Abstract
Nosema apis and Nosema ceranae are two well-known pathogens affecting the health of honeybees. To help understand how honeybee colonies are affected by these pathogens, the aim of this study was to analyze the impact of Nosema ceranae and Nosema apis in hives in the Apulian Region through a Citizen Science approach. First, a form about the health status of the beehives was filled out by beekeepers. After an inspection visit to confirm the signs observed by beekeepers, adult honeybee samples collected from beehives in four Apulian provinces (Taranto, Bari, Foggia and Brindisi) were subjected to light microscopy investigation for the detection of Nosema spp. spores and to molecular analysis using species-specific primers for the discrimination of Nosema apis spores from those of Nosema ceranae. Among the forty-eight samples, thirty-six tested positive for Nosema ceranae, and one sample tested positive for Nosema apis. The forms filled out by beekeepers revealed that only 5/36 beehives that tested positive for Nosema ceranae showed signs of depopulation and reduced honey production, while 19/36 had only low honey yield. This study provides data on Nosema apis and Nosema ceranae prevalence in Italy and correlates the presence of these intestinal pathogens with the most important problems encountered by local beekeepers.
1. Introduction
Apis mellifera is an important eusocial insect that plays a vital role in agriculture by pollinating crops and flowers worldwide [1]. Based on the importance of pollination in maintaining biodiversity, recently, the reduction in the number of honeybee colonies has become a cause for concern [2,3]. The COLOSS Honeybee Research Association monitors the rate of honeybee colony loss each year in many European and some non-European countries. During the last survey conducted in 2020, an overall loss rate of 18.1% was observed out of a total of 837,081 colonies analyzed. The loss rate was higher than the investigations conducted in the previous two years (16.7% in 2019 and 16.4% in 2018, respectively), underscoring the temporal decline in honeybee populations [3,4].
Possible causes of colony loss are abiotic and biotic factors. The abiotic factors include climate change or unfavorable weather conditions, such as long periods of cold and rainy weather, that can lead to a prolonged lack of pollen and nectar [3]. Moreover, the improper use of chemicals such as pesticides and herbicides has been recognized as a relevant factor in the loss of honeybee colonies [3]. Biotic factors, on the other hand, include a whole range of pathogens such as viruses (mainly positive-strand RNA viruses belonging to the families Dicistroviridae and Iflaviridae), bacteria (Melissococcus plutonius and Paenibacillus larvae), acarids (Acarapis woodi and Varroa destructor) and other agents [5,6,7,8]. In the context of biotic factors, biological agents responsible for the reduction of worker bees include the parasitic microsporidia Nosema spp., mainly N. apis and N. ceranae, both responsible for nosemosis in Apis spp. [5].
Nosema spp. are spore-forming microsporidia. Specifically, the spores of Nosema apis have a size of 4–6 × 2–4 µm, whereas the spores of Nosema ceranae are slightly smaller with a size of 3.3–5.5 × 2.3–3.0 μm [9]. Spores are the infectious stage and the only stage capable of existing outside the cell [10]. The infection typically occurs via the fecal–oral route, through ingestion of spore-contaminated feed via trophallaxis in the nest, or after grooming of body hairs [9,10]. As a result, infected honeybees can contaminate food sources (pollen and water) in the outdoor environment, spreading the infection [10]. Moreover, improper beekeeping practices can also promote the spread of infection, such as changing the queen to an infected one or joining an infected hive with an uninfected one [10].
The disease (nosemosis) caused by N. apis and N. ceranae shows different characteristics depending on the microsporidium infecting the hive. N. apis is responsible for the “classical” form of the disease (nosemosis type A), which is widespread, especially in cold and humid areas. It appears more easily during the spring season and in badly managed beehives during the winter season [11]. The seasonality of the disease is due to the sensitivity of this parasite to high temperatures, so during the summer, it does not occur [12]. Individually, the bees most affected by the disease are the worker bees; in fact, in these, both an alteration of the epithelial cells of the intestine and a decrease in digestive enzyme secretions with a consequent deficiency in the absorption of dietary proteins are observed [12]. This deficiency results in atrophy of the pharyngeal glands, followed by the inability of the nurse bees to feed the larvae and queen with royal jelly. Therefore, poor nutrition of the larvae and queen will result in reduced brood size followed by premature aging of the colony [10,12]. Although worker bees are the most affected, the disease never affects the larva and rarely the queen. When the queen bee is affected, she stops laying eggs and dies a few weeks after the infection begins, mostly outside the hive area [10]. The distinctive symptom of the disease caused by Nosema apis is the widespread dysentery that can be observed with fecal spots around the hive, promoting the spread of the disease throughout the colony. The main cause is due to changes occurring in the digestive tract resulting in improper digestion of ingested feed [12].
N. ceranae, on the other hand, is responsible for type “C” nosemosis. This disease affects the gut of bees and leads to a severe weakening of the bee’s immune system, consequently promoting the onset of other diseases of viral etiology [13]. In addition, the affected bees, which have difficulty with digesting, produce less honey and royal jelly, with a progressive weakening of the colony [14]. Type C nosemosis shows several important differences from type A nosemosis. First, affected bees do not suffer from dysentery, so this disease is also known as “dry nosemosis” [12]. Moreover, in contrast to type A nosemosis, which has a seasonal pattern, type C nosemosis has a two-year manifestation and can be divided into four phases [15]. At the end of these phases, during the autumn or early winter of the second year after the onset of infection, there is a significant depopulation of the hive due to an exponential increase in the number of spores in forager bees that die away from the hive [15]. Therefore, few bees, the queen, little feed and brood remain in the hive [12]. Several authors attribute a significant role to the depopulation of beehives caused by N. ceranae, a phenomenon that recently affected apiaries in Europe and Italy [16,17,18]. In Italy, there is a high prevalence of N. ceranae, and the absence of infection with N. apis and co-infection with N. apis/N. ceranae has led some authors to suggest that N. ceranae is decidedly widespread in Italy and has substantially replaced N. apis [16,17,18].
Nowadays, despite the depopulation and the reduction in honey production also affecting the Italian beekeeping sector, studies assessing the prevalence of the major pathogens responsible for these issues are few. Consequently, according to the Regional Beekeeping Observatory and Apulian Beekeepers ‘Association, the urgency of examining the health status of Apulian apiaries was expressed to reduce the loss of honeybee colonies due to various pathogens. Therefore, the present work aims to investigate the prevalence of N. ceranae and N. apis in Apulian apiaries through a Citizen Science (CS) approach. This approach involves close collaboration between the beekeepers, who provide basic information on the health status of the apiaries, and the veterinarian both at the level of Veterinary Services of Health Authorities (control activities) and at the level of the University (research activity). The combination of the expertise allows a broader understanding related to the problems caused by these parasites [19]. The CS approach will help to develop a harmonized European system for assessing the bees’ mortality and the prevalence of major bee diseases through standardized inspection protocols. These will allow both better risk management and prevention of honeybees’ diseases responsible for depopulation and reduced honey production that damages the beekeepers.
2. Material and Methods
2.1. Honeybee Colony Health Status Form
Forty-eight beehives in different provinces belonging to the Apulian Region were selected both for the execution of regular anti-varroa treatments and the adoption of similar beekeeping practices. Then, a form about the health status of honeybee colonies was filled out by the beekeepers for each hive (Supplementary File S1). The form contained the following questions: (a) presence of brood with injury signs (perforated/blackened capping, irregular distribution, abnormal odor, stringy larvae, etc.), (b) presence of honeybees with characteristic symptoms of virosis (dead bees in the pupal stage or small, black bees or bees with deformed wings), (c) presence of Varroa adults on the bees, (d) depopulation events in colony history that cannot be attributed to chemical poisoning (persistent depopulation for more than 10 days), (e) reduction in honey production, and (f) presence of diarrheal droppings on the flight platform and/or inside the hive.
2.2. Sampling
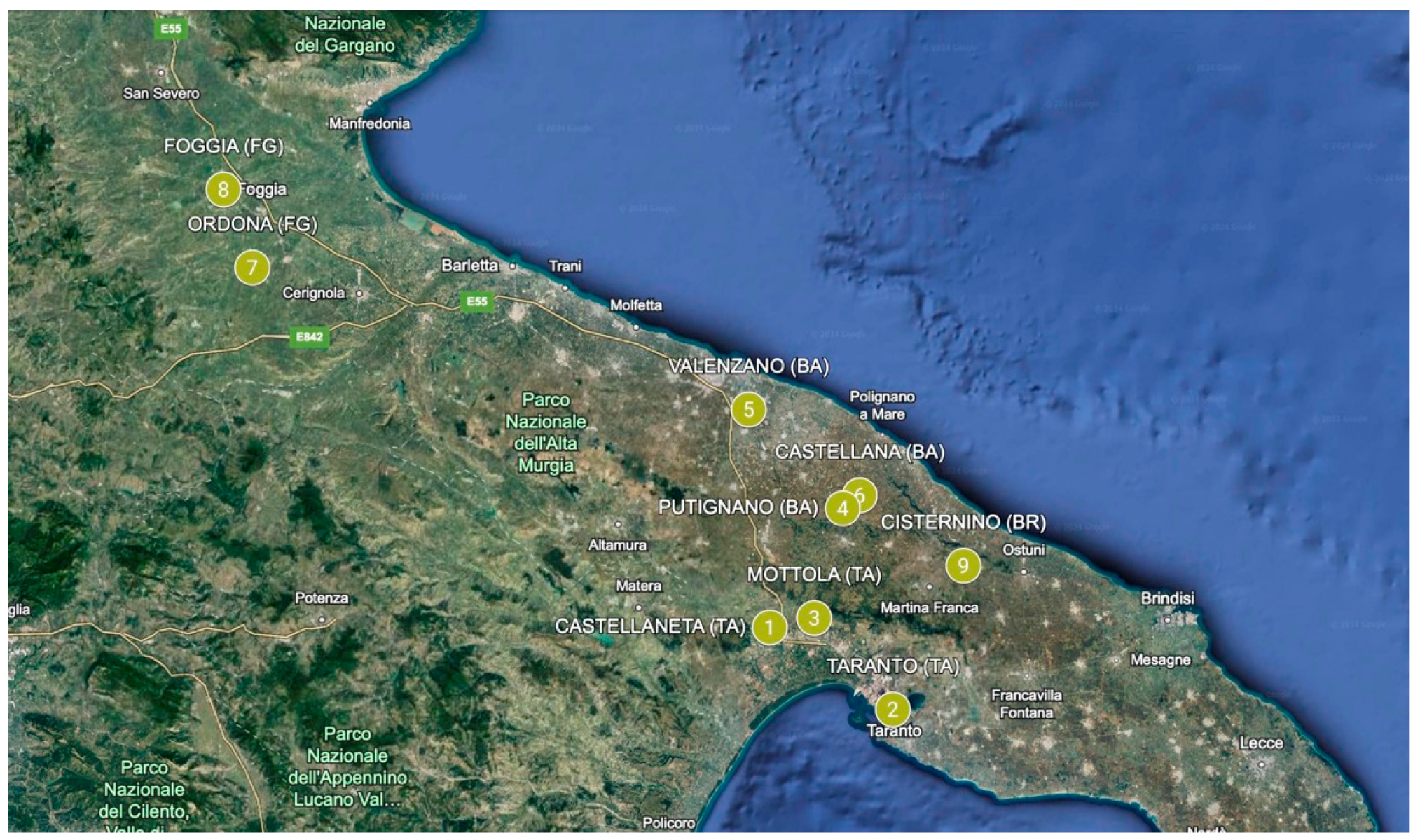
According to Directive 2010/63/EU of the European Parliament, no specific authorization was required for the use of honeybees for this study. During the summer of 2022, after the inspection visit conducted by the Competent Authority to confirm what the beekeepers reported in the forms, honeybees were sampled from apiaries managed by commercial beekeepers (more than thirty hives for apiary). From the province of Taranto, 12 samples were collected, including 6 from the municipality of Castellaneta, 3 from the municipality of Mottola and 3 from the municipality of Taranto. From the province of Bari, 21 samples were collected, including 6 from the municipality of Putignano, 12 from the municipality of Valenzano and 3 from the municipality of Castellana. From the province of Foggia, 12 samples were collected, including 6 from the municipality of Ordona and 6 from the municipality of Foggia. Finally, 3 samples were collected in the municipality of Cisternino belonging to the province of Brindisi (Figure 1).
Figure 1.
Beehive sampling areas in the Apulian Region (Italy). Points 1 to 3 (municipalities belonging to the province of Taranto), points 4 to 6 (municipalities belonging to the province of Bari), points 7 and 8 (municipalities belonging to the province of Foggia), and point 9 (municipality belonging to the province of Brindisi).
Specifically, sampling operations performed directly by the beekeepers and supervised by a group of researchers belonging to the Section of Food Safety of the Department of Veterinary Medicine (University of Bari Aldo Moro) were conducted in line with OIE guidelines [20]. Therefore, at least 20–30 adult forager honeybees were collected from the hive entrance or from peripheral frames if the weather did not permit flight conditions. Immediately after the collection, the honeybees were placed under a freezing regime (−20 °C) and transported to the molecular biology laboratories of the Food Safety Unit of the Department of Veterinary Medicine, University of Bari Aldo Moro.
2.3. Microscopic Investigation
A qualitative analysis with light microscopy was carried out following the OIE 2018 protocol to screen for the presence of Nosema spp. spores in honeybees [20]. The abdomens of honeybees were cut from the bodies. Then, the abdomens were pounded with a mortar and pestle in distilled water (1/1), obtaining a homogenate. A drop of the homogenate was placed on the slide and covered with a coverslip. The preparation was observed under a light microscope (resolution 400×). All samples were also screened by PCR using species-specific primers.
2.4. DNA Extraction
For DNA extraction, the protocol described by Cersini et al. was used [21]. The abdomens of ten honeybees were homogenized with the addition of 10 mL of PBS 1X. Subsequently, 2 mL of homogenate was centrifugated for 10 min at 13,000 rpm. After removing the supernatant, the pellet was weighed, and 40 µL of sodium hydrogen carbonate 0.5 M pH 6.5 and sodium chloride 5 M were added for each 35 mg of pellets, incubated for 15 min at 37° C, and centrifugated at 300 rpm. At the end of the incubation period, 180 µL of lysozyme [10 mg/mL] was added, followed by another incubation phase at 37 °C for 30 min. Subsequently, the DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s indications. The extracted DNA was stored at −20 °C before the execution of the polymerase chain reaction (PCR).
2.5. Amplification by PCR and Booster PCR
The amplification reaction was carried out using the Hot StarTaq Master Mix Kit (Qiagen, Hilden, Germany). The reactions were conducted in a final volume of 25 µL using primers with a final concentration of 5 μM (Table 1) and the following amplification protocol: 15 min at 95 °C, 35 cycles of 30 s at 94 °C, 30 s at 57 °C and 30 s at 72 °C and final elongation for 10 min at 72 °C. Primers amplify a region of the gene coding for ribosomal RNA (rRNA). The amplicon sizes of N. ceranae and N. apis were 104 pb and 142 pb, respectively [22]. To improve analytical sensitivity, a booster PCR was performed by diluting the amplicon 1:100 and following the method described above. The amplified products were displayed through electrophoresis on 2.5% agarose gel (w/v) (Pharmacia, Uppsala, Sweden) in buffer TBE (1X 0.089 M Tris, 0.089 M boric acid, 0.002 M EDTA, pH 8.0) and stained with the intercalant Green Gel Safe 10,000× Nucleic Acid Stain (5 μL/100 mL) (Fisher Molecular Biology, Rome, Italy). The molecular weight marker used was the Gene RulerTM 50 bp DNA Ladder (Thermoscientific, Waltham, MA, USA).

Table 1.
Sequence of primers described by Bourgeois et al. used for PCR and booster PCR reactions.
2.6. Data Analysis
The prevalence of positivity rates was calculated as follows:
Prevalence (%): Number of positive beehives/Number of total beehives
For variables of Boolean type, as in this particular case, statistical analysis was carried out by converting the qualitative data to quantitative, associating the unit value with the truth value, i.e., the positivity rates, the null value otherwise, obtaining a sample of 48 elements. Then, the mean and standard deviation were calculated, with 95% confidence interval (95% CI) based on the hypothesis of Student’s distribution.
3. Results
3.1. Prevalence of Nosema ceranae and Nosema apis
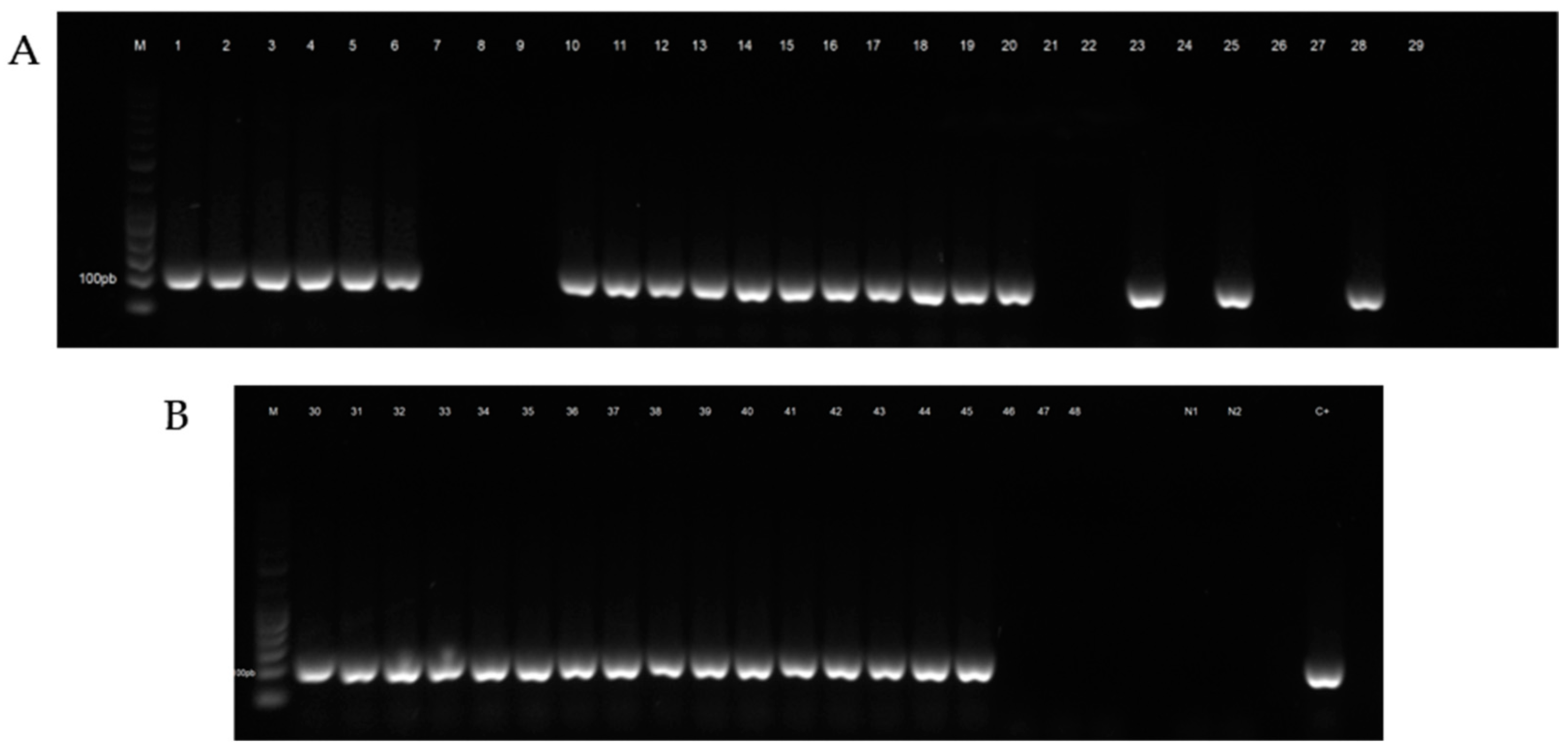
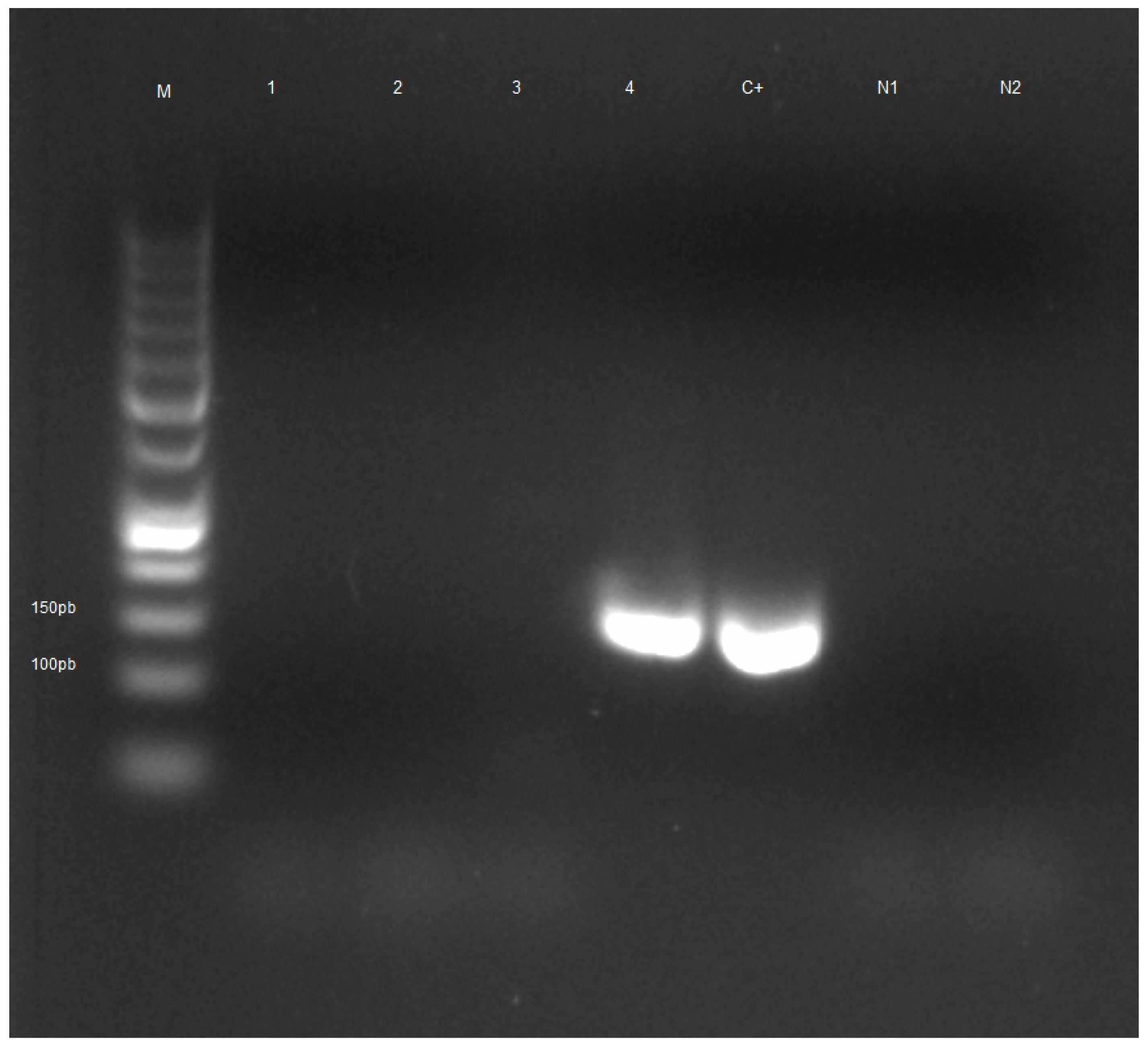
The results obtained after analysis of honeybee samples by light microscopy are summarized in Table 2. The number of samples tested positive for N. ceranae and N. apis after molecular analysis is shown in Table 3. The electrophoretic profile of booster PCR reaction products is shown in Figure 2, Figure 3, Figures S1 and S2.

Table 2.
Number of honeybee samples tested positive for Nosema spp. by light microscopy analysis. Provinces are indexed in red, while municipalities are in black.

Table 3.
Number of honeybee samples tested positive for Nosema apis and/or Nosema ceranae after molecular analysis. Provinces are indexed in red, while municipalities are in black.
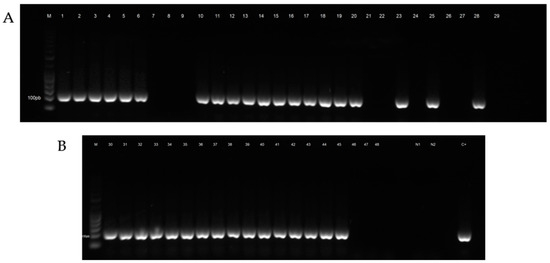
Figure 2.
Electrophoretic profile of booster PCR reaction products of honeybee samples tested for Nosema ceranae. (A) Lane M: Gene RulerTM 50 bp DNA Ladder, Lanes 1–6: positive samples from the municipality of Castellaneta (TA), Lanes 7–9: negative samples from the municipality of Taranto (TA), Lanes 10–12: positive samples from the municipality of Mottola (TA), Lanes 13–18: positive samples from the municipality of Putignano (BA), Lanes 19–29: negative and positive samples from the municipality of Valenzano (BA). (B) Lane 30: positive sample from the municipality of Valenzano (BA), Lanes 31–33: positive samples from the municipality of Castellana (BA), Lanes 34–39: positive samples from the municipality of Ordona (FG), Lanes 40–45: positive samples from the municipality of Foggia (FG), Lanes 46–48: negative samples from the municipality of Cisternino (BR), Lane N1: negative control PCR, Lane N2: negative control booster PCR, Lane C+: positive control.
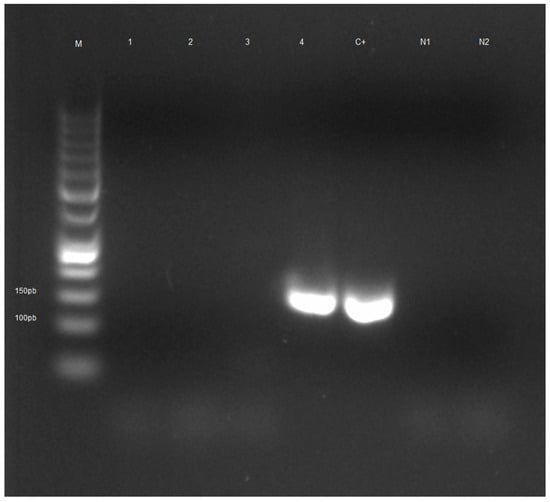
Figure 3.
Electrophoretic profile of booster PCR reaction products of honeybee samples tested for Nosema apis. Lane M: Gene RulerTM 50 bp DNA Ladder, Lanes 1–3: negative samples, Lane 4: positive sample from the municipality of Cisternino (BR), Lane C+: positive control, Lane N1: negative control PCR, Lane N2: negative control booster PCR.
After statistical analysis, the 95% confidence interval for the prevalence of positivity rate for Nosema ceranae in the Apulian Region ranges from 66.62% to 87.38%, with a mean value of 75%, whereas the 95% confidence interval for the prevalence of positivity rate for Nosema apis in the Apulian Region ranges from 0% to 6.88%, with a mean value of 2%.
3.2. Correlation between Beehive Health Status Form and Molecular Analysis
According to the answers given by beekeepers in the form, the following health framework has emerged: -no hives investigated had brood disease and/or viruses symptoms; -all of the hives analyzed had low Varroa infestation; -only 5 hives tested positive for Nosema ceranae (5/36) showed signs of depopulation and reduced honey production; -only limited honey production was reported for 19 hives tested positive for Nosema ceranae (19/36); -the honeybee colony, tested positive for Nosema apis, showed no clinical signs; -all hives tested negative on microscopic and molecular analysis for Nosema had no clinical signs, signs of depopulation or reduction in honey production.
4. Discussion
This study presents for the first time the microscopic and molecular identification of Nosema ceranae and Nosema apis in honeybee samples collected from hives in different provinces of the Apulian Region (Italy). In agreement with other studies, the results obtained show that light microscopy investigation followed by molecular analysis is an effective method for the screening and subsequent identification of the species to which the parasite spores belong. Indeed, because of the strong morphological similarities between the spores of the two microsporidia, the use of light microscopy alone would not be adequate to describe the species of Nosema within the analyzed sample [18]. The length of the polar filament is the only distinguishing morphological factor between Nosema ceranae and Nosema apis spores, and it can only be visualized by electron microscopy, which is difficult for all laboratories to access because of the very high costs associated with instrumentation. Therefore, after visualization of the spores by light microscopy, the samples were analyzed by PCR using species-specific primers [17,18,23,24]. Microscopic investigation showed the presence of Nosema spp. spores in thirty-seven samples; molecular investigation confirmed the presence of Nosema ceranae in thirty-six samples, while one sample showed the presence of Nosema apis.
The high prevalence of positivity rates for Nosema ceranae is a finding that does not come unexpectedly; in fact, since 1993, when the first positivity for this parasite was recorded for Italian honeybees, several studies in different Regions of Italy recorded very high levels of positivity for this parasite in honeybees. For example, studies carried out in Central Italy between the years 2014 and 2015 and in the Emilia Romagna Region (Northern Italy) in 2021 reported prevalence rates of 63% and 42.9%, respectively [18,25,26].
However, the prevalence of Nosema ceranae in honeybees reported in this study is higher than those reported by studies conducted in Regions of Central and Northern Italy. The reasons for this difference in prevalence could be related to several factors, such as the number of hives sampled, sampling methods used, different diagnostic techniques and climatic differences [18]. In this regard, it has been shown that territories with higher temperatures report higher prevalence rates for Nosema ceranae in the analyzed hives than territories with lower temperatures [17,18]. The preference of this pathogen for higher temperatures has also been confirmed in studies conducted in vitro. Fenoy et al., for example, showed how this pathogen has high thermotolerance at 60 and 35 °C as well as high resistance to desiccation. In addition, they showed that the spores have lower viability at temperatures around 4 °C and degenerate if they are frozen [27,28]. Therefore, based on the evidence reported in other studies, it can be supposed that the higher prevalence recorded in the Apulian Region compared to Regions in Central and Northern Italy could be related to the higher average annual temperature normally recorded in the Apulian territory.
Furthermore, the results demonstrate that a high rate of honeybee colonies affected by Nosema ceranae shows reduced honey production, as reported by beekeepers. This is a common subclinical sign of type C nosemosis reported by other studies [29,30]. The reduction in honey production is related to the ability of Nosema spp. to negatively affect the flight ability of foraging bees. In fact, these parasites can subtract from the host glucose and fructose produced by the cleavage of dietary sucrose and, as a result, reduce the synthesis of trehalose, the main carbohydrate in insect hemolymph [31]. The decrease in this key component induces energy stress and impaired flight ability of the infected bee, resulting in reduced nectar reserves necessary for honey production [30,31,32,33].
Another very important finding of this study is depopulation events observed in 5/36 colonies tested positive for N. ceranae, as reported by beekeepers. The mechanisms by which this parasite induces depopulation of affected colonies have been explained by several studies [34]. Indeed, it has been shown how in A. mellifera, N. ceranae induces downregulation of the glycoprotein vitellogenin (Vg), known to be a potent antioxidant and major source of energy [34,35,36,37]. In addition to the vitellogenin reduction, infection with this parasite also induces downregulation of genes involved in royal jelly protein-coding and alters metabolic pathways involved in maintaining carbohydrate, amino acid and lipid levels [34,36]. Finally, Nosema ceranae is responsible for the upregulation of the naked cuticle gene, known as an important suppressor of bee immune function [34].
Therefore, all the evidence suggested by these studies could explain the association between some beehives that tested positive for the parasite and the depopulation observed by beekeepers.
A very important result achieved by this study is the presence of a sample of honeybees collected from one hive in the municipality of Cisterino (Brindisi Province) in which N. apis infection was detected. Indeed, several studies conducted in Italy in recent years recorded the absence of positivity for Nosema apis in the hives analyzed, suggesting that Nosema ceranae has essentially replaced Nosema apis [16,18,38]. Therefore, the positivity recorded by the present study should be clarified with further investigations to be carried out in the Apulian Region and Italy. The usefulness of epidemiological studies also arises because of the abrogation of Veterinary Police Regulation 320/1954 [39]. As of 21 April 2021, with the entry into force of Regulation (EU) 429/2016 (subsequently amended by Regulation (EU) 2018/1629), nosemosis is no longer considered a notifiable disease, consequently posing a potential risk to the welfare of Italian honeybees [40]. The Veterinary Police Regulations provided a set of measures that had to be applied after the positivity of an apiary for Nosema apis, allowing the control of the disease and, at the same time, its eradication [39]. The positivity found in an apiary in the territory of Cisterino (province of Brindisi) highlights the need to continue to collect data about the presence of N. apis in hives distributed on Italian territory to avoid its propagation that could lead to the depopulation of hives with important economic damage for beekeepers.
Moreover, monitoring the trend of Nosema spp. infection in honeybees should also be carried out because of the absence of effective treatments that can eradicate the disease. For example, the antibiotic fumagallin, which has long been shown to be effective against Nosema apis infection, is not authorized in the European Union due to the lack of definition of maximum residual limits in honey and a registered veterinary medicine with this active substance [41]. Consequently, nowadays, a whole range of substances are being studied to overcome this limitation related to the drugs used for the treatment of infected bee colonies [41,42,43].
A major limitation of this study is that the number of spores and, consequently, the infection levels of the Nosema spp. in the bees were not measured. These pathogens can be detected by light microscopy and PCR, but if they are below threshold levels, they normally do not damage colonies [30]. Future investigations should include these types of data. Also, a larger number of samples would be more informative.
5. Conclusions
The loss of honeybee colonies that is occurring worldwide is a major issue and will generate serious economic and ecological problems due to the importance of these pollinating insects for the environment. Within this context, close collaboration between beekeepers, who constantly provide information on the health status of their apiaries, and veterinarians, who identify the cause of the problems encountered, could be the key to reducing the decline of honeybees [19,44,45]. Through this approach, this study has not only increased knowledge about the epidemiological situation of Nosema apis and Nosema ceranae in Italy and the European Union, but it has also correlated the presence of these parasites in the beehives with the most important problems encountered by beekeepers, such as depopulation and reduced honey production. Therefore, even if carried out at the regional level, studies like this will help us gain a better understanding of the characteristics of these pathogens, highlighting the impact of these on honeybee colonies. Indeed, by constantly monitoring beehives not only regionally or nationally but globally, we can take timely action to proactively deal with potential problems and combat bee diseases and pests, helping protect the sectors that depend on pollination services by bees, such as fruit, nut, seed, vegetable and fiber production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14040583/s1, Supplementary File S1: Health status form and Sampling form. Figure S1: Original image of Electrophoretic profile of booster PCR reaction products of honeybee samples tested for Nosema ceranae. Figure S2: Original image of Electrophoretic profile of booster PCR reaction products of honeybee samples tested for Nosema apis.
Author Contributions
Conceptualization, A.P., P.L. and V.T.; methodology, A.P., P.L., A.M. and V.T.; writing—original draft preparation, A.P. and A.M.; writing review—and editing E.B., G.B., G.M.T. and V.T.; supervision, E.B., G.B., G.M.T. and V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Apulian Region, grant number 23705023960.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors thank Dott. Giuseppe Capriglia for his contribution in the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, K.; Li, Y.; Sun, K.; Bao, J.; He, C.; Hou, X. Supplementary honeybee (Apis mellifera L.) pollination enhances fruit growth rate and fruit yield in Paeonia ostii (family: Paeoniaceae). PLoS ONE 2022, 17, e0272921. [Google Scholar] [CrossRef]
- The European Food Safety Authority Insect Pollinator Health. Insect Pollinator Health. 2023. Available online: https://www.efsa.europa.eu/en/topics/insect-pollinator-health (accessed on 30 October 2023).
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and Abiotic Factors Associated with Colonies Mortalities of Managed Honeybee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja Douglas, A.; Cadahía, L.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; et al. Honeybee Colony Loss Rates in 37 Countries Using the COLOSS Survey for Winter 2019–2020: The Combined Effects of Operation Size, Migration and Queen Replacement. J. Apic. Res. 2023, 62, 204–210. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Peyre, Y.; Ahuir-Baraja, A.E.; Garijo, M.M.; Llobat, L. The Role of Nosema Ceranae (Microsporidia: Nosematidae) in Honeybee Colony Losses and Current Insights on Treatment. Vet. Sci. 2022, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Tantillo, G.; Bottaro, M.; Di Pinto, A.; Martella, V.; Di Pinto, P.; Terio, V. Virus Infections of Honeybees Apis mellifera. Ital. J. Food Saf. 2015, 4, 5364. [Google Scholar] [CrossRef] [PubMed]
- de Figueiró Santos, J.; Coelho, F.C.; Bliman, P.-A. Behavioral Modulation of Infestation by Varroa destructor in Bee Colonies. Implications for Colony Stability. PLoS ONE 2016, 11, e0160465. [Google Scholar] [CrossRef]
- Okamoto, M.; Furuya, H.; Sugimoto, I.; Kusumoto, M.; Takamatsu, D. A Novel Multiplex PCR Assay to Detect and Distinguish between Different Types of Paenibacillus larvae and Melissococcus plutonius, and a Survey of Foulbrood Pathogen Contamination in Japanese Honey. J. Vet. Med. Sci. 2022, 84, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Sulborska, A.; Horecka, B.; Cebrat, M.; Kowalczyk, M.; Skrzypek, T.H.; Kazimierczak, W.; Trytek, M.; Borsuk, G. Microsporidia Nosema spp.—Obligate Bee Parasites Are Transmitted by Air. Sci. Rep. 2019, 9, 14376. [Google Scholar] [CrossRef]
- Galajda, R.; Valenčáková, A.; Sučik, M.; Kandráčová, P. Nosema Disease of European Honeybees. J. Fungi 2021, 7, 714. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Nosemosis. 2015. Available online: https://www.fao.org/3/CA3136EN/ca3136en.pdf (accessed on 31 October 2023).
- Mazur, E.D.; Gajda, A.M. Nosemosis in Honeybees: A Review Guide on Biology and Diagnostic Methods. Appl. Sci. 2022, 12, 5890. [Google Scholar] [CrossRef]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Iorizzo, M.; Letizia, F.; Ganassi, S.; Testa, B.; Petrarca, S.; Albanese, G.; Di Criscio, D.; De Cristofaro, A. Recent Advances in the Biocontrol of Nosemosis in Honeybees (Apis mellifera L.). J. Fungi 2022, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How Natural Infection by Nosema ceranae Causes Honeybee Colony Collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Bordin, F.; Zulian, L.; Granato, A.; Caldon, M.; Colamonico, R.; Toson, M.; Trevisan, L.; Biasion, L.; Mutinelli, F. Presence of Known and Emerging Honeybee Pathogens in Apiaries of Veneto Region (Northeast of Italy) during Spring 2020 and 2021. Appl. Sci. 2022, 12, 2134. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European Honeybees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef]
- Papini, R.; Mancianti, F.; Canovai, R.; Cosci, F.; Rocchigiani, G.; Benelli, G.; Canale, A. Prevalence of the Microsporidian Nosema ceranae in Honeybee (Apis mellifera) Apiaries in Central Italy. Saudi J. Biol. Sci. 2017, 24, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Elsner-Adams, E.; Maderson, S. A One-Health Model for Reversing Honeybee (Apis mellifera L.) Decline. Vet. Sci. 2020, 7, 119. [Google Scholar] [CrossRef]
- OIE Nosemosis of Honeybees. In OIE Terrestrial Manual; 2018; pp. 744–749. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 15 October 2023).
- Cersini, A.; Antognetti, V.; Giacomelli, A.; Puccica, S.; Pietropaoli, M.; Milito, M.; Cardeti, G.; Marchesi, U.; Maroni Ponti, A.; Pizzariello, M.; et al. Patologie rare: Un caso di Nosema apis in Italia. Apic. Ital. 2013, 9, 3–6. [Google Scholar]
- Bourgeois, A.L.; Rinderer, T.E.; Beaman, L.D.; Danka, R.G. Genetic Detection and Quantification of Nosema apis and N. ceranae in the Honeybee. J. Invertebr. Pathol. 2010, 103, 53–58. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R.; Szczerba-Turek, A.; Bancerz-Kisiel, A. A Comparison of the Effectiveness of the Microscopic Method and the Multiplex PCR Method in Identifying and Discriminating the Species of Nosema spp. Spores in Worker Bees (Apis mellifera) from Winter Hive Debris. Pol. J. Vet. Sci. 2011, 14, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Utuk, A.E.; Piskin, F.C.; Girisgin, A.O.; Selcuk, O.; Aydin, L. Microscopic and Molecular Detection of Nosema spp. in Honeybees of Turkey. Apidologie 2016, 47, 267–271. [Google Scholar] [CrossRef][Green Version]
- Ferroglio, E.; Zanet, S.; Peraldo, N.; Tachis, E.; Trisciuoglio, A.; Laurino, D.; Porporato, M. Nosema ceranae Has Been Infecting Honeybees Apis mellifera in Italy since at Least 1993. J. Apic. Res. 2013, 52, 60–61. [Google Scholar] [CrossRef]
- Cilia, G.; Tafi, E.; Zavatta, L.; Caringi, V.; Nanetti, A. The Epidemiological Situation of the Managed Honeybee (Apis mellifera) Colonies in the Italian Region Emilia-Romagna. Vet. Sci. 2022, 9, 437. [Google Scholar] [CrossRef]
- Tosun, O.; Yaman, M. The Effects of Temperature and Humidity around the Beehives on the Distribution of Nosema ceranae, and Also Geographical and Seasonal Activity of the Infection in the Eastern Black Sea Region of Turkey. J. Environ. Sci. Eng. B 2016, 5, 513–522. [Google Scholar] [CrossRef]
- Fenoy, S.; Rueda, C.; Higes, M.; Martín-Hernández, R.; del Aguila, C. High-Level Resistance of Nosema ceranae, a Parasite of the Honeybee, to Temperature and Desiccation. Appl. Environ. Microbiol. 2009, 75, 6886–6889. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Carbonell, V.; Valdebenito, J.; Figueroa, C.; Valdovinos, C.; Martín-Hernández, R.; Higes, M.; Delporte, C. Identification of Nosema ceranae in the Valparaíso District, Chile. Arch. Med. Vet. 2014, 46, 487–491. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. Infection and Its Negative Effects on Honeybees (Apis mellifera Iberiensis) at the Colony Level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Parasitic Infection Leads to Decline in Hemolymph Sugar Levels in Honeybee Foragers. J. Insect. Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Naree, S.; Benbow, M.E.; Suwannapong, G.; Ellis, J.D. Mitigating Nosema Ceranae Infection in Western Honeybee (Apis mellifera) Workers Using Propolis Collected from Honeybee and Stingless Bee (Tetrigona Apicalis) Hives. J. Invertebr. Pathol. 2021, 185, 107666. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Fuchs, S. Nosema spp. Influences Flight Behavior of Infected Honey Bee (Apis mellifera) Foragers. Apidologie 2010, 41, 21–28. [Google Scholar] [CrossRef]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the Gut Parasite Nosema ceranae on Honeybee Physiology and Behavior. Curr. Opin. Insect. Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive Protein Protects Functionally Sterile Honeybee Workers from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-Sequence Analysis of Gene Expression from Honeybees (Apis mellifera) Infected with Nosema ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and Behavioral Changes in Honeybees (Apis mellifera) Induced by Nosema ceranae Infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef] [PubMed]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread Dispersal of the Microsporidian Nosema ceranae, an Emergent Pathogen of the Western Honeybee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Presidential Decree No. 320 on Veterinary Police. Gazzetta Ufficiale della Repubblica Italiana No. 142 1954. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1954-06-24&atto.codiceRedazionale=054U0320&elenco30giorni=false (accessed on 30 October 2023).
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). Off. J. Eur. Union 2016, L84, 1–208.
- Formato, G.; Rivera-Gomis, J.; Bubnic, J.; Martín-Hernández, R.; Milito, M.; Croppi, S.; Higes, M. Nosemosis Prevention and Control. Appl. Sci. 2022, 12, 783. [Google Scholar] [CrossRef]
- Shumkova, R.; Balkanska, R.; Hristov, P. The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honeybee Strength and Production Traits. Pathogens 2021, 10, 234. [Google Scholar] [CrossRef]
- Nanetti, A.; Rodriguez-García, C.; Meana, A.; Martín-Hernández, R.; Higes, M. Effect of Oxalic Acid on Nosema ceranae Infection. Res. Vet. Sci. 2015, 102, 167–172. [Google Scholar] [CrossRef]
- Jalbert, K.; Kinchy, A.J.; Perry, S.L. Civil Society Research and Marcellus Shale Natural Gas Development: Results of a Survey of Volunteer Water Monitoring Organizations. J. Environ. Stud. Sci. 2014, 4, 78–86. [Google Scholar] [CrossRef]
- Donnelly, A.; Crowe, O.; Regan, E.; Begley, S.; Caffarra, A. The Role of Citizen Science in Monitoring Biodiversity in Ireland. Int. J. Biometeorol. 2014, 58, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).