Insight into Hormonal Homeostasis and the Accumulation of Selected Heat Shock Proteins in Cold Acclimated and Deacclimated Winter Oilseed Rape (Brassica napus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Sampling
2.3. Measurements
2.3.1. Analysis of the Plant Hormones and Related Metabolites
2.3.2. HSP Analysis
2.4. Statistical Analyses
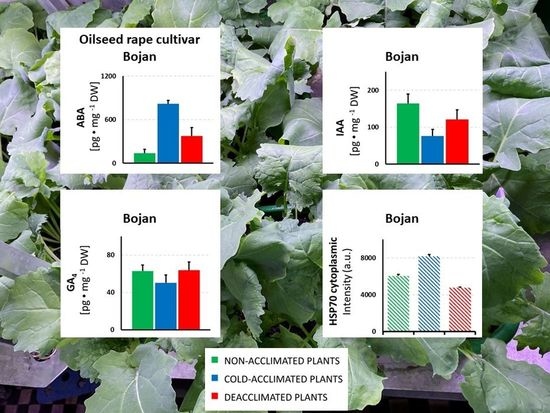
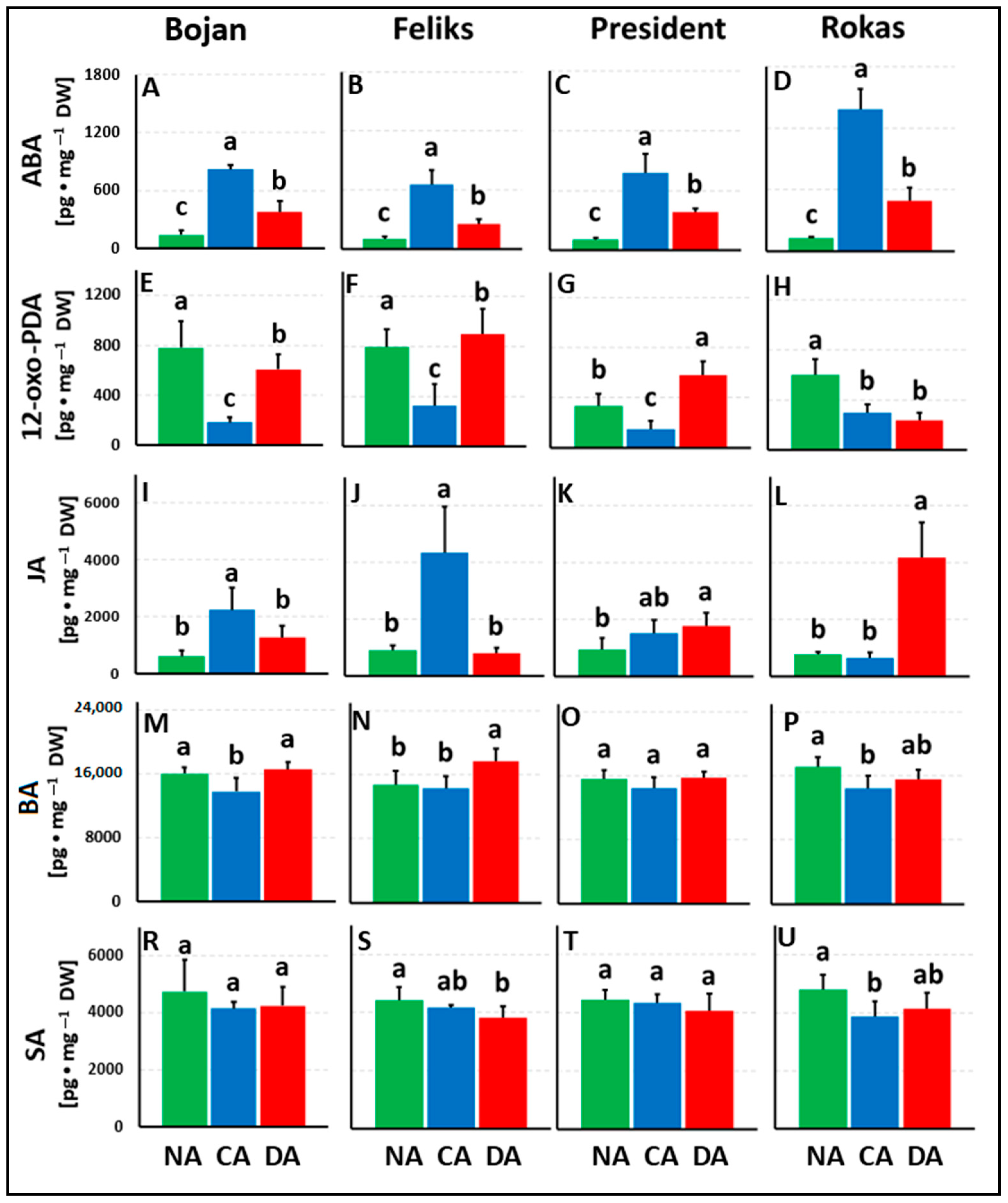
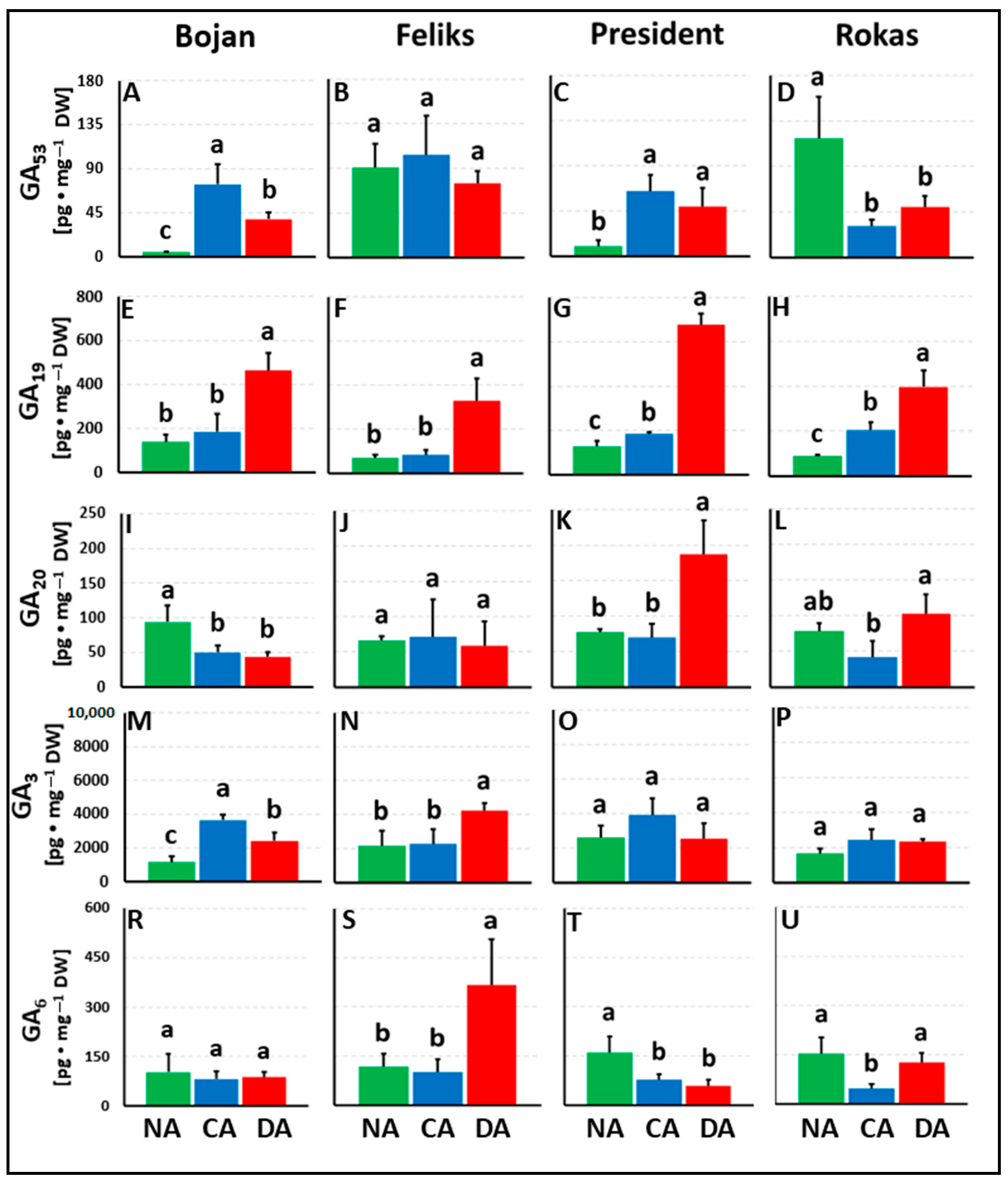
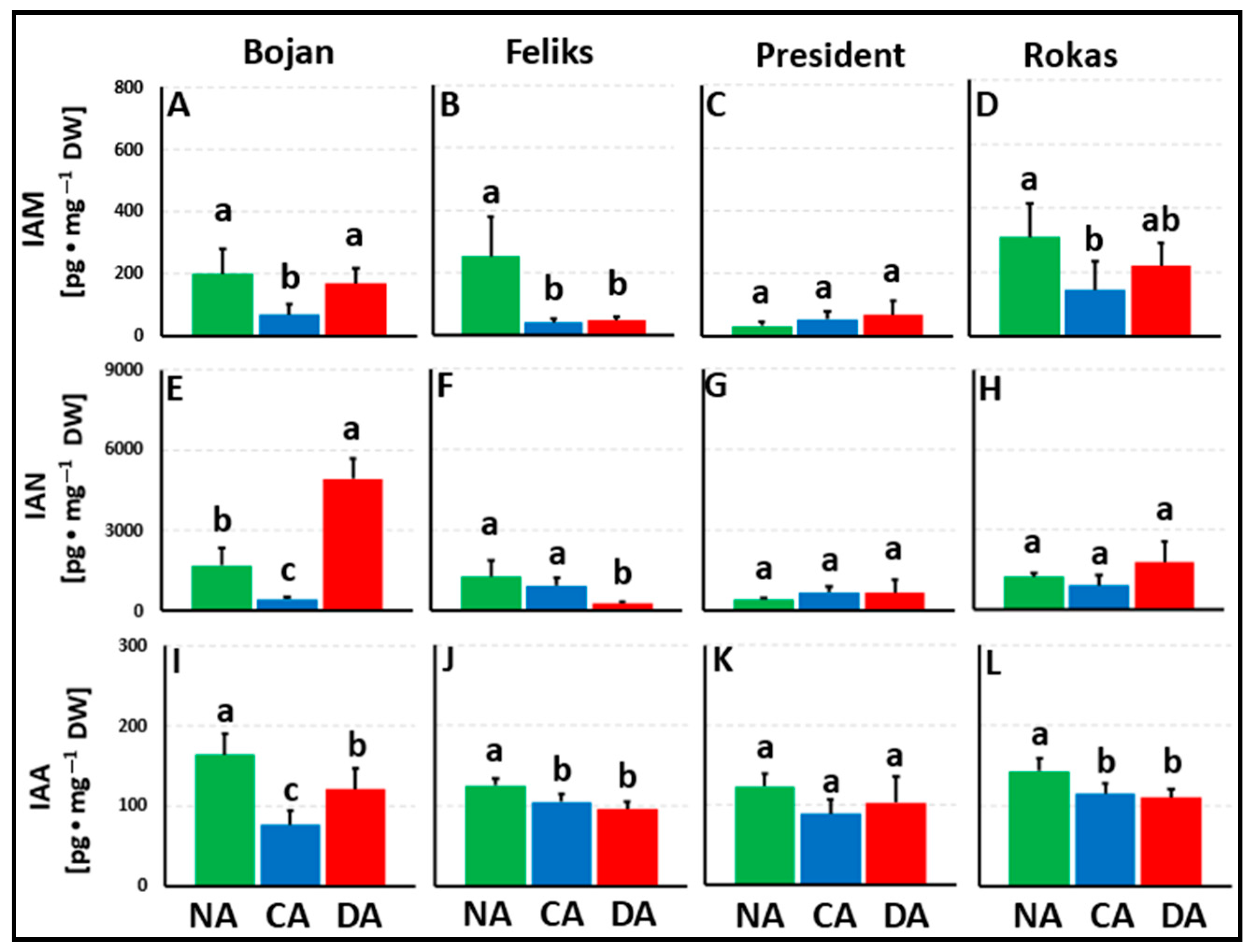
3. Results
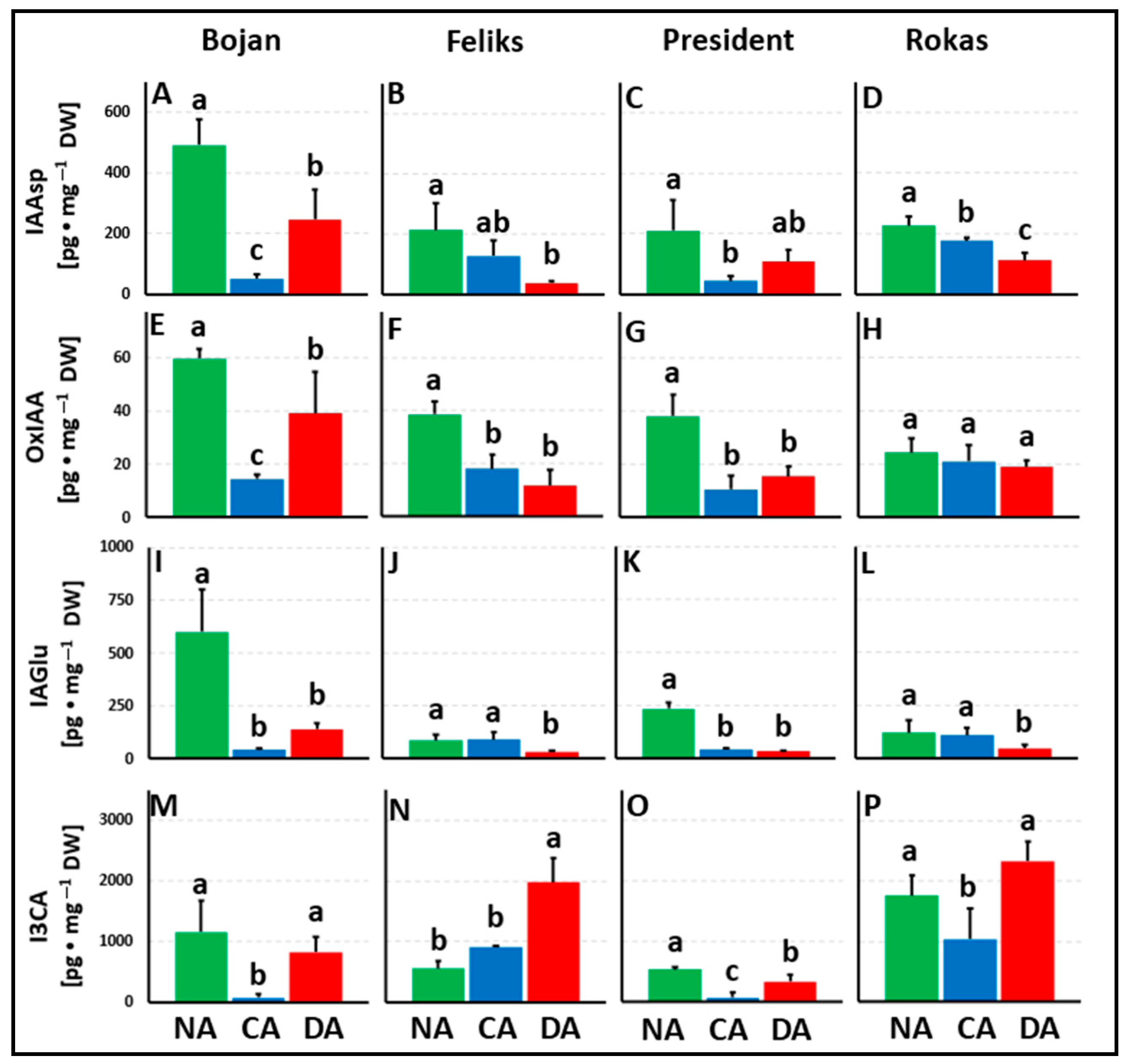
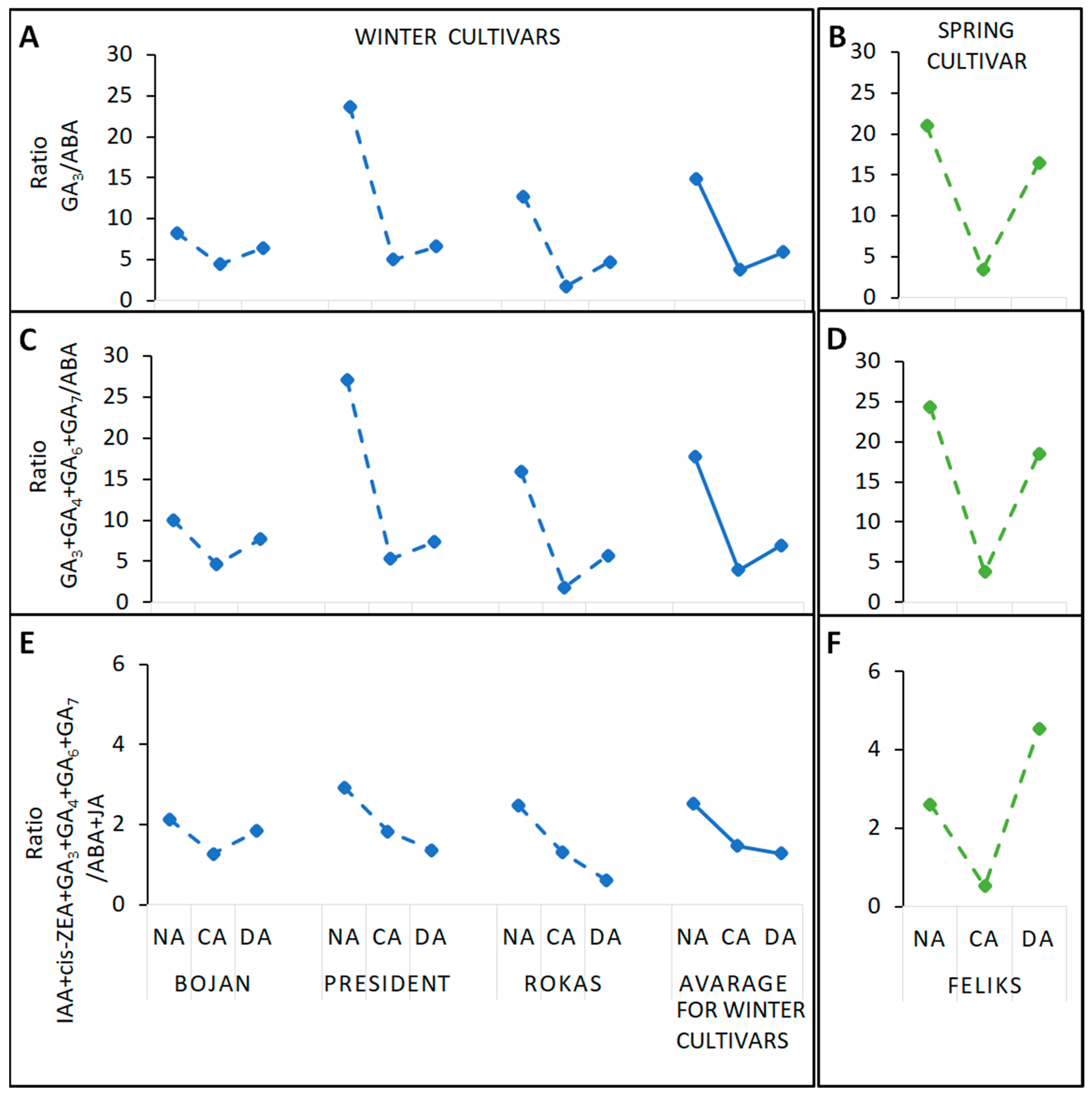
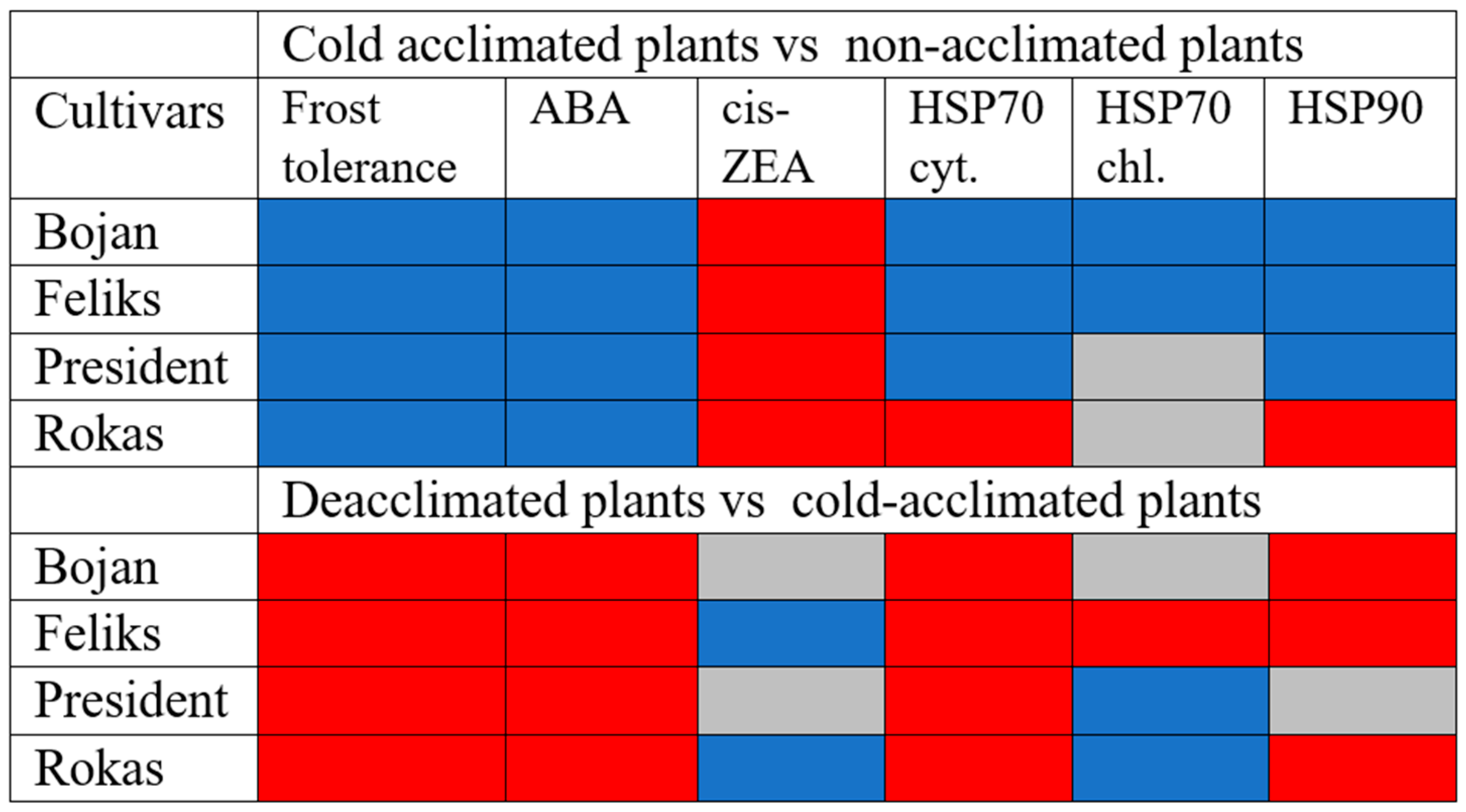
3.1. Hormonal Analyses
3.2. HSP Analyses
4. Discussion
4.1. The Impact of Deacclimation on Plant Hormone Management
4.2. The Impact of Deacclimation on Heat Shock Protein Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomashow, M.F. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998, 118, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rapacz, M.; Jurczyk, B.; Sasal, M. Deacclimation may be crucial for winter survival of cereals under warming climate. Plant Sci. 2017, 256, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, J.; Rys, M.; Pociecha, E.; Kalaji, H.M.; Dąbrowski, P.; Oklestkova, J.; Jurczyk, B.; Janeczko, A. Deacclimation-Induced Changes of Photosynthetic Efficiency, Brassinosteroid Homeostasis and BRI1 Expression in Winter Oilseed Rape (Brassica napus L.)—Relation to Frost Tolerance. Int. J. Mol. Sci. 2022, 23, 5224. [Google Scholar] [CrossRef] [PubMed]
- Vyse, K.; Pagter, M.; Zuther, E.; Hincha, D.K. Deacclimation after cold acclimation—A crucial, but widely neglected part of plant winter survival. J. Exp. Bot. 2019, 70, 4595–4604. [Google Scholar] [CrossRef] [Green Version]
- Popov, V.N.; Antipina, O.V.; Pchelkin, V.P.; Tsydendambaev, V.D. Changes in fatty acid composition of lipids in chloroplast membranes of tobacco plants during cold hardening. Russ. J. Plant Physiol. 2017, 64, 156–161. [Google Scholar] [CrossRef]
- Miki, Y.; Takahashi, D.; Kawamura, Y.; Uemura, M. Temporal proteomics of Arabidopsis plasma membrane during cold- and de-acclimation. J. Proteom. 2019, 197, 71–81. [Google Scholar] [CrossRef]
- Rys, M.; Pociecha, E.; Oliwa, J.; Ostrowska, A.; Jurczyk, B.; Saja, D.; Janeczko, A. Deacclimation of winter oilseed rape-insight into physiological changes. Agronomy 2020, 10, 1565. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Tognetti, V.B.; van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; de Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [Green Version]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, M. The effects of ABA and GA3 treatments on resistance to frost and high-light treatment in oilseed rape leaf discs. Acta Physiol. Plant. 2002, 24, 447–457. [Google Scholar] [CrossRef]
- Gavelienė, V.; Novickienė, L.; Kazlauskienė, D. Effect of auxin physiological analogues on rape growth and reproductive development. Bot. Lith. 2007, 13, 101–107. [Google Scholar]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate regulates the INDUCER OF CBF expression-C-repeat binding factor/dre binding factor1 Cascade and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [Green Version]
- Pagter, M.; Alpers, J.; Erban, A.; Kopka, J.; Zuther, E.; Hincha, D.K. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimated Arabidopsis thaliana. BMC Genom. 2017, 18, 731. [Google Scholar] [CrossRef] [Green Version]
- Pociecha, E.; Janeczko, A.; Dziurka, M.; Gruszka, D. Disturbances in the Biosynthesis or Signalling of Brassinosteroids That Are Caused by Mutations in the HvDWARF, HvCPD and HvBRI1 Genes Increase the Tolerance of Barley to the Deacclimation Process. J. Plant Growth Regul. 2020, 39, 1625–1637. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, L.J.; Pan, Q.H.; Wang, Y.Z.; Zhan, J.C.; Huang, W.D. Accumulation and subcellular localization of heat shock proteins in young grape leaves during cross-adaptation to temperature stresses. Sci. Hortic. 2008, 117, 231–240. [Google Scholar] [CrossRef]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. HSP transcript and protein accumulation in brassinosteroid barley mutants acclimated to low and high temperatures. Int. J. Mol. Sci. 2020, 21, 1889. [Google Scholar] [CrossRef] [Green Version]
- Schöffl, F.; Prandl, R.; Reindl, A. Update on Signal Transduction Regulation of the Heat-Shock Response. Plant Physiol. 1998, 117, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol. Life Sci. 2002, 59, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; DeRocher, A.E.; Keegstra, K.; Vierling, E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc. Natl. Acad. Sci. USA 1990, 87, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroda, M.; Vallon, O.; Wollman, F.A.; Beck, C.F. A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 1999, 11, 1165–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.H.; Li, H.M. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 2008, 146, 1231–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, P.; Sacco, M.; Cherutti, J.F.; Hill, S. Cold-induced accumulation of hsp90 transcripts in Brassica napus. Plant Physiol. 1995, 107, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestková, J.; Novák, O.; Saja, D.; Skoczowski, A.; Tóbiás, I.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef]
- Płażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed hydropriming and smoke water significantly improve low-temperature germination of Lupinus angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. [Google Scholar] [CrossRef] [Green Version]
- Cioć, M.; Dziurka, M.; Pawłowska, B. Changes in Endogenous Phytohormones of Gerbera jamesonii Axillary Shoots Multiplied under Different Light Emitting Diodes Light Quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]
- Dziurka, K.; Dziurka, M.; Muszyńska, E.; Czyczyło-Mysza, I.; Warchoł, M.; Juzoń, K.; Laskoś, K.; Skrzypek, E. Anatomical and hormonal factors determining the development of haploid and zygotic embryos of oat (Avena sativa L.). Sci. Rep. 2022, 12, 548. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Igielski, R.; Kępczyńska, E. Gene expression and metabolite profiling of gibberellin biosynthesis during induction of somatic embryogenesis in Medicago truncatula Gaertn. PLoS ONE 2017, 12, e0182055. [Google Scholar] [CrossRef] [Green Version]
- Mácová, K.; Prabhullachandran, U.; Štefková, M.; Spyroglou, I.; Pěnčík, A.; Endlová, L.; Novák, O.; Robert, H.S. Long-Term High-Temperature Stress Impacts on Embryo and Seed Development in Brassica napus. Front. Plant Sci. 2022, 13, 844292. [Google Scholar] [CrossRef]
- Rood, S.B.; Pearce, D.; Pharis, R.P. Identification of Endogenous Gibberellins from Oilseed Rape. Plant Physiol. 1987, 85, 605–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkowská, D.; Filek, M.; Biesaga-Kościelniak, J.; Marcińska, I.; Macháčková, I.; Krekule, J.; Strnad, M. Cytokinins in shoot apices of Brassica napus plants during vernalization. Plant Sci. 2012, 187, 105–112. [Google Scholar] [CrossRef]
- Rapacz, M.; Waligórski, P.; Janowiak, F. ABA and gibberellin-like substances during prehardening, cold acclimation, de- and reacclimation of oilseed rape. Acta Physiol. Plant. 2003, 25, 151–161. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, X.; Zhang, Z.; Gu, S.; Li, G.; Shi, H. Defense to Sclerotinia sclerotiorum in oilseed rape is associated with the sequential activations of salicylic acid signaling and jasmonic acid signaling. Plant Sci. 2012, 184, 75–82. [Google Scholar] [CrossRef]
- Zheng, X.; Koopmann, B.; von Tiedemann, A. Role of salicylic acid and components of the phenylpropanoid pathway in basal and cultivar-related resistance of oilseed rape (Brassica napus) to Verticillium longisporum. Plants 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Smoleńska-Sym, G.; Gawrońska, H.; Kacperska, A. Modifications of abscisic acid level in winter oilseed rape leaves during acclimation of plants to freezing temperatures. Plant Growth Regul. 1995, 17, 61–65. [Google Scholar] [CrossRef]
- Lalk, I.; Dörffling, K. Hardening, abscisic acid, proline and freezing resistance in two winter wheat varieties. Physiol. Plant. 1985, 63, 287–292. [Google Scholar] [CrossRef]
- Churchill, G.C.; Reaney, M.J.T.; Abrams, S.R.; Gusta, L.V. Effects of abscisic acid and abscisic acid analogs on the induction of freezing tolerance of winter rye (Secale cereale L.) seedlings. Plant Growth Regul. 1998, 25, 35–45. [Google Scholar] [CrossRef]
- Bravo, L.A.; Zúñiga, G.E.; Alberdi, M.; Corcuera, L.J. The role of ABA in freezing tolerance and cold acclimation in barley. Physiol. Plant. 1998, 103, 17–23. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Ignatenko, A.; Talanova, V.; Repkina, N.; Titov, A. Exogenous salicylic acid treatment induces cold tolerance in wheat through promotion of antioxidant enzyme activity and proline accumulation. Acta Physiol. Plant. 2019, 41, 80. [Google Scholar] [CrossRef]
- Taşgín, E.; Atící, Ö.; Nalbantoğlu, B. Effects of salicylic acid and cold on freezing tolerance in winter wheat leaves. Plant Growth Regul. 2003, 41, 231–236. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, X.; Wang, Y.; Huo, Z.; Huang, M.; Cai, J.; Zhou, Q.; Jiang, D. Involvement of salicylic acid in cold priming-induced freezing tolerance in wheat plants. Plant Growth Regul. 2021, 93, 117–130. [Google Scholar] [CrossRef]
- Chen, H.I.; Li, P.F.; Yang, C.H. NAC-Like Gene Gibberellin Suppressing Factor Regulates the Gibberellin Metabolic Pathway in Response to Cold and Drought Stresses in Arabidopsis. Sci. Rep. 2019, 9, 19226. [Google Scholar] [CrossRef] [Green Version]
- Janeczko, A.; Biesaga-Kościelniak, J.; Dziurka, M.; Filek, M.; Hura, K.; Jurczyk, B.; Kula, M.; Oklestkova, J.; Novak, O.; Rudolphi-Skórska, E.; et al. Biochemical and Physicochemical Background of Mammalian Androgen Activity in Winter Wheat Exposed to Low Temperature. J. Plant Growth Regul. 2018, 37, 199–219. [Google Scholar] [CrossRef] [Green Version]
- Sadura, I.; Pociecha, E.; Dziurka, M.; Oklestkova, J.; Novak, O.; Gruszka, D.; Janeczko, A. Mutations in the HvDWARF, HvCPD and HvBRI1 Genes-Involved in Brassinosteroid Biosynthesis/Signalling: Altered Photosynthetic Efficiency, Hormonal Homeostasis and Tolerance to High/Low Temperatures in Barley. J. Plant Growth Regul. 2019, 38, 1062–1081. [Google Scholar] [CrossRef] [Green Version]
- Zanewich, K.P.; Rood, S.B. Vernalization and gibberellin physiology of winter canola. Plant Physiol. 1995, 108, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filek, M.; Biesaga-Koscielniak, J.; Machackova, I.; Krekule, J. Generative Development of WinterRape (Brassica napus L.)—The Role of Vernalization. Int. J. Plant Dev. Biol. 2007, 1, 57–63. [Google Scholar]
- Moblidowska, I. Effects of some growth regulators on frost damage. Cryobiology 1968, 5, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, C.; Cang, J.; Zeng, Y.; Yu, J.; Liu, L.; Zhang, D.; Wang, J. Effects of exogenous GA3 on wheat cold tolerance. J. Agric. Sci. Technol. 2015, 17, 921–934. [Google Scholar]
- Pěnčík, A.; Simonovik, B.; Petersson, S.V.; Henyková, E.; Simon, S.; Greenham, K.; Zhang, Y.; Kowalczyk, M.; Estelle, M.; Zažímalová, E.; et al. Regulation of auxin homeostasis and gradients in Arabidopsis roots through the formation of the indole-3-acetic acid catabolite 2-oxindole-3-acetic acid. Plant Cell 2013, 25, 3858–3870. [Google Scholar] [CrossRef] [Green Version]
- Östin, A.; Kowalyczk, M.; Bhalerao, R.P.; Sandberg, G. Metabolism of indole-3-acetic acid in arabidopsis. Plant Physiol. 1998, 118, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Gamir, J.; Pastor, V.; Cerezo, M.; Flors, V. Identification of indole-3-carboxylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol. Biochem. 2012, 61, 169–179. [Google Scholar] [CrossRef]
- Pastor-Fernández, J.; Pastor, V.; Mateu, D.; Gamir, J.; Sánchez-Bel, P.; Flors, V. Accumulating evidences of callose priming by indole- 3- carboxylic acid in response to Plectospharella cucumerina. Plant Signal. Behav. 2019, 14, 1608107. [Google Scholar] [CrossRef]
- Cohen, S.P.; Leach, J.E. High temperature-induced plant disease susceptibility: More than the sum of its parts. Curr. Opin. Plant Biol. 2020, 56, 235–241. [Google Scholar] [CrossRef]
- Wang, B.; Andargie, M.; Fang, R. The function and biosynthesis of callose in high plants. Heliyon 2022, 8, e09248. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic regulation of ABA and GA in metabolism and signaling pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.S.; Li, Z.Y.; Chen, Y.; Chen, M.; Li, L.C.; Ma, Y.Z. Heat shock protein 90 in plants: Molecular mechanisms and roles in stress responses. Int. J. Mol. Sci. 2012, 13, 15706–15723. [Google Scholar] [CrossRef]
- Aghaie, P.; Tafreshi, S.A.H. Central role of 70-kDa heat shock protein in adaptation of plants to drought stress. Cell Stress Chaperones 2020, 25, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Krishna, P.; Forreiter, C. A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol. 2000, 123, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vítámvás, P.; Prášil, I.T.; Kosová, K.; Planchon, S.; Renaut, J. Analysis of proteome and frost tolerance in chromosome 5A and 5B reciprocal substitution lines between two winter wheats during long-term cold acclimation. Proteomics 2012, 12, 68–85. [Google Scholar] [CrossRef]
- Rathore, N.; Kumar, P.; Mehta, N.; Swarnkar, M.K.; Shankar, R.; Chawla, A. Time-series RNA-Seq transcriptome profiling reveals novel insights about cold acclimation and de-acclimation processes in an evergreen shrub of high altitude. Sci. Rep. 2022, 12, 15553. [Google Scholar] [CrossRef] [PubMed]
- Berka, M.; Kopecká, R.; Berková, V.; Brzobohatý, B.; Černý, M. Regulation of heat shock proteins 70 and their role in plant immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Geisler, M. HSP90 and co-chaperones: A multitaskers’ view on plant hormone biology. FEBS Lett. 2019, 593, 1415–1430. [Google Scholar] [CrossRef] [Green Version]
- Clément, M.; Leonhardt, N.; Droillard, M.J.; Reiter, I.; Montillet, J.L.; Genty, B.; Lauriére, C.; Nussaume, L.; Noël, L.D. The cytosolic/nuclear HSC70 and HSP90 molecular chaperones are important for stomatal closure and modulate abscisic acid-dependent physiological responses in Arabidopsis. Plant Physiol. 2011, 156, 1481–1492. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhuang, L.; Shi, Y.; Huang, B. Up-regulation of HSFA2c and HSPs by ABA contributing to improved heat tolerance in tall fescue and Arabidopsis. Int. J. Mol. Sci. 2017, 18, 1981. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Jia, Z.; Liu, Y.; Wang, M.; Zhao, J.; Zheng, J.; Wang, G. Constitutive expression of ZmsHSP in Arabidopsis enhances their cytokinin sensitivity. Mol. Biol. Rep. 2010, 37, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Jedelský, P.L.; Novák, J.; Schlosser, A.; Brzobohatý, B. Cytokinin modulates proteomic, transcriptomic and growth responses to temperature shocks in Arabidopsis. Plant Cell Environ. 2014, 37, 1641–1655. [Google Scholar] [CrossRef] [PubMed]



| Compound | Type of Ion | Quantifier Transition (Precursor/Product Ions) | Fragmentor Voltage (V) | Collision Energy (V) | MRM Start Time (min.) | |
|---|---|---|---|---|---|---|
| DHZ-N15 | ISTD (10 pmol) | [M + H]+ | 226.2/152 | 124 | 18 | 1.5 |
| cis-ZEA | [M + H]+ | 220.2/136.3 | 85 | 9 | ||
| oxIAA | [M + H]+ | 192.2/146.1 | 54 | 9 | 4.0 | |
| IAM | [M + H]+ | 175.1/130 | 66 | 17 | ||
| t-Z-R-D5 | ISTD (10 pmol) | [M + H]+ | 357.3/225.2 | 116 | 17 | 5.12 |
| cis-ZEA-rib | [M + H]+ | 352.2/220.3 | 120 | 9 | ||
| IAAsp | [M + H]+ | 291.2/130.1 | 54 | 25 | 6.4 | |
| BA-D4 | ISTD (500 pmol) | [M + H]+ | 128.1/84.1 | 61 | 13 | |
| BA | [M + H]+ | 123.1/79.1 | 56 | 13 | ||
| IAGlu | [M + H]+ | 305.2/130.1 | 58 | 29 | ||
| GA3 | [M-H2O + H]+ | 329.3/311.3 | 100 | 14 | 8.15 | |
| GA1-D2 | ISTD (10 pmol) | [M-H2O + H]+ | 333.3/287.2 | 58 | 9 | |
| I3CA | [M + H]+ | 162.2/118.1 | 58 | 9 | ||
| IAA-D5 | ISTD (100 pmol) | [M + H]+ | 181.1/135.1 | 38 | 14 | |
| IAA | [M + H]+ | 176.1/130.3 | 51 | 9 | ||
| SA-D4 | ISTD (500 pmol) | [M + H]+ | 143.2/125.2 | 80 | 14 | |
| SA | [M + H]+ | 139.2/121.2 | 80 | 14 | ||
| GA6-D2 | ISTD (10 pmol) | [M-H2O + H]+ | 331.3/115.1 | 96 | 5 | 10.4 |
| GA6 | [M-H2O + H]+ | 329.3/283.3 | 104 | 14 | ||
| IAN-D4 | ISTD (100 pmol) | [M + H]+ | 161.1/134.1 | 66 | 13 | 12.0 |
| IAN | [M + H]+ | 157.1/130.1 | 71 | 13 | ||
| ABA-D6 | ISTD (30 pmol) | [M-H2O + H]+ | 253.4/191.3 | 80 | 14 | 14.6 |
| ABA | [M-H2O + H]+ | 247.4/187.2 | 80 | 14 | ||
| GA5-D2 | ISTD (10 pmol) | [M-H2O + H]+ | 287.3/115.0 | 96 | 5 | 15.45 |
| GA20 | [M-H2O + H]+ | 287.3/115.0 | 96 | 5 | ||
| GA19 | 345.2/299.1 | 80 | 9 | 16.8 | ||
| JA-D5 | ISTD (100 pmol) | [M + H]+ | 216.3/153.2 | 80 | 5 | 19.5 |
| JA | [M + H]+ | 211.3/151.2 | 80 | 14 | ||
| GA7 | [M-H2O + H]+ | 313.2/223.1 | 104 | 14 | 18.5 | |
| GA4-D2 | ISTD (10 pmol) | [M-H2O + H]+ | 317.3/271.2 | 88 | 9 | |
| GA4 | [M-H2O + H]+ | 315.3/269.3 | 100 | 14 | ||
| GA53 | 303.2/285.1 | 100 | 9 | |||
| GA9 | [M-H2O + H]+ | 271.3/225.2 | 136 | 13 | 22.25 | |
| GA15 | 331.2/285.1 | 115 | 9 | |||
| dinor-12-oxo-OPDA-D5 | ISTD (10 pmol) | [M + H]+ | 270.3/252.2 | 84 | 5 | |
| 12-oxo-PDA | [M + H]+ | 293.3/275.2 | 68 | 9 | 24.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachurska, J.; Sadura, I.; Rys, M.; Dziurka, M.; Janeczko, A. Insight into Hormonal Homeostasis and the Accumulation of Selected Heat Shock Proteins in Cold Acclimated and Deacclimated Winter Oilseed Rape (Brassica napus L.). Agriculture 2023, 13, 641. https://doi.org/10.3390/agriculture13030641
Stachurska J, Sadura I, Rys M, Dziurka M, Janeczko A. Insight into Hormonal Homeostasis and the Accumulation of Selected Heat Shock Proteins in Cold Acclimated and Deacclimated Winter Oilseed Rape (Brassica napus L.). Agriculture. 2023; 13(3):641. https://doi.org/10.3390/agriculture13030641
Chicago/Turabian StyleStachurska, Julia, Iwona Sadura, Magdalena Rys, Michał Dziurka, and Anna Janeczko. 2023. "Insight into Hormonal Homeostasis and the Accumulation of Selected Heat Shock Proteins in Cold Acclimated and Deacclimated Winter Oilseed Rape (Brassica napus L.)" Agriculture 13, no. 3: 641. https://doi.org/10.3390/agriculture13030641
APA StyleStachurska, J., Sadura, I., Rys, M., Dziurka, M., & Janeczko, A. (2023). Insight into Hormonal Homeostasis and the Accumulation of Selected Heat Shock Proteins in Cold Acclimated and Deacclimated Winter Oilseed Rape (Brassica napus L.). Agriculture, 13(3), 641. https://doi.org/10.3390/agriculture13030641