Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey and Honey-Based Samples
2.2. Physicochemical Parameters
2.3. Determination of 5-Hydroxymethylfurfural Content
2.4. Determination of Mineral Content
2.5. Determination of Total Phenolic Content
2.6. Determination of Total Flavonoid Content
2.7. Determination of Total Anthocyanin Content
2.8. DPPH Radical Scavenging Capacity
- A0 = Absorbance of radical blank
- A1 = Absorbance of test sample
2.9. Polyphenol Profile Analysis
2.10. Antibacterial Activity Assay
2.11. In Vitro Antiproliferative Assay—MTT Test
2.12. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Characterisation
3.2. Mineral Content
3.3. Antioxidant Potential
3.4. Polyphenol Profile
3.5. Antibacterial Activity
3.6. Antiproliferative Activity
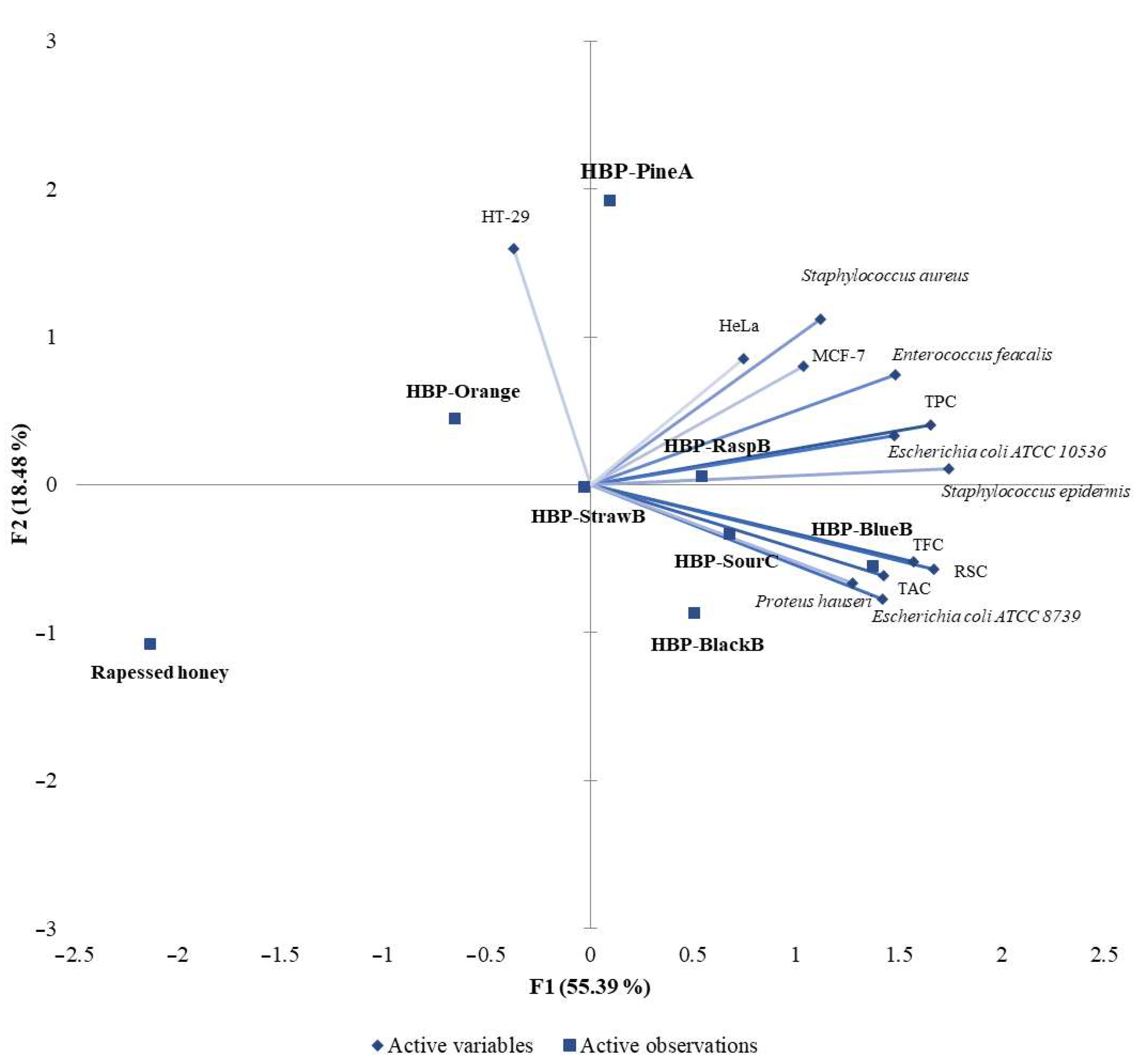
3.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Wojtczuk, M.; Roszko, M.; Bryła, M.; Trajkovska Petkoska, A. Recent Advances and Opportunities Related to the Use of Bee Products in Food Processing. Food Sci. Nutr. 2023, 11, 4372–4397. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and Antimicrobial Effects of Phenolic Compounds Extracts of Northeast Portugal Honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and Therapeutic Effects of Honey Produced by Honey Bees and Stingless Bees: A Comparative Review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular Mechanism-Based Therapeutic Properties of Honey. Biomed. Pharmacother. 2020, 130, 110590. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Hegazi, N.M.; Abd Elghani, G.E.; Farag, M.A. Manuka Honey, a Unique Mono-Floral Honey. A Comprehensive Review of Its Bioactives, Metabolism, Action Mechanisms, and Therapeutic Merits. Food Biosci. 2021, 42, 101038. [Google Scholar] [CrossRef]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, M.; Hajam, Y.A.; Kumar, R. Honey Infused with Herbs: A Boon to Cure Pathological Diseases. Heliyon 2024, 10, e23302. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T.; Stajčić, S.; Vulić, J.; Djilas, S.; Štajner, D.; Popović, B. Evaluation of Phenolic Content, Antioxidant Activity and Sensory Characteristics of Serbian Honey-Based Product. Ind. Crops Prod. 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Guldas, M.; Gurbuz, O.; Cakmak, I.; Yildiz, E.; Sen, H. Effects of Honey Enrichment with Spirulina Platensis on Phenolics, Bioaccessibility, Antioxidant Capacity and Fatty Acids. LWT 2022, 153, 112461. [Google Scholar] [CrossRef]
- Guldas, M.; Demircan, H.; Cakmak, I.; Oral, R.A.; Yildiz, E.; Gurbuz, O.; Tosunoglu, H.; Cavus, F.; Sen, H. Antioxidant and Bioaccessibility Characteristics of Functional Fruit and Vegetable Honeys Produced by Innovative Method. Food Biosci. 2022, 48, 101732. [Google Scholar] [CrossRef]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-Derived Active Substances Incorporated as Antioxidant, Antibacterial or Antifungal Components in Coatings/Films for Food Packaging Applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas Comosus): A Comprehensive Review of Nutritional Values, Volatile Compounds, Health Benefits, and Potential Food Products. Int. Food Res. 2020, 137, 109675. [Google Scholar] [CrossRef]
- Kawasaki, H.; Shimanouchi, T.; Kimura, Y. Recent Development of Optimization of Lyophilization Process. J. Chem. 2019, 2019, 9502856. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre: Bern, Switzerland, 2009. [Google Scholar]
- Rufián-Henares, J.A.; de la Cueva, S.P. Assessment of Hydroxymethylfurfural Intake in the Spanish Diet. Food Addit. Contam. Part A 2008, 25, 1306–1312. [Google Scholar] [CrossRef]
- Petisca, C.; Henriques, A.R.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I.M.P.L.V.O. Assessment of Hydroxymethylfurfural and Furfural in Commercial Bakery Products. J. Food Compos. Anal. 2014, 33, 20–25. [Google Scholar] [CrossRef]
- Ariffin, A.A.; Ghazali, H.M.; Kavousi, P. Validation of a HPLC Method for Determination of Hydroxymethylfurfural in Crude Palm Oil. Food Chem. 2014, 154, 102–107. [Google Scholar] [CrossRef]
- Tomasini, D.; Sampaio, M.R.F.; Caldas, S.S.; Buffon, J.G.; Duarte, F.A.; Primel, E.G. Simultaneous Determination of Pesticides and 5-Hydroxymethylfurfural in Honey by the Modified QuEChERS Method and Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2012, 99, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.; Novaković, A.; Ikonić, P.; Peulić, T.; Škrobot, D.; Radišić, P.; Šikoparija, B.; Jovanov, P.; Maravić, N.; Marić, A. Geographical Origin Authentication of Honey Produced in the Region of Rtanj Mountain (Serbia). J. Food Compos. Anal. 2024, 129, 106088. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Barać, G.; Mastilović, J.; Kevrešan, Ž.; Milić, B.; Kovač, R.; Milović, M.; Kalajdžić, J.; Bajić, A.; Magazin, N.; Keserović, Z. Effects of Plant Growth Regulators on Plum (Prunus. domestica. L.) Grown on Two Rootstocks at Harvest and at the Postharvest Period. Horticulturae 2022, 8, 621. [Google Scholar] [CrossRef]
- Noor, N.; Sarfraz, R.A.; Ali, S.; Shahid, M. Antitumour and Antioxidant Potential of Some Selected Pakistani Honeys. Food Chem. 2014, 143, 362–366. [Google Scholar] [CrossRef]
- Nyarko, K.; Boozer, K.; Greenlief, C.M. Profiling of the Polyphenol Content of Honey from Different Geographical Origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. In Honey Analysis; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Codex Standard 12-1981; Revised Codex Standards for Honey. Codex Alimentarius Commission: Rome, Italy, 2019.
- Missio da Silva, P.; Gonzaga, L.V.; Biluca, F.C.; Schulz, M.; Vitali, L.; Micke, G.A.; Oliveira Costa, A.C.; Fett, R. Stability of Brazilian Apis. mellifera L. Honey during Prolonged Storage: Physicochemical Parameters and Bioactive Compounds. LWT 2020, 129, 109521. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial Effect of Different Types of Honey on Staphylococcus Aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef]
- da Silva, I.C.; Conceição, E.O.A.; Pereira, D.S.; Rogez, H.; Muto, N.A. Evaluation of the Antimicrobial Capacity of Bacteria Isolated from Stingless Bee (Scaptotrigona Aff. Postica) Honey Cultivated in Açai (Euterpe Oleracea) Monoculture. Antibiotics 2023, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) Levels in Honey and Other Food Products: Effects on Bees and Human Health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality Characterization of Artisanal and Retail Turkish Blossom Honeys: Determination of Physicochemical, Microbiological, Bioactive Properties and Aroma Profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- Godoy, C.A.; Valderrama, P.; Boroski, M. HMF Monitoring: Storage Condition and Honey Quality. Food Anal. Methods 2022, 15, 3162–3176. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Barłowska, J.; Litwińczuk, Z. Concentration of Minerals in Nectar Honeys from Direct Sale and Retail in Poland. Biol. Trace Elem. Res. 2018, 186, 579–588. [Google Scholar] [CrossRef]
- Pereira, C.C.; do Nascimento da Silva, E.; de Souza, A.O.; Vieira, M.A.; Ribeiro, A.S.; Cadore, S. Evaluation of the Bioaccessibility of Minerals from Blackberries, Raspberries, Blueberries and Strawberries. J. Food Compos. Anal. 2018, 68, 73–78. [Google Scholar] [CrossRef]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of Phenolic Compounds, Antioxidant and Anti-Inflammatory Activities in Stingless Bee Honey (Meliponinae). Int. Food Res. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of Phenolic Acids and Flavonoids in Honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef]
- Gašić, U.; Stanković, D.; Dabić, D.; Milojković-Opsenica, D.; Natić, M.; Tešić, Z.; Mutić, J. Analytical Possibilities for the Relative Estimation of the Antioxidative Capacity of Honey Varieties Harvested in Different Regions of Serbia. J. Serb. Chem. Soc. 2016, 81, 567–574. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, Antioxidant Activity and Multielement Analysis of Two Types of Early Spring Honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Đogo Mračević, S.; Krstić, M.; Lolić, A.; Ražić, S. Comparative Study of the Chemical Composition and Biological Potential of Honey from Different Regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Higbee, J.; Brownmiller, C.; Solverson, P.; Howard, L.; Carbonero, F. Polyphenolic Profiles of a Variety of Wild Berries from the Pacific Northwest Region of North America. Curr. Res. Food Sci. 2023, 7, 100564. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dżugan, M. Antiviral and Antibacterial Effect of Honey Enriched with Rubus Spp. as a Functional Food with Enhanced Antioxidant Properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of Phenolic Compounds from Bilberry (Vaccinium. myrtillus. L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Aly, A.A.; Ali, H.G.M.; Eliwa, N.E.R. Phytochemical Screening, Anthocyanins and Antimicrobial Activities in Some Berries Fruits. J. Food Meas. Charact. 2019, 13, 911–920. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Wang, W.; Lyu, L.; Wu, W.; Li, W. The Extraction and High Antiproliferative Effect of Anthocyanin from Gardenblue Blueberry. Molecules 2023, 28, 2850. [Google Scholar] [CrossRef]
- Marić, A.; Jovanov, P.; Sakač, M.; Novaković, A.; Hadnađev, M.; Pezo, L.; Mandić, A.; Milićević, N.; Đurović, A.; Gadžurić, S. A Comprehensive Study of Parameters Correlated with Honey Health Benefits. RSC Adv. 2021, 11, 12434–12441. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of Total Phenolics, Anthocyanins, Ellagic Acid and Radical Scavenging Capacity in Various Raspberry (Rubus spp.) Cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Cook, N.C.; Samman, S. Flavonoids–Chemistry, Metabolism, Cardioprotective Effects and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- de Mello Andrade, J.M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 253–265. [Google Scholar]
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a Model to Understand the Synergies Underlying the Antibacterial Mechanism of H2O2-Producing Honeys. Sci. Rep. 2020, 10, 17692. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical Properties, Antibacterial and Cellular Antioxidant Activities of Buckwheat Honey in Comparison to Manuka Honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; de la Luz Cadiz-Gurrea, M.; Mahmoodi-Khaledi, E. Potential Antimicrobial Activity of Honey Phenolic Compounds against Gram Positive and Gram Negative Bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Das, Q.; Islam, M.R.; Marcone, M.F.; Warriner, K.; Diarra, M.S. Potential of Berry Extracts to Control Foodborne Pathogens. Food Control 2017, 73, 650–662. [Google Scholar] [CrossRef]
- do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as Potential Antiproliferative Agents: Scientific Trends. Curr. Opin. Food Sci. 2018, 24, 26–35. [Google Scholar] [CrossRef]
- Haiyan, S.; Funing, M.; Keming, L.; Wei, S.; Guiying, X.; Rulin, Z.; Shenghe, C. Growth of Breast Cancer Cells Inhibited by Bromelains Extracted from the Different Tissues of Pineapple. Folia Biol. 2020, 68, 81–88. [Google Scholar] [CrossRef]
- Gani, M.B.A.; Nasiri, R.; Hamzehalipour Almaki, J.; Majid, F.A.A.; Marvibaigi, M.; Amini, N.; Chermahini, S.H.; Mashudin, M. In Vitro Antiproliferative Activity of Fresh Pineapple Juices on Ovarian and Colon Cancer Cell Lines. Int. J. Pept. Res. Ther. 2015, 21, 353–364. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.-B.; Deng, G.-F.; Ling, W.-H.; Wu, S.; Xu, X.-R.; Chen, F. Antiproliferative Activity of Peels, Pulps and Seeds of 61 Fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Reguengo, L.M.; do Nascimento, R.D.; da Fonseca Machado, A.P.; Junior, M.R. Signaling Pathways and the Potential Anticarcinogenic Effect of Native Brazilian Fruits on Breast Cancer. Int. Food Res. 2022, 155, 111117. [Google Scholar] [CrossRef]
| Honey or HBP * | Moisture (%) | Electrical Conductivity (mS/cm) | pH | Free Acidity (meq/kg) | HMF (mg/kg) |
|---|---|---|---|---|---|
| Rapeseed honey | 19.4 ± 0.20 bc | 0.21 ± 0.01 a | 3.78 ± 0.09 cba | 6.00 ± 0.20 a | 5.02 ± 0.05 a |
| HBP-BlackB | 19.8 ± 0.06 c | 0.37 ± 0.02 b | 3.61 ± 0.20 ba | 18.0 ± 0.79 cd | 5.81 ± 0.12 bc |
| HBP-Orange | 19.6 ± 0.35 bc | 0.60 ± 0.01 c | 3.90 ± 0.07 cb | 16.7 ± 0.38 c | 5.93 ± 0.35 bc |
| HBP-PineA | 16.3 ± 0.40 a | 0.35 ± 0.03 b | 3.97 ± 0.04 c | 12.1 ± 0.27 b | 5.05 ± 0.03 a |
| HBP-RaspB | 18.7 ± 0.20 b | 0.55 ± 0.05 c | 3.74 ± 0.10 cba | 30.7 ± 1.41 g | 6.33 ± 0.14 c |
| HBP-BlueB | 19.2 ± 0.60 bc | 0.36 ± 0.04 b | 3.60 ± 0.06 a | 19.3 ± 0.80 de | 7.10 ± 0.44 d |
| HBP-StrawB | 20.0 ± 0.15 c | 0.58 ± 0.04 c | 3.96 ± 0.08 c | 20.4 ± 0.42 e | 5.61 ± 0.05 ab |
| HBP-SourC | 19.3 ± 0.17 bc | 0.42 ± 0.02 b | 3.99 ± 0.14 c | 24.4 ± 0.59 f | 6.32 ± 0.07 c |
| Honey or HBP * | Na | Ca | K | Mg | Zn | Mn | Fe | Cu | Total |
|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | |||||||||
| Rapeseed honey | 44.0 ± 13.8 a | 101 ± 15.7 a | 307 ± 50.4 a | 32.8 ± 5.1 a | 0.25 ± 0.04 a | 0.37 ± 0.06 a | 2.65 ± 0.63 a | 0.08± 0.01 a | 488 ± 83.8 |
| HBP-BlackB | 63.5 ± 10.4 bc | 178 ± 27.5 c | 627 ± 103 bc | 114 ± 17.6 e | 1.89 ± 0.29 b | 17.8 ± 2.74 e | 4.77 ± 0.74 b | 0.66 ± 0.10 d | 1008 ± 158 |
| HBP-Orange | 88.6 ± 14.5 e | 324 ± 50.2 d | 875 ± 144 de | 93.6 ± 14.5 d | 2.27 ± 0.35 bc | 0.62 ± 0.10 a | 5.33 ± 0.82 b | 0.52 ± 0.08 cd | 1390 ± 218 |
| HBP-PineA | 87.2 ± 14.3 de | 126 ± 19.5 ab | 706 ± 116 cd | 72.7 ± 11.3 b | 2.77 ± 0.43 c | 12.4 ± 1.90 c | 4.44 ± 0.68 ab | 1.09 ± 0.17 e | 1013 ± 159 |
| HBP-RaspB | 85.0 ± 14.0 de | 160 ± 24.8 bc | 1017 ± 167 e | 87.0 ± 13.5 c | 2.75 ± 0.42 c | 15.3 ± 2.36 d | 5.48 ± 0.84 b | 0.52 ± 0.08 cd | 1373 ± 215 |
| HBP-BlueB | 94.2 ± 15.5 e | 185 ± 28.7 c | 466 ± 76.5 ab | 66.9 ± 10.4 b | 4.12 ± 0.63 d | 15.8 ± 2.44 d | 5.52 ± 0.85 b | 0.31 ± 0.05 bc | 838 ± 131 |
| HBP-StrawB | 75.4 ± 12.4 cd | 172 ± 26.7 c | 1029 ± 166 e | 95.8 ± 14.8 d | 1.70 ± 0.26 b | 2.04 ± 0.31 b | 10.4 ± 1.60 c | 0.30 ± 0.05 bc | 1387 ± 217 |
| HBP-SourC | 62.5 ± 10.3 b | 155 ± 23.9 bc | 628 ± 103 bc | 72.8 ± 11.3 b | 0.80 ± 0.12 a | 0.71 ± 0.11 a | 5.61 ± 0.86 b | 0.20 ± 0.03 ab | 925 ± 145 |
| Honey or HBP * | TPC (mg GAE/100 g) | TFC (mg CAE/100 g) | TAC (mg EC/100 g) | RSC (% of DPPH Inhibition) |
|---|---|---|---|---|
| Rapeseed honey | 12.4 ± 0.51 a | 1.83 ± 0.27 a | n.d. | 35.9 ± 0.77 a |
| HBP-BlackB | 93.6 ± 0.90 e | 17.7 ± 1.12 d | 125 ± 0.75 d | 69.3 ± 1.18 e |
| HBP-Orange | 83.5 ± 0.32 c | 8.17 ± 0.38 b | 22.1 ± 1.61 a | 45.0 ± 0.28 b |
| HBP-PineA | 80.7 ± 0.38 b | 7.12 ± 0.59 b | 20.1 ± 1.07 a | 46.6 ± 0.25 b |
| HBP-RaspB | 85.8 ± 0.72 d | 12.9 ± 0.41 c | 85.5 ± 2.27 c | 58.6 ± 0.54 c |
| HBP-BlueB | 98.8 ± 0.66 f | 34.9 ± 0.89 e | 299 ± 3.14 e | 81.0 ± 0.46 g |
| HBP-StrawB | 87.3 ± 0.64 d | 11.0 ± 0.96 b | 50.7 ± 2.54 b | 60.9 ± 0.16 d |
| HBP-SourC | 102 ± 0.18 g | 17.8 ± 1.01 c | 84.6 ± 0.67 c | 77.0 ± 0.34 f |
| Honey or HBP * | Rapeseed Honey | HBP- BlackB | HBP- Orange | HBP- PineA | HBP- RaspB | HBP- BlueB | HBP- StrawB | HBP- SourC |
|---|---|---|---|---|---|---|---|---|
| Ellagic acid (mg/kg) | 0.15 ± 0.06 a | 1.38 ± 0.01 ab | n.d. | 1.12 ± 0.11 ab | 38.4 ± 1.11 d | 2.36 ± 0.31 b | 9.86 ± 0.59 c | 0.47 ± 0.08 a |
| Rutin (mg/kg) | 0.30 ± 0.05 a | 3.46 ± 0.29 c | n.d. | 0.59 ± 0.06 a | 1.73 ± 0.07 b | 3.83 ± 0.38 c | 0.33 ± 0.04 a | 0.56 ± 0.07 a |
| Naringin (mg/kg) | n.d. | 0.13 ± 0.03 a | n.d. | n.d. | n.d. | 0.52 ± 0.05 b | n.d. | 0.21 ± 0.02 a |
| Naringenin (mg/kg) | 0.01 ± 0.00 a | 0.04 ± 0.003 a | 0.04 ±0.003 a | 0.03 ± 0.009 a | 0.01 ± 0.006 a | 0.22 ± 0.01 b | 0.08 ± 0.003 a | 0.06 ± 0.008 a |
| Quercetin (mg/kg) | 0.10 ± 0.01 a | 0.30 ± 0.01 ab | 0.29 ± 0.04 ab | 0.11 ± 0.02 a | 0.32 ± 0.04 ab | 2.23 ± 0.21 c | 0.49 ± 0.03 b | 0.069 ± 0.005 a |
| Protocatechuic acid (mg/kg) | n.d. | 0.27 ± 0.02 b | 0.35 ± 0.05 bc | 0.09 ± 0.01 a | 0.11 ± 0.01 a | 1.26 ± 0.09 d | 0.38 ± 0.01 c | 0.34 ± 0.02 bc |
| Neochlorogenic acid (mg/kg) | n.d. | 0.68 ± 0.07 b | 0.03 ± 0.00 a | n.d. | n.d. | n.d. | n.d. | 0.03 ± 0.00 a |
| Caffeic acid (mg/kg) | 0.47 ± 0.04 a | 0.59 ± 0.05 ab | 1.30 ± 0.09 c | 0.82 ± 0.07 b | 0.90 ± 0.04 b | 4.28 ± 0.18 d | 0.58 ± 0.02 ba | 0.80 ± 0.06 ab |
| Chlorogenic acid (mg/kg) | 0.11 ± 0.01 a | 4.31 ± 0.16 d | 0.36 ± 0.07 abc | 0.25 ± 0.02 ab | 0.19 ± 0.02 ab | 0.38 ± 0.04 bc | 0.22 ± 0.03 ab | 0.53 ± 0.06 c |
| p-Cumaric acid (mg/kg) | 0.21 ± 0.03 a | 0.83 ± 0.09 ab | 1.36 ± 0.11 b | 1.43 ± 0.15 b | 2.21 ± 0.23 c | 1.35 ± 0.11 b | 1.28 ± 0.12 b | 12.8 ± 0.31 d |
| Ferulic acid (mg/kg) | 0.20 ± 0.02 a | 1.04 ± 0.13 c | 0.23 ± 0.03 ab | 0.63 ± 0.07 bc | 2.98 ± 0.19 d | 3.02 ± 0.17 d | 0.32 ± 0.02 ab | 0.30 ± 0.02 ab |
| Sinapic acid (mg/kg) | 0.15 ± 0.01 a | 0.16 ± 0.02 a | 0.28 ± 0.02 ab | 1.15 ± 0.15 d | 0.16 ± 0.02 a | 0.53 ± 0.03 c | 0.41 ± 0.03 bc | 0.15 ± 0.02 a |
| Quercetin-3-arabinoglucoside (mg/kg) | n.d. | n.d. | n.d. | n.d. | n.d. | 1.63 ± 0.18 | n.d. | n.d. |
| Quercetin-3-glucuronide (mg/kg) | n.d. | n.d. | 0.25 ± 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin-3-glucoside (mg/kg) | n.d. | 0.15 ± 0.02 a | 1.61 ± 0.15 b | 0.23 ± 0.02 a | n.d. | 1.83 ± 0.14 b | 0.02 ± 0.00 a | n.d. |
| Quercetin-3-rutinoside (mg/kg) | n.d. | 3.83 ± 0.20 c | n.d. | n.d. | n.d. | 0.92 ± 0.08 b | 0.36 ± 0.03 a | 0.49 ± 0.03 a |
| Total (mg/kg) | 1.70 ± 0.11 | 17.2 ± 0.24 | 6.09 ± 0.05 | 6.46 ± 0.18 | 47.0 ± 0.98 | 24.3 ± 0.82 | 14.3 ± 0.46 | 16.7 ± 0.28 |
| Honey or HBP * | MIC (%) Against Different Strains of Bacteria | |||||
|---|---|---|---|---|---|---|
| Escherichia coli ATCC 8739 | Escherichia coli ATCC 10536 | Enterococcus feacalis ATCC 29212 | Staphylococcus aureus ATCC 25923 | Staphylococcus epidermidis ATCC 12228 | Proteus hauseri ATCC 13315 | |
| Rapeseed honey | >25 | >25 | >25 | >25 | >25 | >25 |
| HBP-BlackB | 25 | 25 | 25 | 25 | 12.5 | 25 |
| HBP-Orange | >25 | >25 | 25 | 25 | 25 | 25 |
| HBP-PineA | >25 | 25 | 25 | 12.5 | 25 | >25 |
| HBP-RaspB | 25 | 25 | 25 | 12.5 | 12.5 | 25 |
| HBP-BlueB | 25 | 25 | 25 | 25 | 12.5 | 25 |
| HBP-StrawB | >25 | 25 | 25 | 12.5 | 25 | 25 |
| HBP-SourC | 25 | 25 | 25 | 25 | 12.5 | 25 |
| Honey or HBP * | Cell Lines | |||
|---|---|---|---|---|
| MCF-7 | HT-29 | HeLa | MRC-5 | |
| Rapeseed honey | >100 e | >100 e | >100 d | >100 b |
| HBP-BlackB | >100 e | >100 e | >100 d | >100 b |
| HBP-Orange | >100 e | 48.4 ± 2.09 c | >100 d | >100 b |
| HBP-PineA | 9.04 ± 0.16 a | 28.3 ± 0.91 a | 34.2 ± 1.25 a | >100 b |
| HBP-RaspB | 24.6 ± 0.52 b | >100 e | 79.9 ± 3.33 c | >100 b |
| HBP-BlueB | 9.95 ± 0.24 a | >100 e | 23.1 ± 0.66 b | >100 b |
| HBP-StrawB | >100 e | >100 e | >100 d | >100 b |
| HBP-SourC | 30.8 ± 1.13 c | 85.2 ± 3.05 d | >100 d | >100 b |
| Standard glucose | 35.6 ± 2.58 d | 32.0 ± 0.40 b | 33.9 ± 1.31 a | 43.7 ± 3.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marić, A.; Sakač, M.; Jovanov, P.; Đermanović, B.; Teslić, N.; Plavšić, D.; Jakimov, D. Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits. Agriculture 2024, 14, 2117. https://doi.org/10.3390/agriculture14122117
Marić A, Sakač M, Jovanov P, Đermanović B, Teslić N, Plavšić D, Jakimov D. Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits. Agriculture. 2024; 14(12):2117. https://doi.org/10.3390/agriculture14122117
Chicago/Turabian StyleMarić, Aleksandar, Marijana Sakač, Pavle Jovanov, Branislava Đermanović, Nemanja Teslić, Dragana Plavšić, and Dimitar Jakimov. 2024. "Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits" Agriculture 14, no. 12: 2117. https://doi.org/10.3390/agriculture14122117
APA StyleMarić, A., Sakač, M., Jovanov, P., Đermanović, B., Teslić, N., Plavšić, D., & Jakimov, D. (2024). Functional Properties of Rapeseed Honey Enriched with Lyophilized Fruits. Agriculture, 14(12), 2117. https://doi.org/10.3390/agriculture14122117







