Research Progress in Soybean by Phytohormone Modulation and Metal Chelation over the Past Decade
Abstract
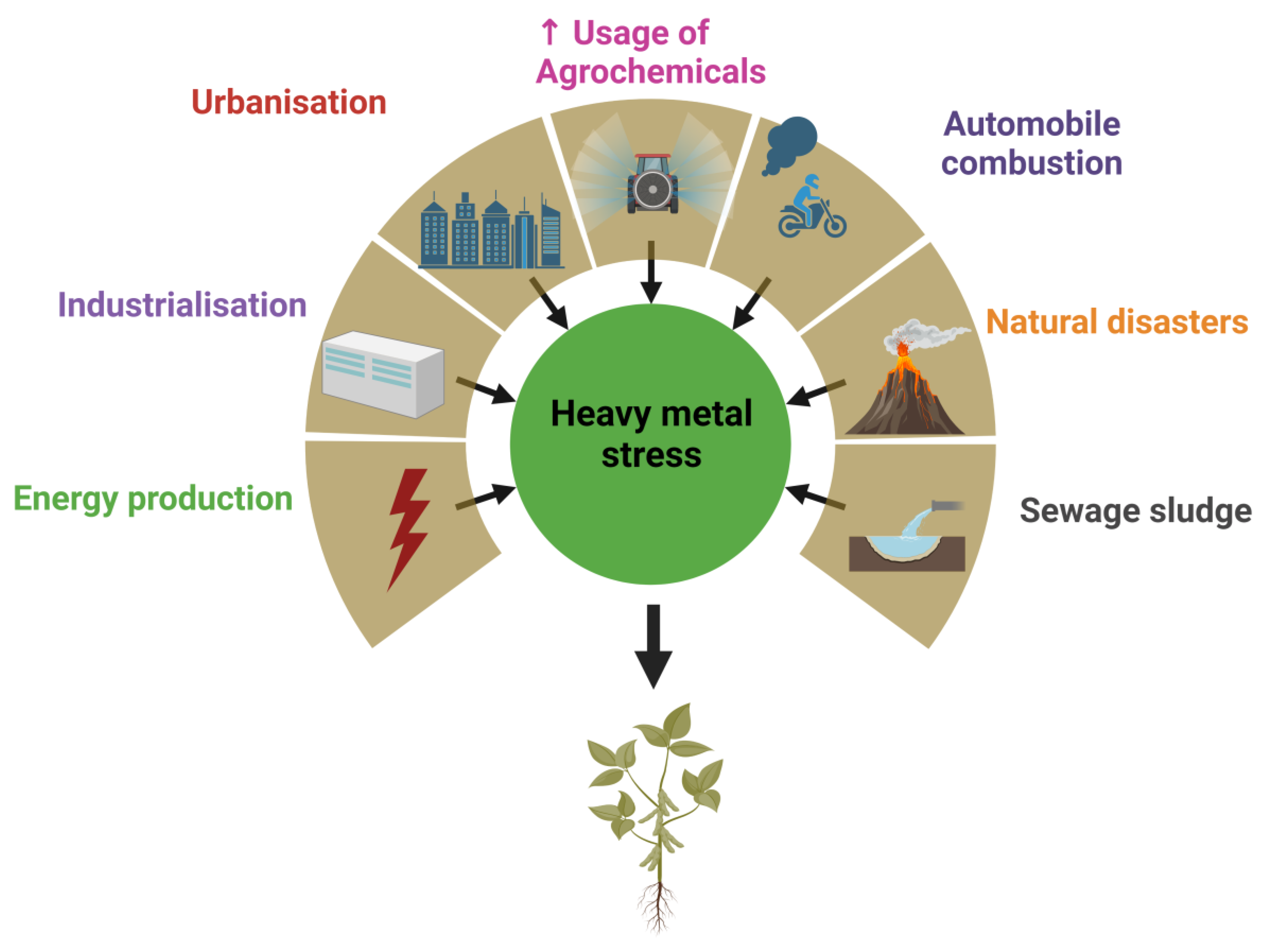
1. Introduction
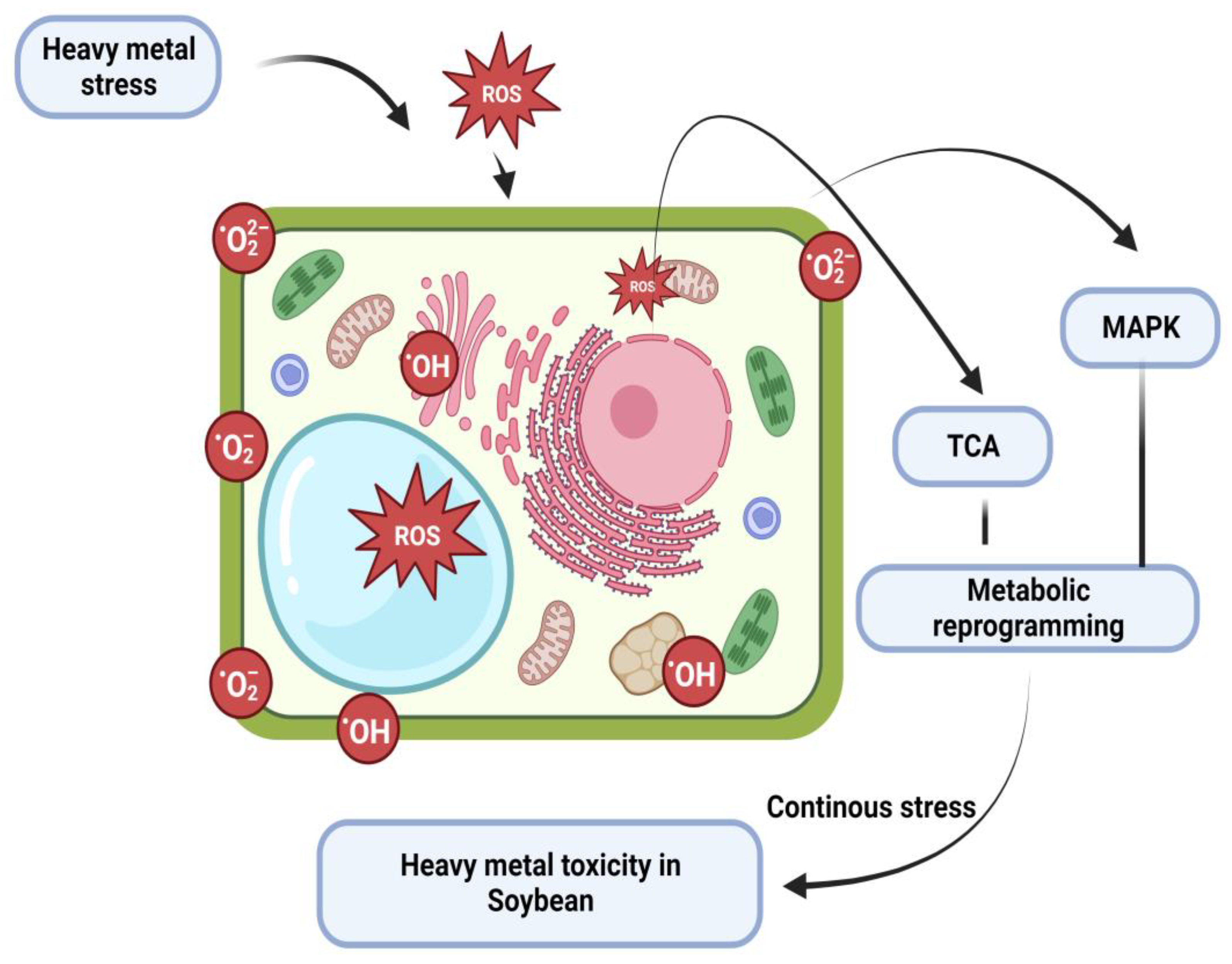
2. Pro-Oxidant and Reactive Oxygen Species
3. Phytohormones in Heavy Metal Tolerance
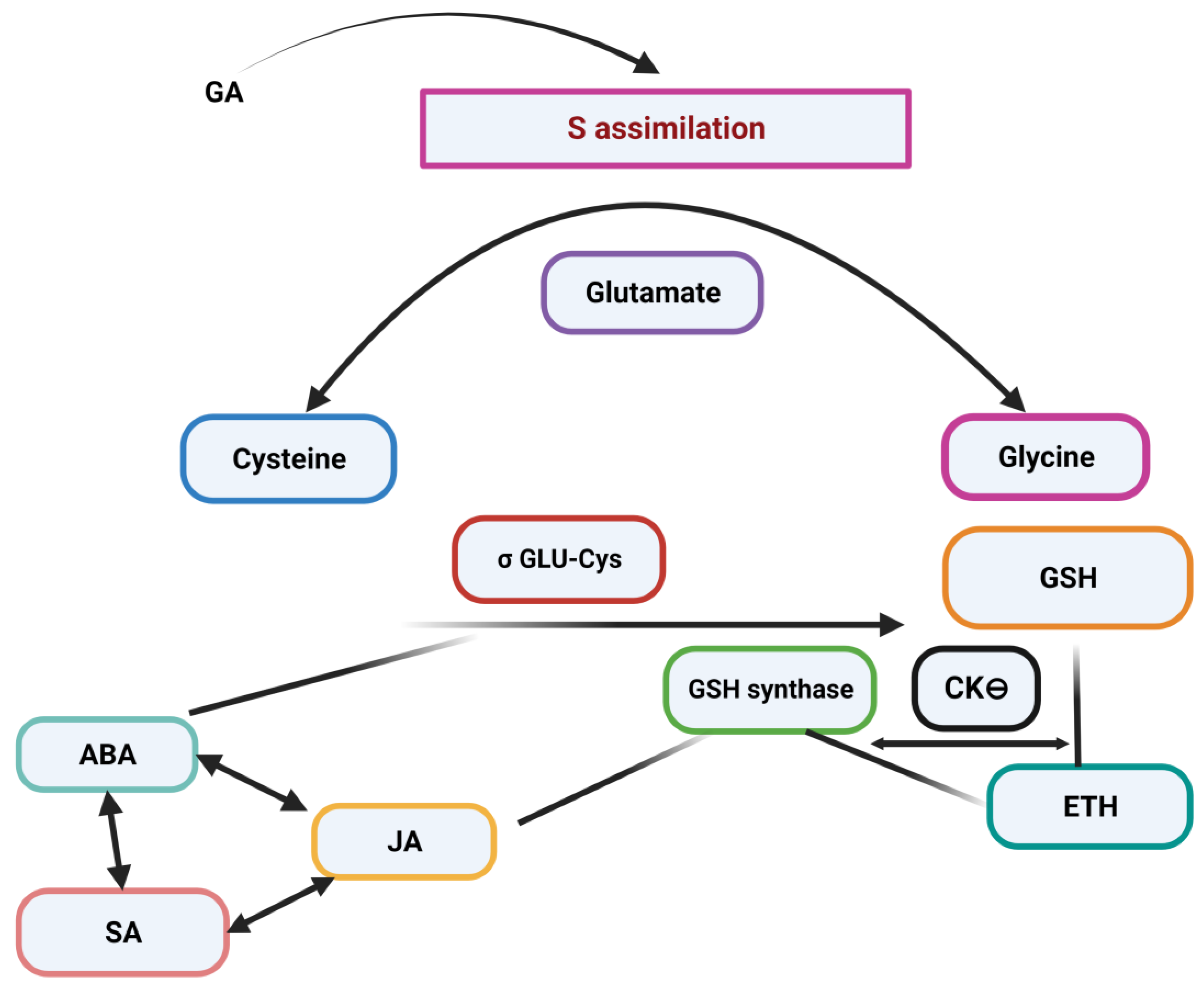
4. Metal Chelation and Activation of Phytohormones
5. Conclusions
6. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ghori, N.-H.; Ghori, T.; Hayat, M.; Imadi, S.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormetic effects of abiotic environmental stressors in woody plants in the context of climate change. J. For. Res. 2023, 34, 7–19. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, K.-W.; Rhee, J.-S.; Hwang, D.-S.; Lee, Y.-M.; Park, H.G.; Ahn, I.-Y.; Lee, J.-S. Environmental stressors (salinity, heavy metals, H2O2) modulate expression of glutathione reductase (GR) gene from the intertidal copepod Tigriopus japonicus. Aquat. Toxicol. 2006, 80, 281–289. [Google Scholar] [CrossRef]
- Gupta, D.K.; Corpas, F.J.; Palma, J.M. Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Nocito, F.F.; Lancilli, C.; Crema, B.; Fourcroy, P.; Davidian, J.-C.; Sacchi, G.A. Heavy metal stress and sulfate uptake in maize roots. Plant Physiol. 2006, 141, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, P. Mitigating the effect of biofertilizers on morphological and biochemical level in pearl millet grown under mercury toxicity. J. Pharmacogn. Phytochem. 2020, 9, 955–961. [Google Scholar]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Heavy metal mediated phytotoxic impact on winter wheat: Oxidative stress and microbial management of toxicity by Bacillus subtilis BM2. RSC Adv. 2019, 9, 6125–6142. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Nan, G.; Cao, M.; Gao, Y.; Guo, L.; Meng, X.; Yang, G. The metal distribution and the change of physiological and biochemical process in soybean and mung bean plants under heavy metal stress. Int. J. Phytoremediation 2018, 20, 1113–1120. [Google Scholar] [CrossRef]
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055. [Google Scholar]
- Appenroth, K.-J. What are “heavy metals” in plant sciences? Acta Physiol. Plant. 2010, 32, 615–619. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, J.I.A.; Bhat, R.A.; Dervash, M.A.; Ganei, S.A. Vehicular stress a cause for heavy metal accumulation and change in physico-chemical characteristics of road side soils in Pahalgam. Environ. Monit. Assess. 2018, 190, 353. [Google Scholar] [CrossRef]
- Varma, S.; Jangra, M. Heavy metals stress and defense strategies in plants: An overview. J. Pharmacogn. Phytochem. 2021, 10, 608–614. [Google Scholar]
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11, 147. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wang, C.; Liao, W. Recent progress in the knowledge on the alleviating effect of nitric oxide on heavy metal stress in plants. Plant Physiol. Biochem. 2020, 147, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Goud, E.L.; Devi, P.; Dey, S.R.; Dwivedi, P. Heavy Metals: Transport in Plants and Their Physiological and Toxicological Effects. In Plant Metal and Metalloid Transporters; Springer: Singapore, 2022; pp. 23–54. [Google Scholar]
- Daulta, R.; Prakash, M.; Goyal, S. Metal content in soils of Northern India and crop response: A review. Int. J. Environ. Sci. Technol. 2023, 20, 4521–4548. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Wang, Z.; Yuan, Y.; Zhang, Z.; Liang, Q.; Yang, X.; Duan, Z.; Liu, Y.; Kong, F. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 2022, 20, 256–282. [Google Scholar] [CrossRef]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I.-J. Phytohormones producing rhizobacteria alleviate heavy metals stress in soybean through multilayered response. Microbiol. Res. 2023, 266, 127237. [Google Scholar]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Cardoso, A.A.; da-Silva, C.J.; de Oliveira, L.M.; Dari, B.; Sihi, D.; Karimi, N. Heavy metals and photosynthesis: Recent developments. In Plant Responses to Xenobiotics; Springer: Singapore, 2019; pp. 107–134. [Google Scholar]
- Rai, R.; Agrawal, M.; Agrawal, S. Impact of heavy metals on physiological processes of plants: With special reference to photosynthetic system. In Plant Responses to Xenobiotics; Springer: Singapore, 2016; pp. 127–140. [Google Scholar]
- Shah, F.U.R.; Ahmad, N.; Masood, K.R.; Peralta-Videa, J.R.; Ahmad, F.u.D. Heavy metal toxicity in plants. In Plant Responses to Xenobiotics; Springer: Dordrecht, The Netherlands, 2010; pp. 71–97. [Google Scholar]
- Kalaivanan, D.; Ganeshamurthy, A.N. Mechanisms of heavy metal toxicity in plants. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 85–102. [Google Scholar]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Or Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect of heavy metals on plant growth: An overview. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar]
- Miransari, M. Soybean production and heavy metal stress. In Abiotic and Biotic Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–216. [Google Scholar]
- Reddy, M.; Dunn, S. Heavy metal and micronutrient uptake in soybeans as influenced by sewage sludge amendment. Sci. Total Environ. 1983, 30, 85–98. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Nouman, W.; Bashir, T.; Gul, R.; Gull, T.; Olson, M.E.; Shaheen, M.; Rosa, E.; Domínguez-Perles, R.; Soliman, W.S. Metalliferous conditions induce regulation in antioxidant activities, polyphenolics and nutritional quality of Moringa oleifera L. Int. J. Phytoremediation 2020, 22, 1348–1361. [Google Scholar] [CrossRef]
- Abbas, A.M.; Abd-Elmabod, S.K.; El-Ashry, S.M.; Soliman, W.S.; El-Tayeh, N.; Castillo, J.M. Capability of the invasive tree Prosopis glandulosa Torr. to remediate soil treated with sewage sludge. Sustainability 2019, 11, 2711. [Google Scholar] [CrossRef]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Gautam, V.; Bali, S.; Sharma, A.; Khanna, K.; Arora, S.; Thukral, A.K.; Ohri, P.; Karpets, Y.V. ROS signaling in plants under heavy metal stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017; pp. 185–214. [Google Scholar]
- Steffens, B. The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front. Plant Sci. 2014, 5, 685. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy metal stress signaling in plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. [Google Scholar]
- Yang, Z.; Chu, C. Towards understanding plant response to heavy metal stress. Abiotic Stress Plants–Mech. Adapt. 2011, 10, 24204. [Google Scholar]
- Pinto, E.; Sigaud-kutner, T.C.; Leitao, M.A.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK cascades and transcriptional factors: Regulation of heavy metal tolerance in plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Nakagami, H.; Hirt, H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004, 136, 3276–3283. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Hussain, S.; Hussain, B.; Riaz, L.; Ashraf, M.N.; Khaliq, M.A.; Du, Z.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of Polyamines and Phytohormones in Plant Response to Abiotic Stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Noor, W.; Majeed, G.; Lone, R.; Tyub, S.; Kamili, A.N.; Azeez, A. Interactive Role of Phenolics and PGPR in Alleviating Heavy Metal Toxicity in Wheat. In Plant Phenolics in Abiotic Stress Management; Springer: Singapore, 2023; pp. 287–320. [Google Scholar]
- Arshad, A.; Mushtaq, N.; Sajjad, M.; Ahad, A.; Ilyas, M.; Gul, A. Role of exogenous phytohormones in mitigating stress in plants. In Phytohormones and Stress Responsive Secondary Metabolites; Elsevier, Academic Press: Cambridge, MA, USA, 2023; pp. 111–131. [Google Scholar]
- Naz, M.; Akhtar, K.; Khan, A.; Zaib, S.; Tariq, M.; Raza, M.A.; Zhou, J.; Dai, Z.; Du, D. Genetic engineering strategies for regulation of phytohormones in plants exposed to biotic and abiotic stresses. In Phytohormones and Stress Responsive Secondary Metabolites; Elsevier, Academic Press: Cambridge, MA, USA, 2023; pp. 265–274. [Google Scholar]
- Vaishnav, D.; Chowdhury, P. Types and Function of Phytohormone and Their Role in Stress. In Plant Stress Responses and Defense Mechanisms; Intechopen: London, UK, 2023. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Huang, K.-X.; Zhang, Y.-R.; Yang, L.; Zhou, J.-L.; Yang, Q.; Gao, F. Efficient microalgal lipid production driven by salt stress and phytohormones synergistically. Bioresour. Technol. 2023, 367, 128270. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Xie, Y. The Role of New Members of Phytohormones in Plant Amelioration under Abiotic Stress with an Emphasis on Heavy Metals. Pol. J. Environ. Stud. 2020, 29, 1009–1020. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.-E.; Dawood, M.F.-A.; Hussein, W.E.; Mansour, A.; Amin, D.H. Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Sethi, M.; Kaur, C.; Hagroo, R.P.; Singh, M.P. Endophyte mediated plant health via phytohormones and biomolecules. In Microbial Endophytes and Plant Growth; Elsevier, Academic Press: Cambridge, MA, USA, 2023; pp. 151–166. [Google Scholar]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.N. Phytohormonal roles in plant responses to heavy metal stress: Implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Ma, Y. Metal tolerance mechanisms in plants and microbe-mediated bioremediation. Environ. Res. 2023, 222, 115413. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Nguyen, Q.T.; Dang, D.H.; Emery, R.N. Phytohormones enhance heavy metal responses in Euglena gracilis: Evidence from uptake of Ni, Pb and Cd and linkages to hormonomic and metabolomic dynamics. Environ. Pollut. 2023, 320, 121094. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, A.L.; Mun, B.-G.; Bilal, S.; Shaffique, S.; Kwon, E.-H.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Melatonin and nitric oxide: Dual players inhibiting hazardous metal toxicity in soybean plants via molecular and antioxidant signaling cascades. Chemosphere 2022, 308, 136575. [Google Scholar] [CrossRef]
- Vezza, M.E.; Alemano, S.; Agostini, E.; Talano, M.A. Arsenic toxicity in soybean plants: Impact on chlorophyll fluorescence, mineral nutrition and phytohormones. J. Plant Growth Regul. 2021, 41, 2719–2731. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-U.; Lee, M.; Kim, Y.-Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef]
- You, Y.; Wang, Y.; Zhang, S.; Sun, X.; Liu, H.; Guo, E.Y.; Du, S. Different pathways for exogenous ABA-mediated down-regulation of cadmium accumulation in plants under different iron supplies. J. Hazard. Mater. 2022, 440, 129769. [Google Scholar] [CrossRef]
- Cheng, L.; Pu, L.; Li, A.; Zhu, X.; Zhao, P.; Xu, X.; Lei, N.; Chen, J. Implication of exogenous abscisic acid (ABA) application on phytoremediation: Plants grown in co-contaminated soil. Environ. Sci. Pollut. Res. 2022, 29, 8684–8693. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.; Pandey, M. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.; Hasnain, Z.; Elsayed, E.A.; El Enshasy, H.A. Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra (L.) K. Koch in chromium contaminated soil. Molecules 2021, 26, 1569. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Tripathi, D.K.; Baluska, F.; Mukherjee, S. Molecular mechanisms of auxin mediated regulation of heavy metal and metalloid stress in plants. Environ. Exp. Bot. 2022, 196, 104796. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Zhao, L.; Yang, S.; Song, Y. Impact of heavy metal stresses on the growth and auxin homeostasis of Arabidopsis seedlings. Biometals 2015, 28, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef]
- Demecsová, L.; Tamás, L. Reactive oxygen species, auxin and nitric oxide in metal-stressed roots: Toxicity or defence. BioMetals 2019, 32, 717–744. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Pacheco-Aguilar, J.R.; Alatorre-Cobos, F.; Hernández-Morales, A. The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ. Geochem. Health 2022, 44, 3743–3764. [Google Scholar] [CrossRef]
- Wang, P.; Yu, W.; Zhang, J.; Rengel, Z.; Xu, J.; Han, Q.; Chen, L.; Li, K.; Yu, Y.; Chen, Q. Auxin enhances aluminium-induced citrate exudation through upregulation of GmMATE and activation of the plasma membrane H+-ATPase in soybean roots. Ann. Bot. 2016, 118, 933–940. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Al-Harrasi, A.; Kim, C.K.; Lee, I.-J. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J. Hazard. Mater. 2019, 379, 120824. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Ahmed, M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soybean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Han, X.; Wang, J.; Zhang, Y.; Kong, Y.; Dong, H.; Feng, X.; Li, T.; Zhou, C.; Yu, J.; Xin, D. Changes in the m6A RNA methylome accompany the promotion of soybean root growth by rhizobia under cadmium stress. J. Hazard. Mater. 2023, 441, 129843. [Google Scholar] [CrossRef]
- Khan, A.L.; Lee, I.-J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 2011, 248, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Saeidi-Sar, S.; Khavari-Nejad, R.; Fahimi, H.; Ghorbanli, M.; Majd, A. Interactive effects of gibberellin A 3 and ascorbic acid on lipid peroxidation and antioxidant enzyme activities in Glycine max seedlings under nickel stress. Russ. J. Plant Physiol. 2007, 54, 74–79. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Phytohormone-producing fungal endophytes and hardwood-derived biochar interact to ameliorate heavy metal stress in soybeans. Biol. Fertil. Soils 2014, 50, 1155–1167. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef]
- Saeidi-Sar, S.; Fahimi, H.; Khavari-Nejad, R.; Majd, A.; Ghorbanli, M. Amelioration of nickel toxicity in soybean plants by gibberellin and ascorbic acid. Rostaniha 2005, 6, 67–76. [Google Scholar]
- Abu-Muriefah, S.S. Phytohormonal priming improves germination and antioxidant enzymes of soybean (Glycine max) seeds under lead (Pb) stress. Biosci. Res. 2017, 14, 42–56. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P. Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef] [PubMed]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.N.; Guerrero, Y.R.; González, L.M.; de la Noval, W.T. Brassinosteroids and plant responses to heavy metal stress. An overview. Open J Met. 2013, 3, 34–41. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Hu, J.; Kaushik, P.; Ahmad, A.; Guan, Y.; Ahmad, P. Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation. Plants 2022, 11, 2292. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, W.; Yao, Q.; Guo, B.; Valliyodan, B.; Wang, Z.; Nguyen, H.T. Genome-wide transcriptional profiling for elucidating the effects of brassinosteroids on Glycine max during early vegetative development. Sci. Rep. 2019, 9, 16085. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2018, 46, 197–212. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.; Mak, M.; Deng, F. Molecular interaction and evolution of jasmonate signaling with transport and detoxification of heavy metals and metalloids in plants. Front. Plant Sci. 2021, 12, 665842. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wianowska, D.; Dawidowicz, A.L.; Radkiewicz, S.; Mardarowicz, M.; Krupa, Z. The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. J. Plant Physiol. 2005, 162, 1338–1346. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Kaur, R.; Kumar, V.; Khanna, K.; Bakshi, P.; Singh, R.; Arora, S.; Kaur, R.; Bhardwaj, R. Role of salicylic acid in heavy metal stress tolerance: Insight into underlying mechanism. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 123–144. [Google Scholar]
- Popova, L.P.; Maslenkova, L.T.; Yordanova, R.Y.; Ivanova, A.P.; Krantev, A.P.; Szalai, G.; Janda, T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol. Biochem. 2009, 47, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Lee, K.E.; Lee, J.D.; Lee, I.J. Enterobacter asburiae KE 17 association regulates physiological changes and mitigates the toxic effects of heavy metals in soybean. Plant Biol. 2015, 17, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Curá, J.A. Role of beneficial microorganisms and salicylic acid in improving rainfed agriculture and future food safety. Microorganisms 2020, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Nazli, F.; Mustafa, A.; Ahmad, M.; Hussain, A.; Jamil, M.; Wang, X.; Shakeel, Q.; Imtiaz, M.; El-Esawi, M.A. A review on practical application and potentials of phytohormone-producing plant growth-promoting rhizobacteria for inducing heavy metal tolerance in crops. Sustainability 2020, 12, 9056. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Drazic, G.; Mihailovic, N. Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci. 2005, 168, 511–517. [Google Scholar] [CrossRef]
- Dražić, G.; Mihailović, N. Salicylic acid modulates accumulation of Cd in seedlings of Cd-tolerant and Cd-susceptible soybean genotypes. Arch. Biol. Sci. 2009, 61, 431–439. [Google Scholar] [CrossRef]
- Gupta, S.; Meena, M.K.; Datta, S. The Alleviating Effect of Salicylic Acid on Early Seedling Growth of Soybean under Zinc and Lead Stress. Bull. Env. Pharm. Life Sci. 2017, 6, 48–54. [Google Scholar]
- Liu, N.; Song, F.; Zhu, X.; You, J.; Yang, Z.; Li, X. Salicylic acid alleviates aluminum toxicity in soybean roots through modulation of reactive oxygen species metabolism. Front. Chem. 2017, 5, 96. [Google Scholar] [CrossRef]
- Lan, T.; You, J.; Kong, L.; Yu, M.; Liu, M.; Yang, Z. The interaction of salicylic acid and Ca2+ alleviates aluminum toxicity in soybean (Glycine max L.). Plant Physiol. Biochem. 2016, 98, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef]
- Keramat, B.; Kalantari, K.M.; Arvin, M.J. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr. J. Microbiol. Res. 2009, 3, 240–244. [Google Scholar]
- Keramat, B.; Kalantari, K.M.; Arvin, M.J. Effects of methyl jasmonate treatment on alleviation of cadmium damages in soybean. J. Plant Nutr. 2010, 33, 1016–1025. [Google Scholar] [CrossRef]
- Mir, M.A.; Sirhindi, G.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J. Plant Growth Regul. 2018, 37, 1195–1209. [Google Scholar] [CrossRef]
- Silva, M.L.d.S.; Vitti, G.C.; Trevizam, A.R. Heavy metal toxicity in rice and soybean plants cultivated in contaminated soil. Rev. Ceres 2014, 61, 248–254. [Google Scholar] [CrossRef]
- Cid, C.V.; Pignata, M.L.; Rodriguez, J.H. Effects of co-cropping on soybean growth and stress response in lead-polluted soils. Chemosphere 2020, 246, 125833. [Google Scholar]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, Y.; Yang, X.; Cui, Z. Optimization of combined phytoremediation for heavy metal contaminated mine tailings by a field-scale orthogonal experiment. Ecotoxicol. Environ. Saf. 2019, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Colls, J.; Hall, D. Application of a chlorophyll fluorescence sensor to detect chelate-induced metal stress in Zea mays. Photosynthetica 2004, 42, 139–145. [Google Scholar] [CrossRef]
- Arya, S.K.; Roy, B. Manganese induced changes in growth, chlorophyll content and antioxidants activity in seedlings of broad bean (Vicia faba L.). J. Environ. Biol. 2011, 32, 707. [Google Scholar] [PubMed]
- Shakya, K.; Chettri, M.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Janda, T.; Szalai, G. Interactions between plant hormones and thiol-related heavy metal chelators. Plant Growth Regul. 2018, 85, 173–185. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Finnegan, P.M.; Liu, W.; Li, X.; Yong, J.W.; Xu, J.; Zhang, Q.; Wen, Y.; Qin, K.; Guo, J. Mechanisms underlying enhanced Cd translocation and tolerance in roots of Populus euramericana in response to nitrogen fertilization. Plant Sci. 2019, 287, 110206. [Google Scholar] [CrossRef]
- Salbitani, G.; Maresca, V.; Cianciullo, P.; Bossa, R.; Carfagna, S.; Basile, A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. Int. J. Mol. Sci. 2023, 24, 5302. [Google Scholar] [CrossRef]
- Yu, M.; He, Z.; Li, S.; Lu, Z.; Chen, J.; Qu, T.; Xu, J.; Qiu, W.; Han, X.; Zhuo, R. SpHsfA4c from Sedum plumbizincicola Enhances Cd Tolerance by the AsA–GSH Pathway in Transgenic Populus× canescens. Agronomy 2023, 13, 760. [Google Scholar] [CrossRef]
- Lu, Q.; Weng, Y.; You, Y.; Xu, Q.; Li, H.; Li, Y.; Liu, H.; Du, S. Inoculation with abscisic acid (ABA)-catabolizing bacteria can improve phytoextraction of heavy metal in contaminated soil. Environ. Pollut. 2020, 257, 113497. [Google Scholar] [CrossRef]
- Polle, A.; Schützendübel, A. Heavy metal signalling in plants: Linking cellular and organismic responses. In Plant Responses to Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2003; pp. 187–215. [Google Scholar]
- Wang, J.; Lin, L.; Luo, L.; Liao, M.a.; Lv, X.; Wang, Z.; Liang, D.; Xia, H.; Wang, X.; Lai, Y. The effects of abscisic acid (ABA) addition on cadmium accumulation of two ecotypes of Solanum photeinocarpum. Environ. Monit. Assess. 2016, 188, 182. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Maksymiec, W.; Krupa, Z. Jasmonic acid and heavy metals in Arabidopsis plants-a similar physiological response to both stressors? J. Plant Physiol. 2002, 159, 509–515. [Google Scholar] [CrossRef]
- Musa, M.; Jan, F.G.; Hamayun, M.; Jan, G.; Khan, S.A.; Rehman, G.; Ali, S.; Lee, I.-J. An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress. Agronomy 2023, 13, 883. [Google Scholar] [CrossRef]
- Naz, H.; Sayyed, R.; Khan, R.U.; Naz, A.; Wani, O.A.; Maqsood, A.; Maqsood, S.; Fahad, A.; Ashraf, S.; Show, P.L. Mesorhizobium improves chickpea growth under chromium stress and alleviates chromium contamination of soil. J. Environ. Manag. 2023, 338, 117779. [Google Scholar] [CrossRef] [PubMed]
- Beniwal, R.; Yadav, R.; Ramakrishna, W. Multifarious effects of arsenic on plants and strategies for mitigation. Agriculture 2023, 13, 401. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; González-Villagra, J.; Ulloa-Inostroza, E.M.; Delgado, M.; Inostroza-Blancheteau, C.; Ivanov, A.G. Phytohormone Involvement in Plant Responses to Soil Acidity. In Plant Hormones and Climate Change; Springer: Singapore, 2023; pp. 301–323. [Google Scholar]
- Ren, Z.; Cheng, R.; Chen, P.; Xue, Y.; Xu, H.; Yin, Y.; Huang, G.; Zhang, W.; Zhang, L. Plant-associated Microbe System in Treatment of Heavy Metals–contaminated Soil: Mechanisms and Applications. Water Air Soil Pollut. 2023, 234, 39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaffique, S.; Kang, S.-M.; Hoque, M.I.U.; Imran, M.; Aaqil khan, M.; Lee, I.-J. Research Progress in Soybean by Phytohormone Modulation and Metal Chelation over the Past Decade. Agriculture 2023, 13, 1325. https://doi.org/10.3390/agriculture13071325
Shaffique S, Kang S-M, Hoque MIU, Imran M, Aaqil khan M, Lee I-J. Research Progress in Soybean by Phytohormone Modulation and Metal Chelation over the Past Decade. Agriculture. 2023; 13(7):1325. https://doi.org/10.3390/agriculture13071325
Chicago/Turabian StyleShaffique, Shifa, Sang-Mo Kang, Md. Injamum Ul Hoque, Muhamad Imran, Muhamad Aaqil khan, and In-Jung Lee. 2023. "Research Progress in Soybean by Phytohormone Modulation and Metal Chelation over the Past Decade" Agriculture 13, no. 7: 1325. https://doi.org/10.3390/agriculture13071325
APA StyleShaffique, S., Kang, S.-M., Hoque, M. I. U., Imran, M., Aaqil khan, M., & Lee, I.-J. (2023). Research Progress in Soybean by Phytohormone Modulation and Metal Chelation over the Past Decade. Agriculture, 13(7), 1325. https://doi.org/10.3390/agriculture13071325







