Tolerance of Forage Grass to Abiotic Stresses by Melatonin Application: Effects, Mechanisms, and Progresses
Abstract
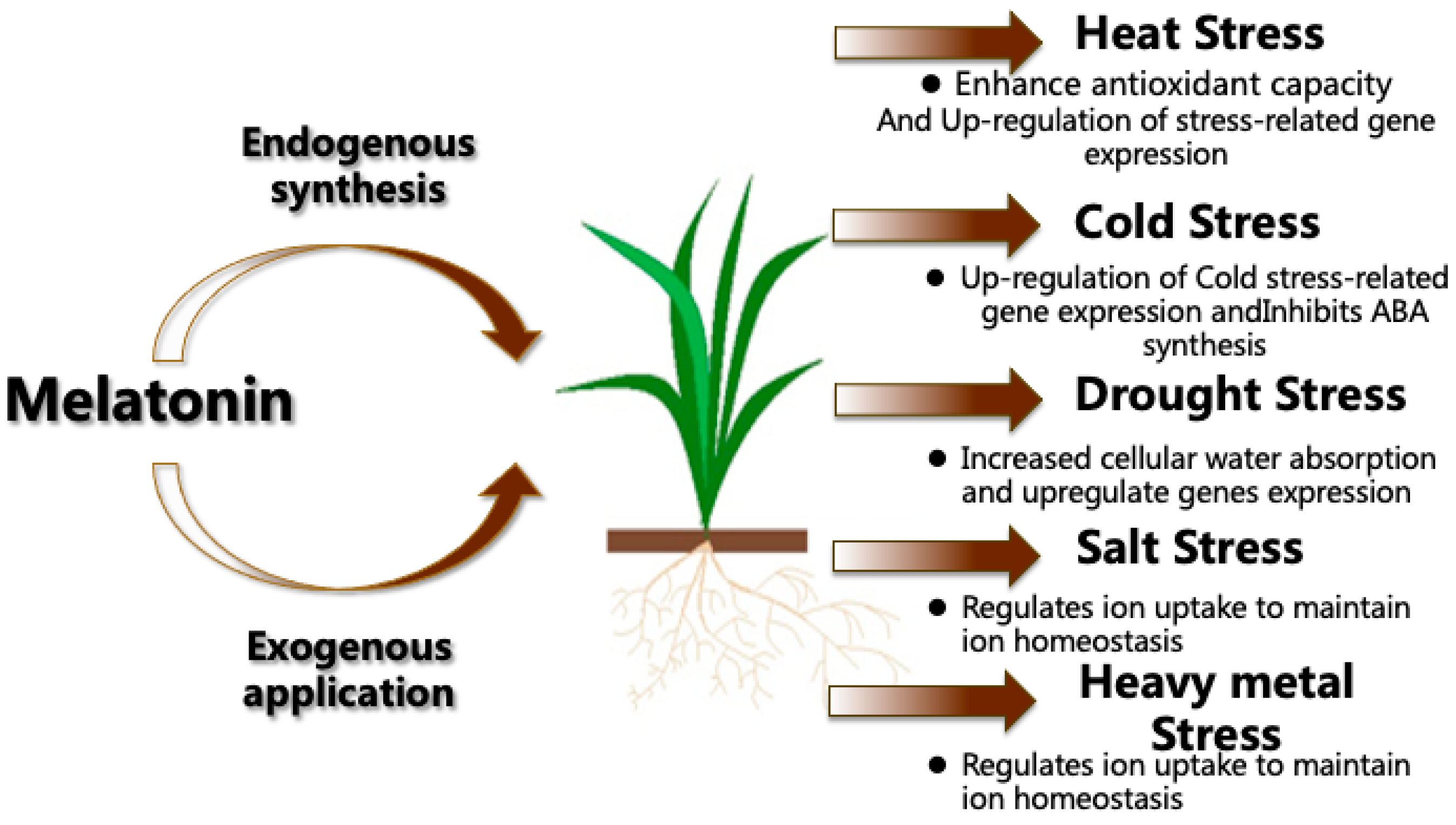
:1. Introduction
2. Melatonin Regulates the Growth and Development of Forage Grass
2.1. Seed Germination and Seedling Growth
2.2. The Mechanism of Melatonin Regulation in the Growth and Development Stage
3. Melatonin Regulates the Photosynthesis of Forage Grass
3.1. The Effects of Melatonin Application on Photosynthesis
3.2. The Mechanism of Melatonin Regulation on Photosynthesis
4. Melatonin Regulates the Antioxidant Mechanism of Forage Grass
The Effects of Melatonin on Antioxidant Mechanism
5. Melatonin Regulates Ion Homeostasis of Forage Grass
5.1. The Effects of Melatonin on Ion Homeostasis
5.2. The Mechanism of Melatonin Regulation on Ion Homeostasis
6. Melatonin Interacts with Other Plant Hormones
6.1. The Effects of Melatonin on Other Plant Hormones
6.2. The Mechanism of Melatonin Regulation on Other Plant Hormones
7. Conclusions
Funding
Conflicts of Interest
References
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.N. Climate Change and Abiotic Stress Mechanisms in Plants. Emerg. Top. Life Sci. 2019, 3, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, K.; Samireddypalle, A. Impact of Climate Change on Forage Availability for Livestock. In Climate Change Impact on Livestock: Adaptation and Mitigation; Sejian, V., Gaughan, J., Baumgard, L., Prasad, C., Eds.; Springer: New Delhi, India, 2015; pp. 97–112. [Google Scholar]
- Dutta, P.; Chakraborti, S.; Chaudhuri, K.M.; Mondal, S. Physiological Responses and Resilience of Plants to Climate Change. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 3–20. [Google Scholar]
- Yang, T.; Dong, J.; Huang, L.; Li, Y.; Yan, H.; Zhai, J.; Wang, J.; Jin, Z.; Zhang, G. A Large Forage Gap in Forage Availability in Traditional Pastoral Regions in China. Fundam. Res. 2023, 3, 188–200. [Google Scholar] [CrossRef]
- Miao, L.; Sun, Z.; Ren, Y.; Schierhorn, F.; Müller, D. Grassland Greening on the Mongolian Plateau despite Higher Grazing Intensity. Land Degrad. Dev. 2021, 32, 792–802. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Bai, Y.; Wang, P. Assessing the Sustainability of Grass-Based Livestock Husbandry in Hulun Buir, China. Phys. Chem. Earth Parts A/B/C 2020, 120, 102907. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens melanocyteS1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Back, K.; Yool, L.H.; Jin, H.O. Functional Characterization of Tobacco (Nicotiana benthamiana) Serotonin N-Acetyltransferases (NbSNAT1 and NbSNAT2). Melatonin Res. 2021, 4, 507–521. [Google Scholar] [CrossRef]
- Chen, S. Effects of Exogenous Melatonin in the Germination and Seedling Growth of Bermudagrass under Lead Stress Treatments. Master’s Thesis, Sichuan Agriculture University, Ya’an, China, 2021. [Google Scholar]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile Roles of Melatonin in Growth and Stress Tolerance in Plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Cen, H.; Wang, T.; Liu, H.; Wang, H.; Tian, D.; Li, X.; Cui, X.; Guan, C.; Zang, H.; Li, M.; et al. Overexpression of MsASMT1 Promotes Plant Growth and Decreases Flavonoids Biosynthesis in Transgenic Alfalfa (Medicago sativa L.). Front. Plant Sci. 2020, 11, 489. [Google Scholar] [CrossRef]
- Mackiewicz-Walec, E.; Olszewska, M. Biostimulants in the Production of Forage Grasses and Turfgrasses. Agriculture 2023, 13, 1796. [Google Scholar] [CrossRef]
- Leal, V.; Santos, D.; Paim, T.; dos Santos, L.; Alves, E.; Claudio, F.; Junior, G.; Fernandes, P.; Salviano, P. Economic Results of Forage Species Choice in Crop-Livestock Integrated Systems. Agriculture 2023, 13, 637. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a Regulator of Plant Ionic Homeostasis: Implications for Abiotic Stress Tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, Q. Effect of Exogenous Melatonin on Germination of Pennisetuma Alopecurides under NaCl Stress. J. China Agric. Univ. 2014, 19, 54–60. [Google Scholar]
- Xiao, Z.; Sui, X.; Shi, G.; Chen, A.; Wu, L.; Zhang, B. Effects of Different Concentrations and Immersion Durations of Exogenous Melatonin on Seed Germination of Bromus Inermis under Drought Stress. Acta Agrestia Sin. 2022, 30, 655. [Google Scholar]
- Shi, Y. Effects of Melatonin on Seed Germination and Seedling Growth and Physiological Charateristics of ALFALFA under Salt Stress. Master’s Thesis, Yangzhou University, Yangzhou, China, 2023. [Google Scholar]
- Roy, M.; Niu, J.; Irshad, A.; Kareem, H.A.; Hassan, M.U.; Xu, N.; Sui, X.; Guo, Z.; Amo, A.; Wang, Q. Exogenous Melatonin Protects Alfalfa (Medicago sativa L.) Seedlings from Drought-Induced Damage by Modulating Reactive Oxygen Species Metabolism, Mineral Balance and Photosynthetic Efficiency. Plant Stress 2021, 2, 100044. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A. Melatonin Enhances Seed Germination and Seedling Growth of Medicago Sativa under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef]
- Li, L. Effects of Melatonin Soaking on Alfalfa Seed Under NaCl Stress. Master’s Thesis, Xinjiang Agriculture University, Ürümqi, China, 2022. [Google Scholar] [CrossRef]
- Cen, H.; Wang, T.; Liu, H.; Tian, D.; Zhang, Y. Melatonin Application Improves Salt Tolerance of Alfalfa (Medicago sativa L.) by Enhancing Antioxidant Capacity. Plants 2020, 9, 220. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin Improves the Germination Rate of Cotton Seeds under Drought Stress by Opening Pores in the Seed Coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- Huangfu, L.; Zhang, Z.; Zhou, Y.; Zhang, E.; Chen, R.; Fang, H.; Li, P.; Xu, Y.; Yao, Y.; Zhu, M. Integrated Physiological, Metabolomic and Transcriptomic Analyses Provide Insights into the Roles of Exogenous Melatonin in Promoting Rice Seed Germination under Salt Stress. Plant Growth Regul. 2021, 95, 19–31. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin Confers Plant Tolerance against Cadmium Stress via the Decrease of Cadmium Accumulation and Reestablishment of microRNA-Mediated Redox Homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive Effects of Melatonin and Cytokinin on Alleviating Drought-Induced Leaf Senescence in Creeping Bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin Suppression of Heat-Induced Leaf Senescence Involves Changes in Abscisic Acid and Cytokinin Biosynthesis and Signaling Pathways in Perennial Ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial Effect of Melatonin on Growth and Chlorophyll Content in Wheat (Triticum aestivum L.) Grown under Salt Stress Conditions. Gesunde Pflanz. 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Sun, C.; Lv, T.; Huang, L.; Liu, X.; Jin, C.; Lin, X. Melatonin Ameliorates Aluminum Toxicity through Enhancing Aluminum Exclusion and Reestablishing Redox Homeostasis in Roots of Wheat. J. Pineal Res. 2020, 68, e12642. [Google Scholar] [CrossRef]
- Sehar, Z.; Fatma, M.; Khan, S.; Mir, I.; Abdi, G.; Khan, N. Melatonin Influences Methyl Jasmonate-Induced Protection of Photosynthetic Activity in Wheat Plants against Heat Stress by Regulating Ethylene-Synthesis Genes and Antioxidant Metabolism. Sci. Rep. 2023, 13, 7468. [Google Scholar] [CrossRef]
- Cao, X. Physiological Responses in Alfalfa to Exogenous Melatonin under Drought Stress. Master’s Thesis, Northwest A&F University, Yulin, China, 2021. [Google Scholar]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin Improves Antioxidant Capacity and Ion Homeostasis and Enhances Salt Tolerance in Maize Seedlings. Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial Effects of Melatonin in Overcoming Drought Stress in Wheat Seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Talaat, N. Co-Application of Melatonin and Salicylic Acid Counteracts Salt Stress-Induced Damage in Wheat (Triticum aestivum L.) Photosynthetic Machinery. J. Soil Sci. Plant Nutr. 2021, 21, 2893–2906. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-Mediated Nitric Oxide Improves Tolerance to Cadmium Toxicity by Reducing Oxidative Stress in Wheat Plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Zuo, Z.; Sun, L.; Wang, T.; Miao, P.; Zhu, X.; Liu, S.; Song, F.; Mao, H.; Li, X. Melatonin Improves the Photosynthetic Carbon Assimilation and Antioxidant Capacity in Wheat Exposed to Nano-ZnO Stress. Molecules 2017, 22, 1727. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a Quest for Stress Tolerance Genes: Membrane Transporters in Sensing and Adapting to Hostile Soils. J. Exp. Bot. 2016, 67, 1015–1031. [Google Scholar] [CrossRef]
- Fu, J.; Gates, R.N.; Xu, Y.; Hu, T. Diffusion Limitations and Metabolic Factors Associated with Inhibition and Recovery of Photosynthesis Following Cold Stress in Elymus Nutans Griseb. J. Photochem. Photobiol. B Biol. 2016, 163, 30–39. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2021, 22, 413–429. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin in Plants and Other Phototrophs: Advances and Gaps Concerning the Diversity of Functions. J. Exp. Bot. 2015, 66, 627–646. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Reina, M.; Martínez, A. A New Free Radical Scavenging Cascade Involving Melatonin and Three of Its Metabolites (3OHM, AFMK and AMK). Comput. Theor. Chem. 2018, 1123, 111–118. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sheeja, A.A.; Janardanan, D. Hydroxyl Radical Scavenging Activity of Melatonin and Its Related Indolamines. Free Radic. Res. 2020, 54, 373–383. [Google Scholar] [CrossRef]
- Dong, L.I.; Yanfang, W.; Hongtao, S.; Yilin, M.A.; Lijun, W.; Shimin, Z.; Ling, L. Alleviation Effects of Exogenous Melatonin and 2, 4-Epibrassinolide on Flue-Cured Tobacco Seedlings under Drought Stress. Acta Tabacaria Sin. 2019, 25, 77–85. [Google Scholar]
- Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules 2018, 23, 1580. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Zhou, Y.; Liu, P.; Yao, H.; Miao, Y.; Fu, J. Effects of Melatonin on Growth and Antioxidant System of Perennial Ryegrass Seedlings under Cold and Drought Stresses. Acta Agrestia Sin. 2020, 28, 1337. [Google Scholar]
- Arora, D.; Bhatla, S.C. Melatonin and Nitric Oxide Regulate Sunflower Seedling Growth under Salt Stress Accompanying Differential Expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Niu, J.; Chen, Z.; Guo, Z.; Xu, N.; Sui, X.; Roy, M.; Kareem, H.A.; Hassan, M.U.; Cui, J.; Wang, Q. Exogenous Melatonin Promotes the Growth of Alfalfa (Medicago sativa L.) under NaCl Stress through Multiple Pathways. Ecotoxicol. Environ. Saf. 2022, 242, 113938. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative Roles of Nitric Oxide and Hydrogen Sulfide in Melatonin-induced Tolerance of Pepper (Capsicum annuum L.) Plants to Iron Deficiency and Salt Stress Alone or in Combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.-X.; Pan, Y.-T.; Ahammed, G.J.; Qi, Z.-Y.; Zhou, J. Melatonin Alleviates Low-Sulfur Stress by Promoting Sulfur Homeostasis in Tomato Plants. Sci. Rep. 2018, 8, 10182. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K.; Burzyński, M. Different Effect of Cadmium and Copper on H+-ATPase Activity in Plasma Membrane Vesicles from Cucumis Sativu s Roots. J. Exp. Bot. 2012, 63, 4133–4142. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Gao, X.; Lan, J.; Fu, B. Comparative Physiological and Transcriptome Analysis Reveal the Molecular Mechanism of Melatonin in Regulating Salt Tolerance in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 919177. [Google Scholar] [CrossRef]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin Regulates Root Architecture by Modulating Auxin Response in Rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin Regulates Root Meristem by Repressing Auxin Synthesis and Polar Auxin Transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef]
- Wang, L.-F.; Li, T.-T.; Zhang, Y.; Guo, J.-X.; Lu, K.-K.; Liu, W.-C. CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 4014. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin Induces Disease Resistance to Botrytis Cinerea in Tomato Fruit by Activating Jasmonic Acid Signaling Pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Jalili, S.; Ehsanpour, A.A.; Morteza Javadirad, S. Melatonin Improves Salt Tolerance of in Vitro Root Culture of Alfalfa (Medicago sativa L.). Biologia 2023, 78, 961–970. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin Inhibits Seed Germination by Crosstalk with Abscisic Acid, Gibberellin, and Auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, D.; Delaplace, P.; Pan, Y.; Zhou, Y.; Tang, W.; Chen, K.; Chen, J.; Xu, Z.; Ma, Y.; et al. Melatonin Enhances Drought Tolerance by Affecting Jasmonic Acid and Lignin Biosynthesis in Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 202, 107974. [Google Scholar] [CrossRef]
| Plant | Type of Stress | MEL Level | Main Effects | Ref |
|---|---|---|---|---|
| Pennisetum | Salt | 300 μmol/L | The germination rate and germination potential of seeds increased by 20% and 10%, respectively | [19] |
| Awnless brome | Drought | 75 μmol/L-24 h | The germination rate, germination potential, germination index, and vitality index of seeds significantly improved | [20] |
| Agrostis stolonifera | Drought | 20 μmol/L | Increased light efficiency and chlorophyll content decreased the expression of chlorophyll degrading genes | [27] |
| Perennial ryegrass | Drought | 100 μmol/L | Increased chlorophyll content, enhanced antioxidant enzyme activity, and removed reactive oxygen species | [28] |
| Wheat | Salt | 200 μmol/L | The chlorophyll content of varieties Shahkar-13 and Khaista17 increased by 25% and 37%, respectively | [29] |
| Wheat | Aluminum | 30 μmol/L | Melatonin treatment decreased root tip-Al contents by 19.0% and 15.5% | [30] |
| Wheat | Heat | 100 μmol/L | MT treatment reduced H2O2 by 47.2%, O2 by 52.8%, and TBARS by 48.1%. | [31] |
| ‘Chifeng’ | Salt | 300 μmol/L | The germination rate and germination potential of seeds increased by 53.8% and 53.5%, and the germination index increased by 55.8% | [21] |
| ‘Sanditi’ | Drought | 100 μmol/L | Seedling height, stem diameter and root length significantly increased | [22] |
| ‘Xiangjiangdaye’ | Salt | 50 μmol/L | The germination rate, germination potential, and germination index increased by 20.97%, 18.67%, and 231%, respectively | [24] |
| ‘Zhongmu No.2’ | Salt | 50 μmol/L | The germination rate and germination potential of seeds increased by 42% and 19.9%, respectively | [25] |
| ‘Bara310S’ | Drought | 100 μmol/L | The chlorophyll content increased by 22.92%, and the photosynthetic efficiency improved | [32] |
| ‘Longdong’ | Drought | 100 μmol/L | The chlorophyll content increased by 58.22%, and the photosynthetic efficiency improved | [32] |
| Cotton | Salt | 50 μmol/L | The germination rate and vigor index of seeds increased by 11% and 17%, respectively | [26] |
| Rice | Salt | 5 μmol/L | The seed germination rate increased by 20% | [25] |
| Watermelon | Salt | 150 μmol/L | Pn and Gs increased by 40.8% and 21.6%, respectively | [33] |
| Maize | Salt | 1 μmol/L | Improved light and efficiency, and Pn increased by 19% | [34] |
| Plants | Main Gene | Effect | Ref |
|---|---|---|---|
| alfalfa | DRE, ARF, HD-ZF, MYB, REM | stress-related genes alleviate salt stress damage | [35] |
| alfalfa | MsASMT1 | melatonin receptor gene, overexpression could increase the endogenous melatonin content | [15] |
| Agrostis stolonifera | Chlorophyllase (Chlase), Pheophytinase (PPH), Chlorophyll peroxidase (Chl-PRX) | under drought stress, reduced the expression of chlorophyll degradation genes | [41] |
| perennial ryegrass | LpSAG12.1 and Lph36 | Under high-temperature stress, inhibited the expression of chlorophyll degradation genes | [27] |
| Lolium multiflorum | CBF9, CBF14, and COR14a | cold-response genes | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Li, L.; Guo, F.; Hou, X. Tolerance of Forage Grass to Abiotic Stresses by Melatonin Application: Effects, Mechanisms, and Progresses. Agriculture 2024, 14, 171. https://doi.org/10.3390/agriculture14020171
Fan Y, Li L, Guo F, Hou X. Tolerance of Forage Grass to Abiotic Stresses by Melatonin Application: Effects, Mechanisms, and Progresses. Agriculture. 2024; 14(2):171. https://doi.org/10.3390/agriculture14020171
Chicago/Turabian StyleFan, Yufeng, Lingling Li, Fenghui Guo, and Xiangyang Hou. 2024. "Tolerance of Forage Grass to Abiotic Stresses by Melatonin Application: Effects, Mechanisms, and Progresses" Agriculture 14, no. 2: 171. https://doi.org/10.3390/agriculture14020171




