huang, Y.-M.; Ni, Q.-D.; He, X.-H. Mycorrhizal-Mediated Lower Proline Accumulation in Poncirus trifoliata under Water Deficit Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PLoS ONE 2013, 8, e80568. [Google Scholar] [CrossRef] [Green Version]
- Ohtomo, R.; Kobae, Y.; Morimoto, S.; Oka, N. Infection unit density as an index of infection potential of arbuscularmycorrhizal fungi. Microbes Environ. 2018, 33, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Ahanger, M.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Kong, J.; Pei, Z.; Du, M.; Sun, G.; Zhang, X. Effects of arbuscular mycorrhizal fungi on the drought resistance of the mining area repair plant Sainfoin. Int. J. Min. Sci. Technol. 2014, 24, 485–489. [Google Scholar] [CrossRef]
- Nasim, G. The role of Arbuscular mycorrhizae in inducing resistance to drought and salinity stress in crops. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M.S.A., Eds.; Springer: New York, NY, USA, 2010; pp. 119–141. [Google Scholar]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Jeddi, F.B.; Sahraoui, A.L.H. Mycorrhizal biofertilization improves grain yield and quality of hulless Barley (Hordeum vulgare ssp. nudum L.) under water stress conditions. J. Cereal Sci. 2022, 104, 103436. [Google Scholar]
- Marulanda, A.; Azcon, R.; Ruiz-Lozano, J.M. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol. Plantarum 2003, 19, 526–533. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Latati, M.; Polityko, P.; Kucher, D.; Hezla, L.; Norezzine, A.; Iguer-Ouada, M. Influence of three cultivation technologies to control Fusarium spp. in winter wheat (Triticum aestivum L.) production under Moscow conditions. Res. Crops 2020, 21, 17–25. [Google Scholar]
- Temirbekova, S.K.; Kulikov, I.M.; Afanasyeva, Y.V.; Ashirbekov, M.Z.; Beloshapkina, O.O.; Kalashnikova, E.A.; Sardarova, I.; Begeulov, M.S.; Kucher, D.E.; Ionova, N.E.; et al. The Biological Traumatization of Crops Due to the Enzyme Stage of Enzyme-Mycotic Seed Depletion. Pathogens 2022, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Temirbekova, S.K.; Kulikov, I.M.; Ashirbekov, M.Z.; Afanasyeva, Y.V.; Beloshapkina, O.O.; Tyryshkin, L.G.; Zuev, E.V.; Kirakosyan, R.N.; Glinushkin, A.P.; Potapova, E.S.; et al. Evaluation of Wheat Resistance to Snow Mold Caused by Microdochium nivale (Fr) Samuels and I.C. Hallett under Abiotic Stress Influence in the Central Non-Black Earth Region of Russia. Plants 2022, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- David, M. Osmotic adjustment capacity and cuticular transpiration in several wheat cultivars cultivated in Algeria. Rom. Agric. Res. 2009, 26, 29–33. [Google Scholar]
- Merouche, A.; Debaeke, P.; Messahel, M.; Kelkouli, M. Response of durum wheat varieties to water in semi-arid Algeria. Afr. J. Agric. Res. 2014, 9, 2879–2892. [Google Scholar]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]

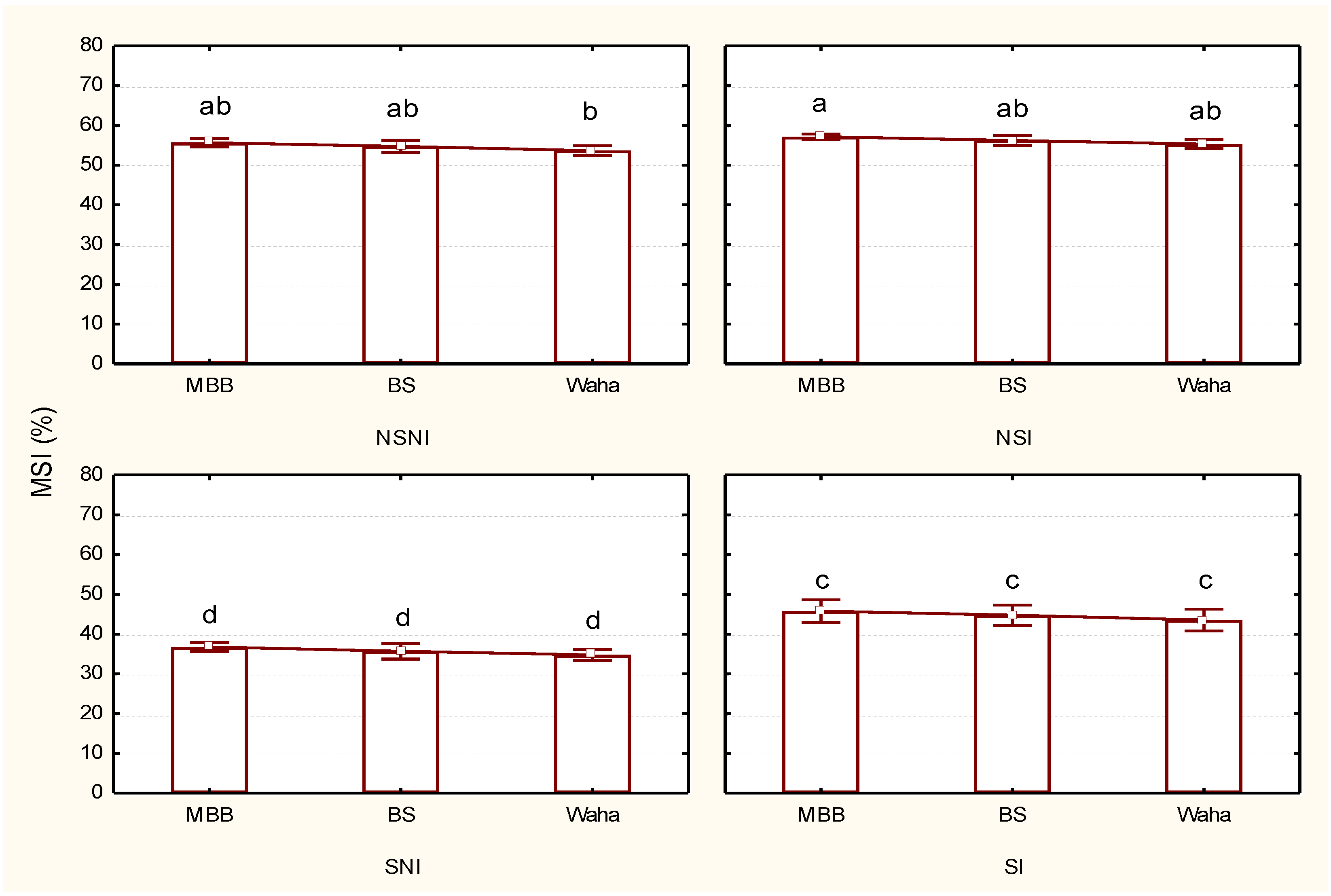

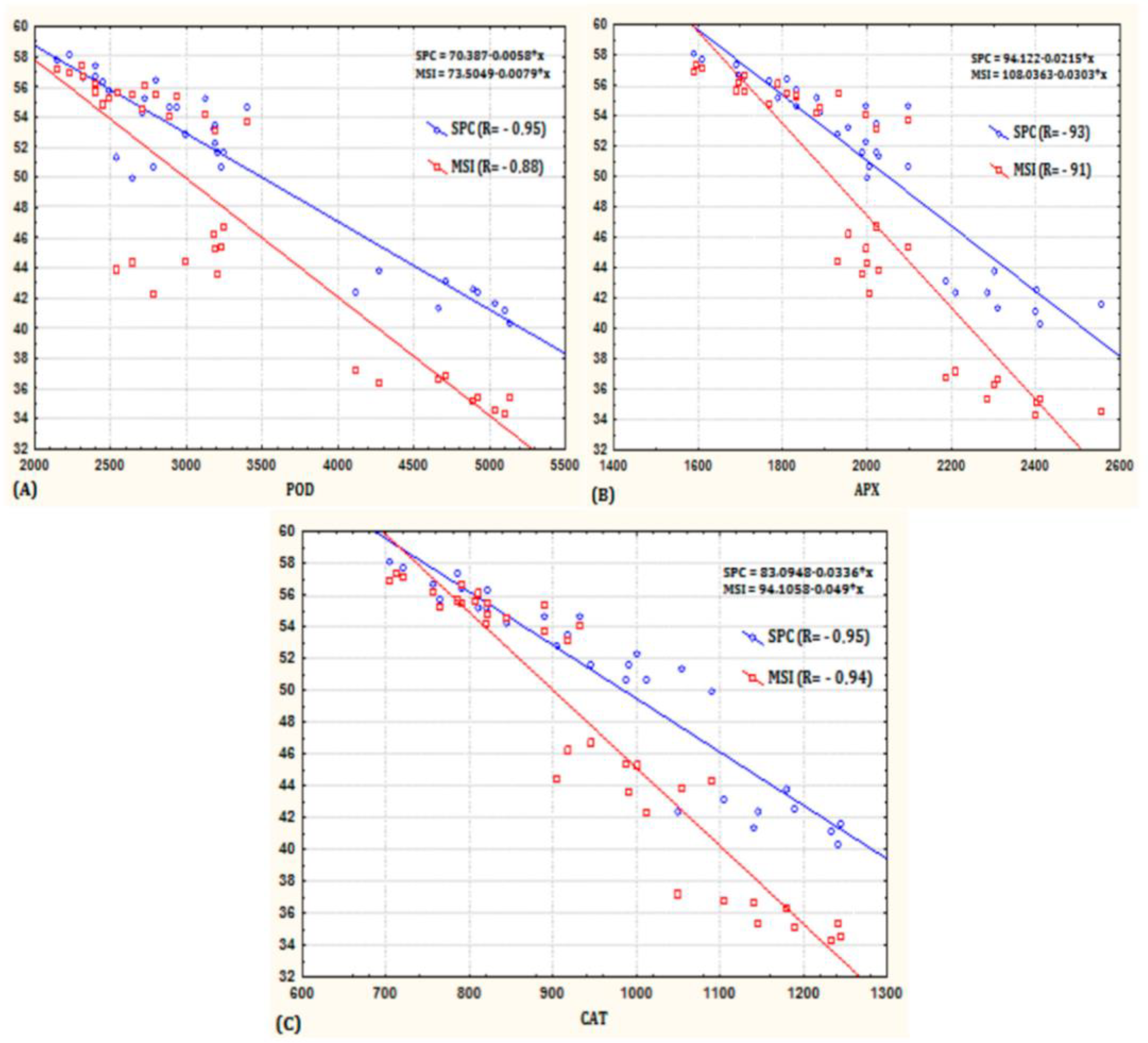
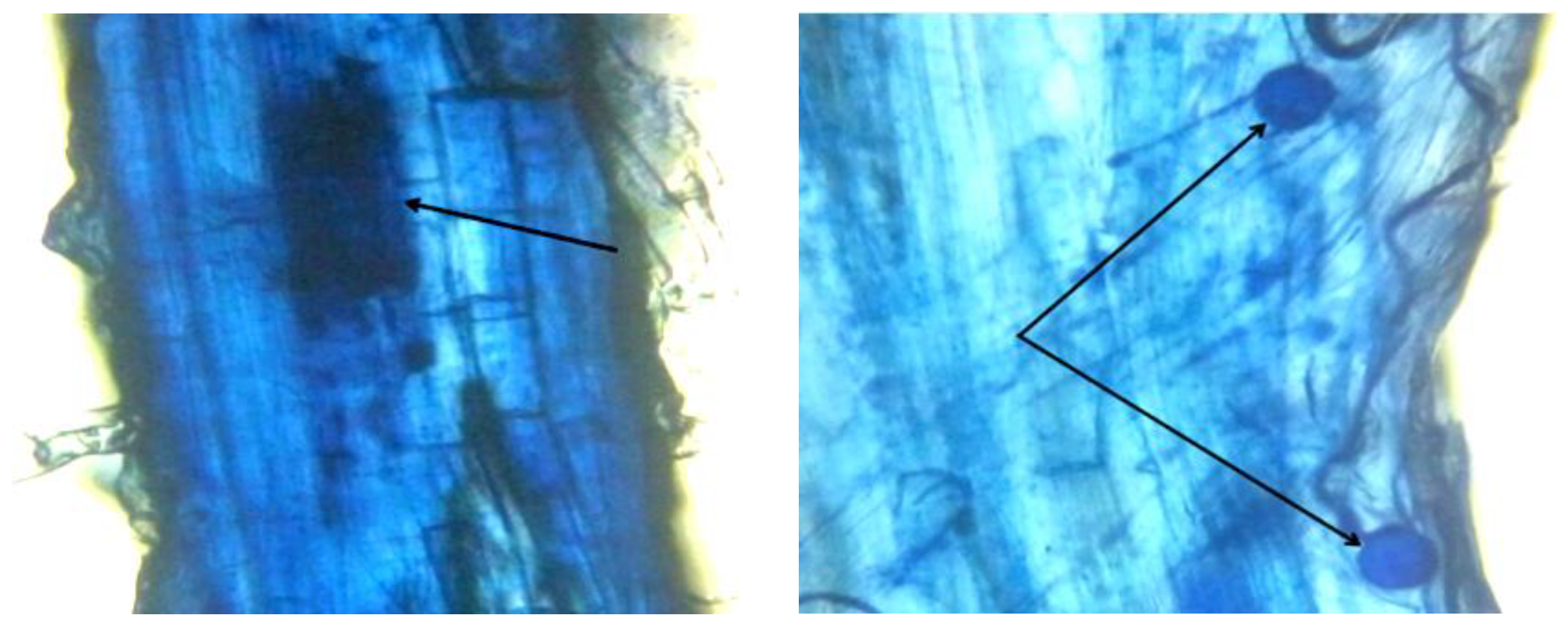
| Varieties | Treatments | HAP (Cm) | LIN (Cm) | APDW (g) | RDW (g) | LE (Cm) |
|---|---|---|---|---|---|---|
| MBB | NSNI | 37.3 ± 2.31 a | 19.6 ± 3.21 a | 1.38 ± 0.06 b | 0.78 ± 0.28 ab | 12.8 ± 0.15 ab |
| NSI | 39.6 ± 5.86 a | 23.3 ± 2.08 a | 1.63 ± 0.34 ab | 0.93 ± 0.21 a | 13.1 ± 0.76 a | |
| SNI | 19.6 ± 2.08 b | 10.8 ± 0.76 c | 1.04 ± 0.15 c | 0.71 ± 0.16 ab | 12.2 ± 1.21 b | |
| SI | 26.3 ± 2.52 b | 13.6 ± 2.08 b | 1.09 ± 0.30 c | 0.75 ± 0.27 ab | 12.7 ± 0.66 ab | |
| BS | NSNI | 33.6 ± 2.08 a | 15 ± 1 a | 1.71 ± 0.17 a | 0.63 ± 0.09 b | 12.6 ± 0.58 ab |
| NSI | 35 ± 1 a | 15.3 ± 2.08 a | 1.76 ± 0.33 a | 0.70 ± 0.16 ab | 13.1 ± 0.76 a | |
| SNI | 25.6 ± 3 b | 12 ± 1 c | 0.97 ± 0.33 c | 0.56 ± 0.08 b | 12 ± 1.45 b | |
| SI | 30.2 ± 2.08 b | 12.6 ± 2.65 b | 1.48 ± 0.15 ab | 0.64 ± 0.04 b | 12.2 ± 0.58 b | |
| W | NSNI | 34.2 ± 3.51 a | 14.6 ± 1.15 a | 1.43 ± 0.09 b | 0.53 ± 0.13 b | 12.3 ± 0.3 ab |
| NSI | 35.7 ± 5 a | 15.2 ± 0.58 a | 1.56 ± 0.38 ab | 0.58 ± 0.15 b | 12.6 ± 0.58 ab | |
| SNI | 30.6 ± 3.61 b | 12.4 ± 0.58 c | 1.21 ± 0.10 bc | 0.46 ± 0.14 c | 11.4 ± 02 c | |
| SI | 32.1 ± 2.52 b | 13.7 ± 2 b | 1.24 ± 0.11 bc | 0.49 ± 0.06 c | 11.9 ± 54 b | |
| p-Value | Variety | 0.5 | ≤0.001 | 0.1 | ≤0.001 | 0.2 |
| Treatment | ≤0.005 | ≤0.001 | ≤0.005 | 0.2 | ≤0.005 | |
| Var × Treat | ≤0.001 | ≤0.001 | 0.6 | 0.2 | ≤0.005 |
| Varieties | Treatments | Chl (mg/g MF) | Prol (μM/g MF) |
|---|---|---|---|
| MBB | NSNI | 44.65 ± 6.09 ab | 2.22 ± 0.43 b |
| NSI | 46.08 ± 3.68 a | 1.67 ± 0.74 b | |
| SNI | 42.68 ± 7.37 b | 6.28 ± 1.62 a | |
| SI | 43.34 ± 6.42 ab | 3.87 ± 0.61 b | |
| BS | NSNI | 43.22 ± 1.46 ab | 2.90 ± 0.59 b |
| NSI | 44.65 ± 0.44 ab | 1.33 ± 0.30 b | |
| SNI | 40.12 ± 2.13 c | 9.02 ± 4.80 a | |
| SI | 42.94 ± 1.21 b | 3.50 ± 2.02 b | |
| W | NSNI | 43.67 ± 3.51 ab | 2.67 ± 1.15 b |
| NSI | 44.03 ± 0.65 ab | 1.67 ± 0.58 b | |
| SNI | 41.44 ± 1.61 bc | 8.33 ± 0.58 a | |
| SI | 42.67 ± 1.52 b | 4.97 ± 0.52 b | |
| p-Value | Variety | 0.5 | 0.1 |
| Treatment | ≤0.005 | ≤0.005 | |
| Variety × Treatment | 0.2 | 0.3 |
| Varieties | Treatments | TCR % | AR % | VR % | HGR % |
|---|---|---|---|---|---|
| MBB | NSNI | 17.47 ± 3.28 d | 0.6 ± 0.72 e | 6.92 ± 2.42 d | 11.56 ± 0.86 cd |
| NSI | 46.56 ± 2.98 bc | 3.92 ± 2.79 c | 14.23 ± 4.97 b | 30.04 ± 3.51 b | |
| SNI | 15.04 ± 3.69 d | 0.25 ± 1.18 e | 4.33 ± 0.58 d | 9.72 ± 3.82 d | |
| SI | 54.73 ± 11.08 b | 3.70 ± 1.04 c | 16.44 ± 4.30 a | 36.86 ± 14.47 a | |
| BS | NSNI | 19.17 ± 4.01 d | 0.46 ± 1.31 e | 8.75 ± 3.46 cd | 13.66 ± 0.74 cd |
| NSI | 35.69 ± 10.54 c | 5.15 ± 4.62 b | 14.70 ± 7.01 b | 18.35 ± 8.21 c | |
| SNI | 16.43 ± 5.80 d | 0.8 ± 2.45 e | 3.32 ± 2.32 de | 10.10 ± 3.50 cd | |
| SI | 60.98 ± 9.02 a | 9.42 ± 6.71 a | 15.39 ± 8.06 ab | 37.96 ± 5.64 a | |
| W | NSNI | 14.90 ± 6.03 d | 5.60 ± 2.86 b | 5.60 ± 2.86 d | 9.31 ± 3.52 d |
| NSI | 25.94 ± 0.46 c | 2.55 ± 1.18 cd | 10.73 ± 1.94 c | 12.66 ± 2.14 cd | |
| SNI | 12.18 ± 7.26 e | 2.55 ± 6.32 cd | 2.81 ± 5.24 e | 6.67 ± 3.38 e | |
| SI | 50.30 ± 17.46 b | 9.01 ± 8.26 a | 14.47 ± 10.84 b | 29.55 ± 14.10 b | |
| p-Value | Variety | ≤0.005 | 0.2 | 0.1 | ≤0.005 |
| Treatment | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | |
| Variety × Treatment | 0.2 | 0.3 | 0.4 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ould Amer, S.; Aliat, T.; Kucher, D.E.; Bensaci, O.A.; Rebouh, N.Y. Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.). Agriculture 2023, 13, 552. https://doi.org/10.3390/agriculture13030552
Ould Amer S, Aliat T, Kucher DE, Bensaci OA, Rebouh NY. Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.). Agriculture. 2023; 13(3):552. https://doi.org/10.3390/agriculture13030552
Chicago/Turabian StyleOuld Amer, Samira, Toufik Aliat, Dmitry E. Kucher, Oussama A. Bensaci, and Nazih Y. Rebouh. 2023. "Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.)" Agriculture 13, no. 3: 552. https://doi.org/10.3390/agriculture13030552
APA StyleOuld Amer, S., Aliat, T., Kucher, D. E., Bensaci, O. A., & Rebouh, N. Y. (2023). Investigating the Potential of Arbuscular Mycorrhizal Fungi in Mitigating Water Deficit Effects on Durum Wheat (Triticum durum Desf.). Agriculture, 13(3), 552. https://doi.org/10.3390/agriculture13030552








