Correlation between Kinetics of Pectin Degradation and Texture Loss of Okra (Abelmoschus esculentus L.) Puree during Thermal Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Blanching Test and Production of Okra Puree
2.2.2. Moisture Content
2.2.3. Extraction of Okra Pectin
2.2.4. Pectin Yield
2.2.5. Reactivity of Extracted Pectin to Temperature and pH
Formulation of Model Medium
Thermal Treatments
2.2.6. Chemical Characterization
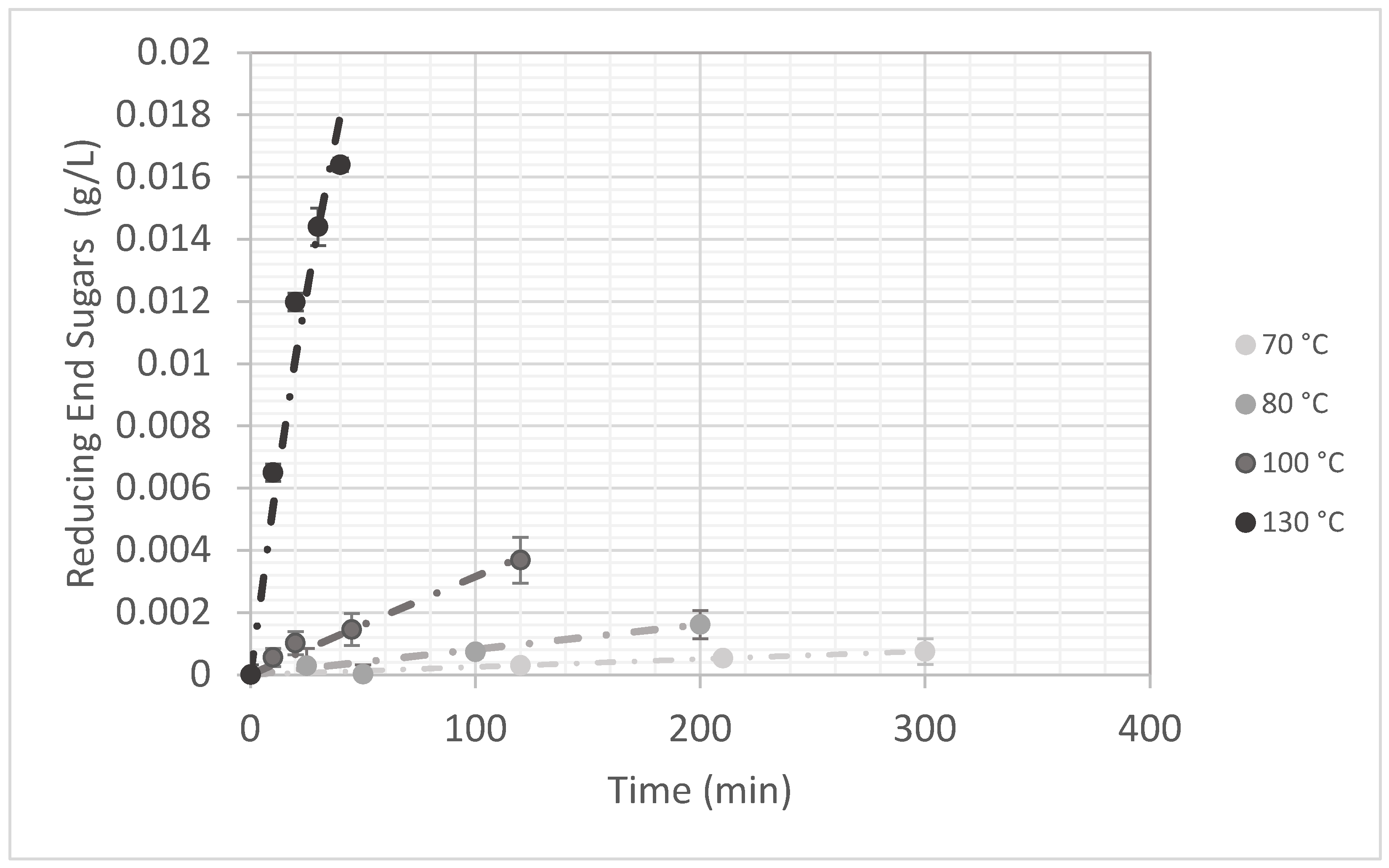
Reducing End Groups (Galactose Equivalent)
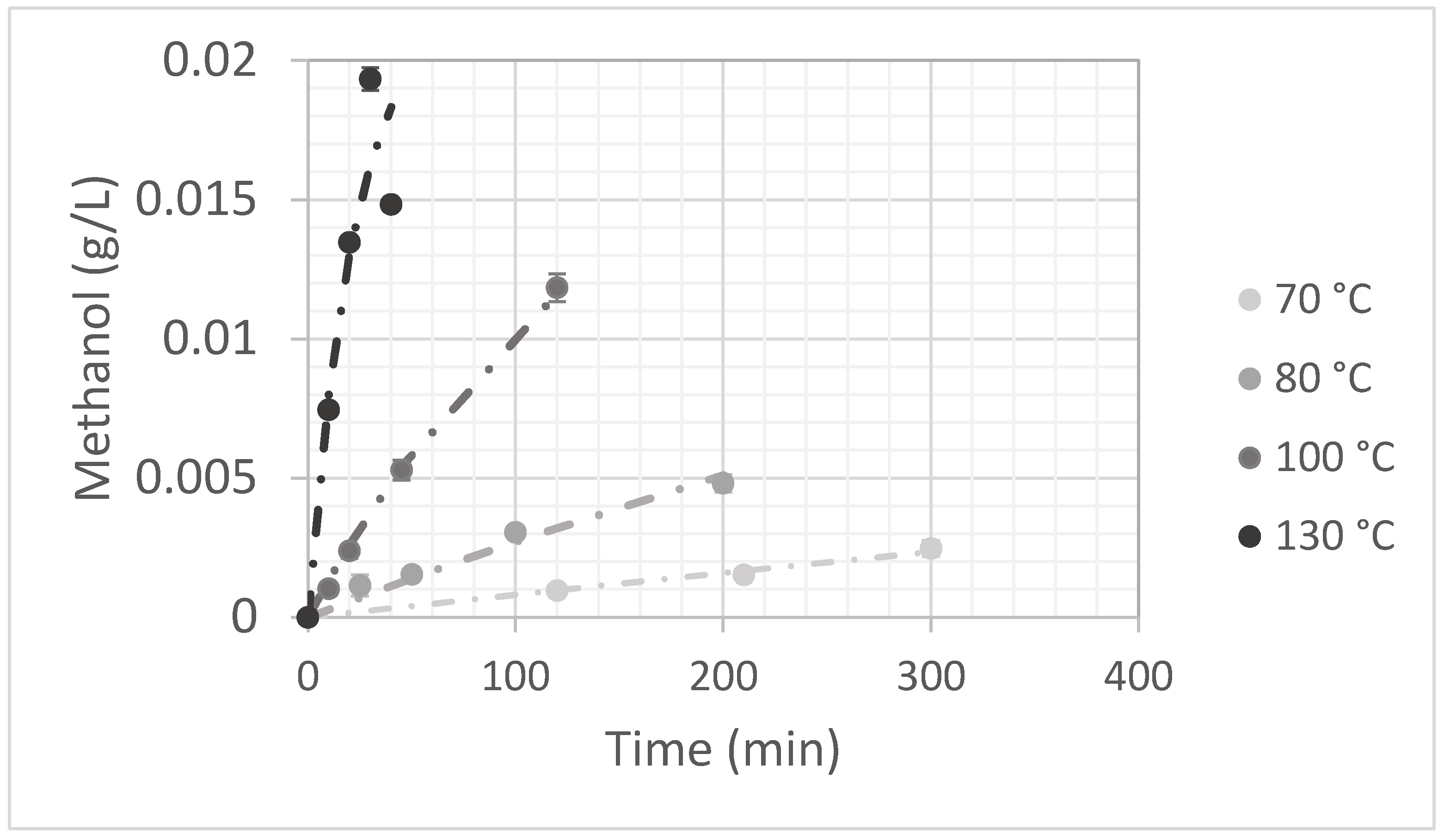
Methanol Production
2.2.7. Thermal Treatments of Okra Puree
2.2.8. Textural Measurements on Okra Puree
- Recording of a stretching test with a camera: after having been placed in a steel cylinder (diameter = 3 cm; depth = 5.2 cm), each sample was left to relax for 2 min. Next, a Stable Micro Systems (Surrey, UK) TA.XT plus texture analyzer equipped with a cylindrical PVC probe (diameter = 2 cm) was used to perform a stretch test (probe descent rate = 10 mm∙s−1, 2 mm penetration of the probe into the sample after detection of a trigger force of 19.6 mN; ascent rate of the probe = 40 mm∙s−1). A penetration of 2 mm of the probe into the sample, before stretching, is intended to ensure good adhesion between the probe and the suspension.
- Extraction of the image corresponding to the maximum length of elongation of the filament, reached during the ascent of the probe. The VLC Media Player (VLC 3.0.16) software was used to capture and extract the image from the stretch test film.
2.2.9. Mathematical Modeling
3. Results and Discussion
3.1. Extraction of Okra Hydrocolloid
3.2. Kinetic of Okra Pectin Degradation: Demethylation and Hydrolysis
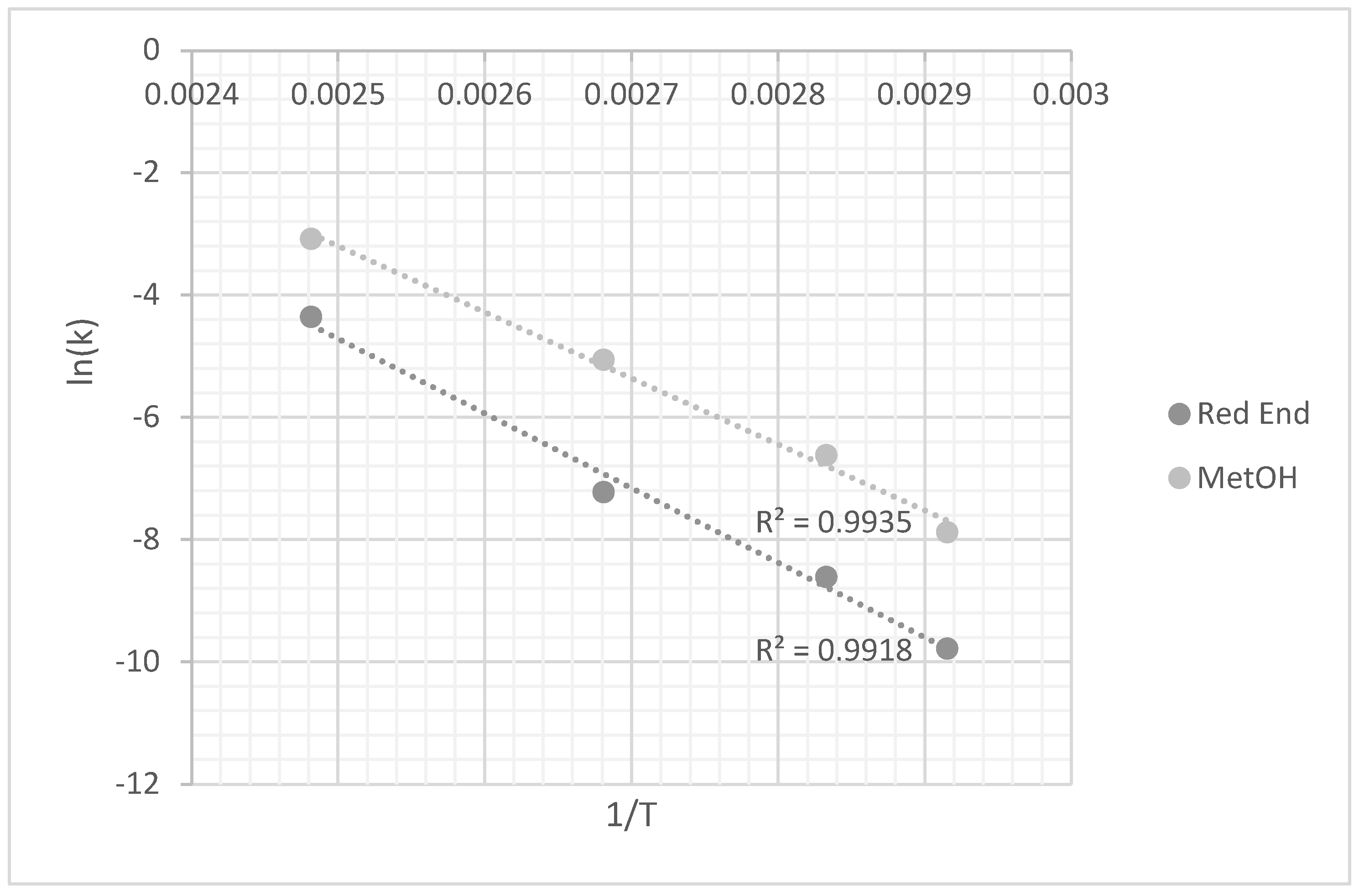
3.2.1. Temperature Effect
Demethylation Reaction
Depolymerization Reaction
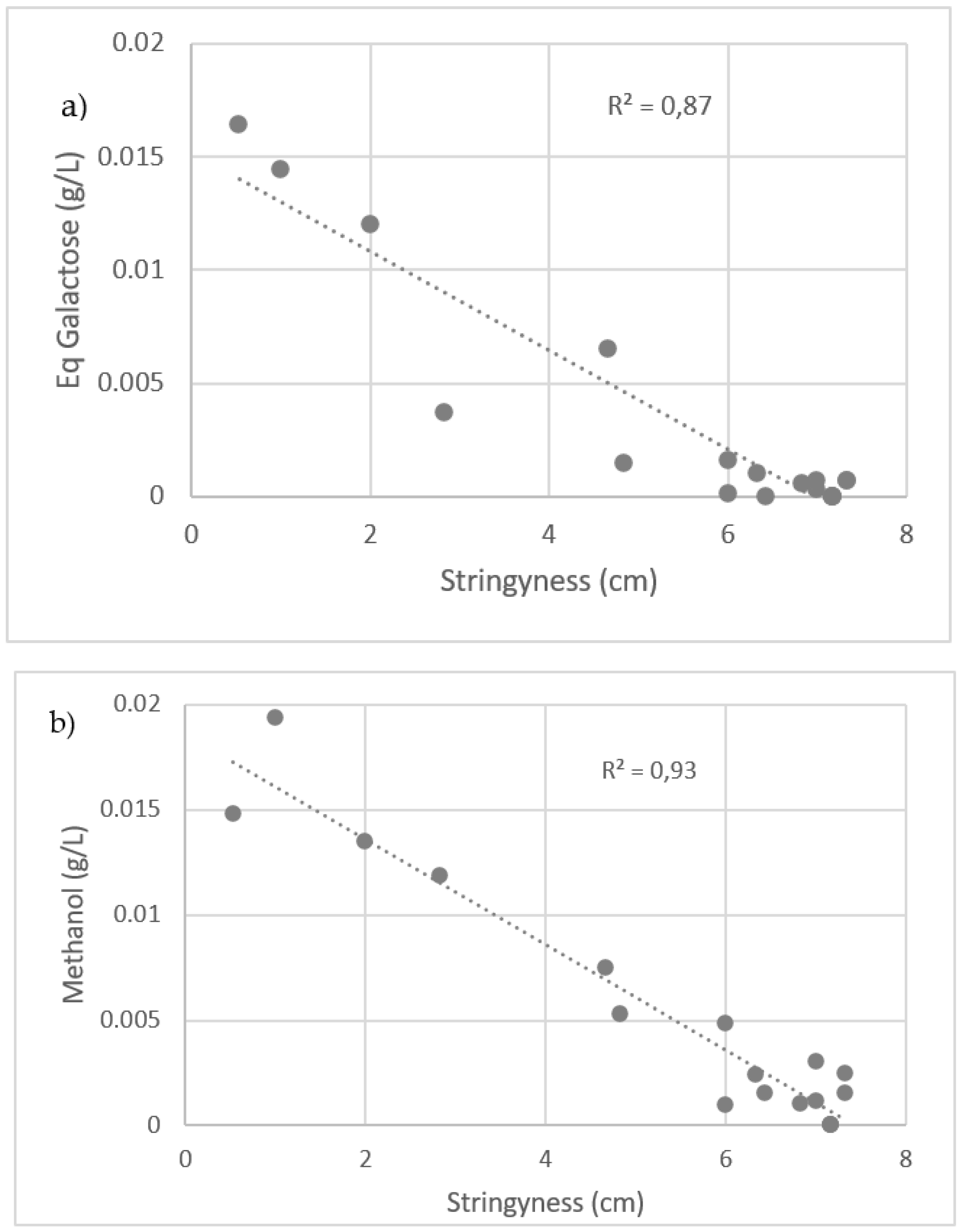
3.3. Stringiness Loss and Hydrocolloid Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Dagnoko, S.; Haougui, A.; Ratnadass, A.; Pasternak, D.; Kouame, C. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res. 2010, 5, 3590–3598. [Google Scholar]
- Olawuyi, I.F.; Park, J.J.; Hahn, D.; Lee, W.Y. Physicochemical and Functional Properties of Okra Leaf Polysaccharides Extracted at Different pHs. Chemistry 2022, 4, 405–418. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgi, A.Z.; Beyene, F. Nutritional Quality and Health Benefits of Okra (Abelmoschus Esculentus): A Review. Int. J. Nutr. Food Sci. 2015, 4, 208–215. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Xu, R.; Wang, H.; Chen, J.-Y.; Tu, Z.-C. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A Review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef] [PubMed]
- Sugri, I.; Abdulai, M.S.; Larbi, A.; Hoeschle-Zeledon, I.; Kusi, F.; Agyare, R.Y. Participatory variety selection of okra (Abelmoschus esculentus L.) genotypes for adaptation to the semi-arid agro-ecology of Northern Ghana. Afr. J. Plant Sci. 2015, 9, 466–475. [Google Scholar] [CrossRef]
- Uzo, J.O.; Ojiako, G.U. A physical method for measuring Okra fruit quality. J. Food Sci. 1980, 45, 390–391. [Google Scholar] [CrossRef]
- Owoeye, A.I.; Caurie, M.C.; Allagheny, N.N.; Onyezili, F.N. Chemical and Physical Parameters Affecting the Viscosity of Mixed Okra and Tomato Homogenate. J. Sci. Food Agric. 1990, 53, 283–286. [Google Scholar] [CrossRef]
- Kumar, D.S.; Tony, D.E.; Kumar, A.P.; Kumar, K.A.; Rao, D.B.S.; Nadendla, R. A Review on: Abelmoschus esculentus (Okra). Int. Res. J. Pharm. Appl. Sci. 2013, 3, 129–132. [Google Scholar]
- Romdhane, M.H.; Chahdoura, H.; Barros, L.; Dias, M.I.; Corrêa, R.C.G.; Morales, P.; Ciudad-Mulero, M.; Flamini, G.; Majdoub, H.; Ferreira, I.C.F.R. Chemical Composition, Nutritional Value, and Biological Evaluation of Tunisian Okra Pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Inyang, U.E.; Ike, C.I. Effect of blanching, dehydration method and temperature on the ascorbic acid, colour, sliminess and other constituents of okra fruit. Int. J. Food Sci. Nutr. 1998, 49, 125–130. [Google Scholar] [CrossRef]
- Aamir, M.; Boonsupthip, W. Effect of microwave drying on quality kinetics of okra. J. Food Sci. Technol. 2017, 54, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- USDA, Agricultural Research Service. 2018. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169260/nutrients (accessed on 1 July 2024).
- Ciqual, Anses. Available online: https://ciqual.anses.fr/ (accessed on 28 August 2024).
- Liu, Y.; Qi, J.; Luo, J.; Qin, W.; Luo, Q.; Zhang, Q.; Wu, D.; Lin, D.; Li, S.; Dong, H.; et al. Okra in Food Field: Nutritional Value, Health Benefits and Effects of Processing Methods on Quality. Food Rev. Int. 2019, 37, 67–90. [Google Scholar] [CrossRef]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a Potential Functional Food Source of Mucilage and Bioactive Compounds with Technological Applications and Health Benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Xu, K.; Martinez, M.M.; Yang, B.; Guo, M. Fine structure, physicochemical and antioxidant properties of LM-pectins from okra pods dried under different techniques. Carbohydr. Polym. 2020, 241, 116272. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbas, M.M.; Moumneh, M.; Hamad, H.J.; Abughoush, M.; Abuawad, B.; Al-Nawasrah, B.A.; Al-Jaloudi, R.; Iqbal, S. Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods 2023, 12, 3711. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chauhan, V.; Tiwari, B.K.; Chauhan, S.S.; Simon, S.; Bilal, S.; Abidi, A.B. An Overview on Okra (Abelmoschus esculentus) and it’s Importance as a Nutritive Vegetable in the World. Int. J. Pharm. Biol. Sci. 2014, 4, 227–233. [Google Scholar]
- Xiong, B.; Zhang, W.; Wu, Z.; Liu, R.; Yang, C.; Hui, A.; Huang, H.; Xian, Z. Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef]
- Nie, X.-R.; Fu, Y.; Wu, D.-T.; Huang, T.-T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Chen, H.; Qin, W. Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. [Google Scholar] [CrossRef]
- Woolfe, M.L.; Chaplin, M.F.; Otchere, G. Studies on the mucilages extracted from okra fruits (Hibiscus esculentus L.) and baobab leaves (Adansonia digitata L.). J. Sci. Food Agric. 1977, 28, 519–529. [Google Scholar] [CrossRef]
- Benchasri, S. Okra (Abelmoschus esculentus (L.) Moench) as a Valuable Vegetable of the World. Ratar. Povrt. 2012, 49, 105–112. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef] [PubMed]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Okra pectin contains an unusual substitution of its rhamnosyl residue with acetyl and alpha-linked galactosyl groups. Carbohydr. Res. 2009, 344, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Smout, C.; Elliot, F.; Loey, A.V.; Hendrickx, M. Non-enzymatic Depolymerization of Carrot Pectin: Toward a Better Understanding of Carrot Texture During Thermal Processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2019, 100, 105396. [Google Scholar] [CrossRef]
- Ashour, E.A.; Bin-Jumah, M.; Abou Sayed-Ahmed, E.T.; Osman, A.O.; Taha, A.E.; Momenah, M.A.; Allam, A.A.; Swelum, A.A.; Abd El-Hack, M.E. Effects of dried okra fruit (Abelmoschus esculentus L.) powder on growth, carcass characteristics, blood indices, and meat quality of stored broiler meat. Poult. Sci. 2020, 99, 3060–3069. [Google Scholar] [CrossRef]
- Tufaro, D.; Bassoli, A.; Cappa, C. Okra (Abelmoschus esculentus) Powder Production and Application in Gluten-Free Bread: Effect of Particle Size. Food Bioprocess Technol. 2022, 15, 904–914. [Google Scholar] [CrossRef]
- Guebebia, S.; Gharsallaoui, A.; Dumas, E.; Baghi, F.; Zourgui, L.; Romdhane, M.; Agusti, G.; Ghnimi, S. Microencapsulation of Phenolic Compounds Extracted from Okra (Abelmoschus esculentus L.) Leaves, Fruits and Seeds. Appl. Sci. 2023, 13, 12273. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824–1832. [Google Scholar] [CrossRef]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726–735. [Google Scholar] [CrossRef]
- Öncü Glaue, Ş.; Akcan, T.; Tavman, Ş. Thermal Properties of Ultrasound-Extracted Okra Mucilage. Appl. Sci. 2023, 13, 6762. [Google Scholar] [CrossRef]
- King, K. Method for rapid extraction of pectic substances from plant materials. Food Chem. 1987, 26, 109–118. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef] [PubMed]
- Fangel, J.U.; Jones, C.Y.; Ulvskov, P.; Harholt, J.; Willats, W.G.T. Analytical implications of different methods for preparing plant cell wall material. Carbohydr. Polym. 2021, 261, 117866. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Savouré, T.; Dornier, M.; Vachoud, L.; Collignan, A. Clustering of instrumental methods to characterize the texture and the rheology of slimy okra (Abelmoschus esculentus) suspensions. J. Texture Stud. 2020, 51, 426–443. [Google Scholar] [CrossRef]
- De Roeck, A.; Duvetter, T.; Fraeye, I.; Van der Plancken, J.; Sila, D.N.; Van Loey, A.; Hendrickx, M. Effect of high-pressure/high-temperature processing on chemical pectin conversions in relation to fruit and vegetable texture. Food Chem. 2009, 115, 207–213. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Sila, D.N.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part II—Structure–Function Relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Kiss, J. β-Eliminative Degradation of Carbohydrates Containing Uronic Acid Residues. Adv. Carbohyd. Chem. 1974, 29, 229–303. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic Degradation of Citrus Pectin and Pectate during Prolonged Heating: Effects of pH, Temperature, and Degree of Methyl Esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef] [PubMed]
- Krall, S.M.; McFeeters, R.F. Pectin Hydrolysis: Effect of Temperature, Degree of Methylation, pH, and Calcium on Hydrolysis Rates. J. Agric. Food Chem. 1998, 46, 1311–1315. [Google Scholar] [CrossRef]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of pectin properties and processing conditions on thermal pectin degradation. Food Chem. 2007, 105, 555–563. [Google Scholar] [CrossRef]
- Zid, M.B.; Dhuique-Mayer, C.; Bellagha, S.; Sanier, C.; Collignan, A.; Servent, A.; Dornier, M. Effects of Blanching on Flavanones and Microstructure of Citrus aurantium Peels. Food Bioprocess Technol. 2015, 8, 2246–2255. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1999.
- Faravash, R.S.; Ashtiani, F.Z. The influence of acid volume, ethanol-to-extract ratio and acid-washing time on the yield of pectic substances extraction from peach pomace. Food Hydrocoll. 2008, 22, 196–202. [Google Scholar] [CrossRef]
- Pagan, J.; Ibarz, A.; Llorca, M.; Coll, L. Quality of industrial pectin extracted from peach pomace at different pH and temperatures. J. Sci. Food Agric. 1999, 79, 1038–1042. [Google Scholar] [CrossRef]
- Approximate pH of Foods and Food Products; US FDA/CFSAN: Silver Spring, MD, USA, 2003.
- Waffenschmidt, S.; Jaenicke, L. Assay of Reducing Sugars in the Nanomole Range with 2,2′-Bicinchoninate. Anal. Biochem. 1987, 165, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Klavons, J.A.; Bennett, R.D. Determination of Methanol Using Alcohol Oxidase and Its Application to Methyl Ester Content of Pectins. J. Agric. Food Chem. 1986, 34, 597–599. [Google Scholar] [CrossRef]
- Jiménez, N.; Bohuon, P.; Lima, J.; Dornier, M.; Vaillant, F.; Pérez, A.M. Kinetics of Anthocyanin Degradation and Browning in Reconstituted Blackberry Juice Treated at High Temperatures (100–180 °C). J. Agric. Food Chem. 2010, 58, 2314–2322. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- van Boekel, M.A.J.S. Kinetic Modeling of Reactions in Foods, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- de Levie, R. Advanced Excel for Scientific Data Analysis, 3rd ed.; Atlantic Academic LLC: Virginia Beach, VA, USA, 2012. [Google Scholar]
- U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center. FoodData Central. 2024. Available online: https://fdc.nal.usda.gov/ (accessed on 1 July 2024).
- Constenla, D.; Lozano, J.E. Kinetic model of pectin demethylation. Lat. Am. Appl. Res. 2003, 33, 91–95. [Google Scholar]


| Temperature (°C) | Time (min) | ||||
|---|---|---|---|---|---|
| 70 | 0 | 120 | 210 | 300 | |
| 80 | 0 | 30 | 60 | 110 | 200 |
| 100 | 0 | 10 | 20 | 45 | 120 |
| 130 | 0 | 10 | 20 | 30 | 40 |
| T (°C) | kmet (min−1) | R2 |
|---|---|---|
| 70 | 3.8 × 10−4 (1.5 × 10−5) | 0.99 |
| 80 | 1.3 ×10−3 (7.8 × 10−5) | 0.98 |
| 100 | 6.3 × 10−3 (1.5 × 10−4) | 0.99 |
| 130 | 4.6 × 10−2 (7.5 × 10−3) | 0.92 |
| T (°C) | khyd (min−1) | R2 |
|---|---|---|
| 70 | 5.62 × 10−5 (3.32 × 10−5) | 0.88 |
| 80 | 1.83 × 10−4 (5.74 × 10−5) | 0.93 |
| 100 | 7.30 × 10−4 (4.04 × 10−5) | 0.98 |
| 130 | 1.28 × 10−2 (8.27 × 10−4) | 0.97 |
| Demethylation | Depolymerization | |
|---|---|---|
| kref (min−1) | 4.37 × 10−3 (1.31 × 10−4) | 4.56 × 10−4 (5.19 × 10−5) |
| Ea (kJ mol−1) | 82.7 (1.1) | 117.3 (4.0) |
| R2 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic Markovic, M.; Servent, A.; Savoure, T.; Collignan, A.; Dornier, M.; Achir, N. Correlation between Kinetics of Pectin Degradation and Texture Loss of Okra (Abelmoschus esculentus L.) Puree during Thermal Treatments. Agriculture 2024, 14, 1687. https://doi.org/10.3390/agriculture14101687
Petrovic Markovic M, Servent A, Savoure T, Collignan A, Dornier M, Achir N. Correlation between Kinetics of Pectin Degradation and Texture Loss of Okra (Abelmoschus esculentus L.) Puree during Thermal Treatments. Agriculture. 2024; 14(10):1687. https://doi.org/10.3390/agriculture14101687
Chicago/Turabian StylePetrovic Markovic, Milena, Adrien Servent, Timoty Savoure, Antoine Collignan, Manuel Dornier, and Nawel Achir. 2024. "Correlation between Kinetics of Pectin Degradation and Texture Loss of Okra (Abelmoschus esculentus L.) Puree during Thermal Treatments" Agriculture 14, no. 10: 1687. https://doi.org/10.3390/agriculture14101687






