Nitrogen Addition Decreased Respiration and Heterotrophic Respiration but Increased Autotrophic Respiration in a Cabbage (Brassica pekinensis Rupr) Experiment in the Northeast Plains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design
2.3. Measurement of Rs and Rh
2.3.1. Experimental Setup
2.3.2. Instrumentation and Measurement Conditions
2.3.3. Rs and Rh Measurement Protocol
2.4. Soil Sampling and Analysis
2.5. ANPP and BNPP Estimations
2.6. Data Analysis
3. Results and Discussion
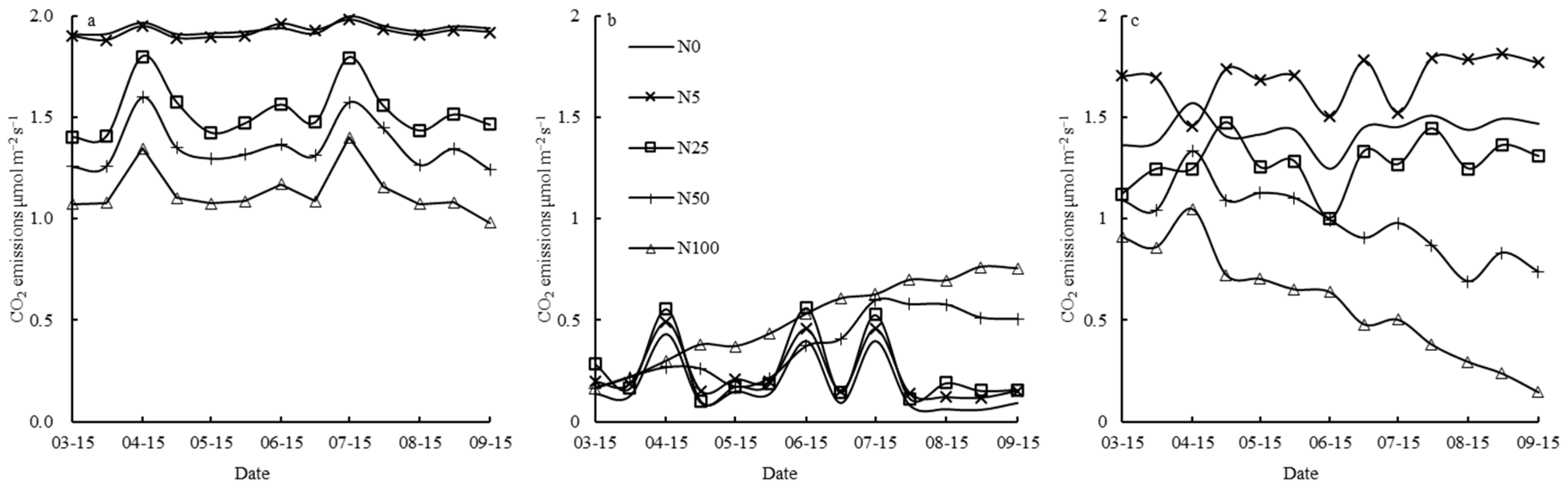
3.1. N Fertilizer Effects on Rs and Its Constituent Parts (Ra and Rh)
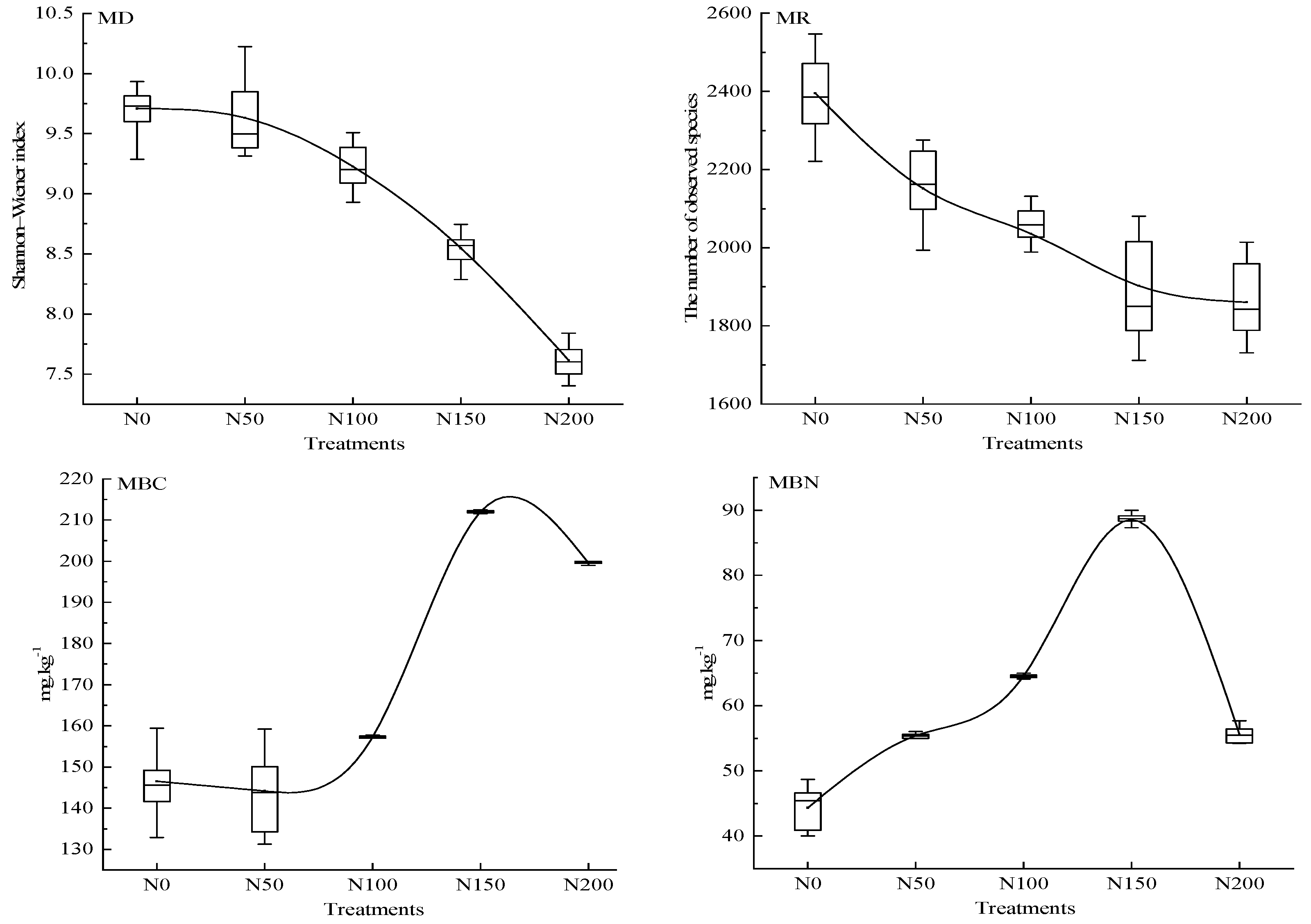
3.2. Changes in Biotic and Abiotic Factors in Response to N Addition
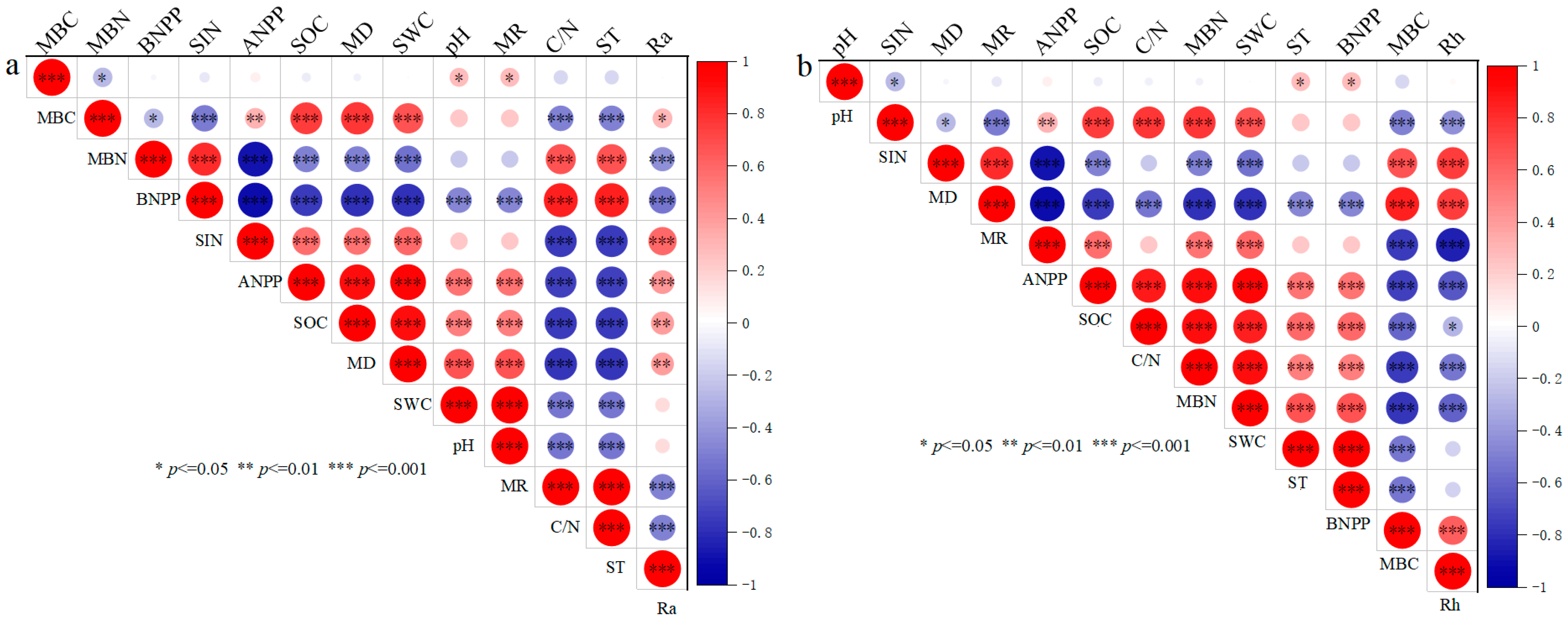
3.3. Pearson Correlation Analysis for Ra and Rh
3.4. Controlling Factors and Pathways for the Ra and Rh
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.-J.; Wu, L.-M.; Ding, Y.-F.; Weng, F.; Wu, X.-R.; Li, G.-H.; Liu, Z.-H.; Tang, S.; Ding, C.-Q.; Wang, S.-H. Top-dressing nitrogen fertilizer rate contributes to decrease culm physical strength by reducing structural carbohydrate content in japonica rice. J. Integr. Agric. 2016, 15, 992–1004. [Google Scholar] [CrossRef]
- Cui, S.; Shi, Y.; Groffman, P.M.; Schlesinger, W.H.; Zhu, Y.-G. Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910–2010). Proc. Natl. Acad. Sci. USA 2013, 110, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Singh, R.S.; Nathanail, C.P. Mine spoil acts as a sink of carbon dioxide in Indian dry tropical environment. Sci. Total Environ. 2014, 468, 1162–1171. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, Y.N.; Xu, J. Organic fertilizer application increases the soil respiration and net ecosystem carbon dioxide absorption of paddy fields under water-saving irrigation. Environ. Sci. Pollut. Res. 2018, 25, 9958–9968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Wang, Z.; Zheng, H.; Chen, Y.; Chen, X.; Wang, L.; Li, H.; Zhang, J. Nitrogen addition alleviates microbial nitrogen limitations and promotes soil respiration in a subalpine coniferous forest. Forests 2019, 10, 1038. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kruse, M.L.; Sawyer, J.E. Effect of nitrogen fertilizer application on growing season soil carbon dioxide emission in a corn–soybean rotation. J. Environ. Qual. 2008, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Kirkman, K.; Hagenah, N.; Tsvuura, Z. Soil respiration declines with increasing nitrogen fertilization and is not related to productivity in long-term grassland experiments. Soil Biol. Biochem. 2017, 115, 415–422. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, S.; Wang, R. Diverse soil respiration responses to extreme precipitation patterns in arid and semiarid ecosystems. Appl. Soil Ecol. 2021, 163, 103928. [Google Scholar] [CrossRef]
- Kou, T.; Zhu, J.; Xie, Z.; Hasegawa, T.; Heiduk, K. Effect of elevated atmospheric CO2 concentration on soil and root respiration in winter wheat by using a respiration partitioning chamber. Plant Soil 2007, 299, 237–249. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Liu, J.; Shangguan, Z. Response of soil respiration to nitrogen fertilization: Evidence from a 6-year field study of croplands. Geoderma 2021, 384, 114829. [Google Scholar] [CrossRef]
- Wang, J.; Song, B.; Ma, F.; Tian, D.; Li, Y.; Yan, T.; Quan, Q.; Zhang, F.; Li, Z.; Wang, B. Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow. Funct. Ecol. 2019, 33, 2239–2253. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Meng, D.; Dang, S.; Zhou, J.; Osborne, B.; Ren, Y.; Liang, T.; Yu, K. Effect of soil microorganisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis. Ecol. Evol. 2020, 10, 13602–13612. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Xu, X.; Yu, Y.; Zhang, Y.; Sun, Z.; Tao, X. Forest soil respiration response to increasing nitrogen deposition along an urban–rural gradient. Glob. Ecol. Conserv. 2021, 27, e01575. [Google Scholar] [CrossRef]
- Lu, X.; Wen, L.; Sun, H.; Fei, T.; Liu, H.; Ha, S.; Tang, S.; Wang, L. Responses of soil respiration to phosphorus addition in global grasslands: A meta-analysis. J. Clean. Prod. 2022, 131413. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Amelung, W. Correction to: Effect of sieving and sample storage on soil respiration and its temperature sensitivity (Q10) in mineral soils from Germany. Biol. Fertil. Soils 2019, 55, 833. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Hu, J.; Tang, C.; Zhang, S.; Fu, S.; Jiang, P.; Ge, T.; Luo, Y.; Song, X. Rates of soil respiration components in response to inorganic and organic fertilizers in an intensively-managed Moso bamboo forest. Geoderma 2021, 403, 115212. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Garrett, R.D.; Niles, M.T.; Gil, J.D.B.; Gaudin, A.; Chaplin-Kramer, R.; Assmann, A.; Assmann, T.S.; Brewer, K.; de Faccio Carvalho, P.C.; Cortner, O.; et al. Social and ecological analysis of commercial integrated crop livestock systems: Current knowledge and remaining uncertainty. Agric. Syst. 2017, 155, 136–146. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Troy, S.M.; Lawlor, P.G.; O’Flynn, C.J.; Healy, M.G. Impact of biochar addition to soil on greenhouse gas emissions following pig manure application. Soil Biol. Biochem. 2013, 60, 173–181. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Y.; Wang, Y.; Ali, S.; Liu, Q.; Guo, S. Nitrogen application increases soil respiration but decreases temperature sensitivity: Combined effects of crop and soil properties in a semiarid agroecosystem. Geoderma 2019, 353, 320–330. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Z.; Zhuang, D.; Wang, J.; Xie, S.; Liu, G. Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol. Fertil. Soils 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Wei, S.; Tie, L.; Liao, J.; Liu, X.; Du, M.; Lan, S.; Li, X.; Li, C.; Zhan, H.; Huang, C. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 2020, 450, 171–182. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.H.; Gao, X.; Li, G.; Shi, J.; Wei, J.; Tan, D.; Wang, M.; Jiang, L. Effects of nitrogen levels on yield, quality and soil environment of Chinese cabbage in open field. Acta Hortic. 2017, 1192, 19. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, F.; Zhou, R.; Wu, Y.; Hou, X.; Li, J.; Lin, W.-H.; Wu, Z. Effects of Reduced Nitrogen with Bio-Organic Fertilizer on Soil Properties, Yield and Quality of Non-Heading Chinese Cabbage. Agronomy 2021, 11, 2196. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zhang, F.; He, T. Short-term nitrogen fertilization decreased root and microbial respiration in a young Cunninghamia lanceolata plantation. Chin. J. Plant Ecol. 2015, 39, 1104–1112. [Google Scholar]
- Wenchen, S.; Xiaojuan, T.; Jinsong, Z.; Ping, M.; Jun, L. Autotrophic and heterotrophic components of soil respiration caused by rhizosphere priming effects in a plantation. Plant Soil Environ. 2017, 63, 383–389. [Google Scholar]
- Zhang, Q.; Zhou, J.; Li, X.; Liu, C.; Lin, W.; Zheng, W.; Chen, Y.; Yang, Y. Nitrogen addition accelerates the nitrogen cycle in a young subtropical Cunninghamia lanceolata (Lamb.) plantation. Ann. For. Sci. 2019, 76, 1–15. [Google Scholar] [CrossRef]
- Bao, S. Soil Agriculturalization Analysis; China Agriculture Press: Beijing, China, 1981. [Google Scholar]
- Trocha, L.K.; Bułaj, B.; Kutczyńska, P.; Mucha, J.; Rutkowski, P.; Zadworny, M. The interactive impact of root branch order and soil genetic horizon on root respiration and nitrogen concentration. Tree Physiol. 2017, 37, 1055–1068. [Google Scholar] [CrossRef]
- Peng, F.; You, Q.G.; Xu, M.H.; Zhou, X.H.; Xue, X. Effects of experimental warming on soil respiration and its components in an alpine meadow in the permafrost region of the Qinghai-Tibet Plateau. Eur. J. Soil Sci. 2014, 66, 145–154. [Google Scholar] [CrossRef]
- Bolloju, S.; Rohan, R.; Wu, S.-T.; Yen, H.-X.; Dwivedi, G.D.; Lin, Y.A.; Lee, J.-T. A green and facile approach for hydrothermal synthesis of LiFePO4 using iron metal directly. Electrochim. Acta 2016, 220, 164–168. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Pérez-Rodríguez, P.; Insolia, E.; Soto-Gómez, D.; Fernández-Calviño, D.; Zornoza, R. The impact of crop diversification, tillage and fertilization type on soil total microbial, fungal and bacterial abundance: A worldwide meta-analysis of agricultural sites. Agric. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Sun, W.; Ren, C. The impact of energy consumption structure on China’s carbon emissions: Taking the Shannon–Wiener index as a new indicator. Energy Rep. 2021, 7, 2605–2614. [Google Scholar] [CrossRef]
- Garrett, L.G.; Lin, Y.; Matson, A.L.; Strahm, B.D. Nitrogen isotope enrichment predicts growth response of Pinus radiata in New Zealand to nitrogen fertiliser addition. Biol. Fertil. Soils 2023, 59, 555–566. [Google Scholar] [CrossRef]
- Hunecke, C.; Engler, A.; Jara-Rojas, R.; Poortvliet, P.M. Understanding the role of social capital in adoption decisions: An application to irrigation technology. Agric. Syst. 2017, 153, 221–231. [Google Scholar] [CrossRef]
- Liao, H.; Li, C.; Ai, S.; Li, X.; Ai, X.; Ai, Y. A simulated ecological restoration of bare cut slope reveals the dosage and temporal effects of cement on ecosystem multifunctionality in a mountain ecosystem. J. Environ. Manag. 2023, 325, 116672. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, A.; Sudargini, Y. Partial least squares structural squation modeling (PLS-SEM) analysis for social and management research: A literature review. J. Ind. Eng. Manag. Res. 2021, 2, 114–123. [Google Scholar]
- Ferretti, M.; Marchetto, A.; Arisci, S.; Bussotti, F.; Calderisi, M.; Carnicelli, S.; Cecchini, G.; Fabbio, G.; Bertini, G.; Matteucci, G. On the tracks of Nitrogen deposition effects on temperate forests at their southern European range–an observational study from Italy. Glob. Change Biol. 2014, 20, 3423–3438. [Google Scholar] [CrossRef]
- Guenet, B.; Gabrielle, B.; Chenu, C.; Arrouays, D.; Balesdent, J.; Bernoux, M.; Bruni, E.; Caliman, J.P.; Cardinael, R.; Chen, S. Can N2O emissions offset the benefits from soil organic carbon storage? Glob. Chang. Biol. 2021, 27, 237–256. [Google Scholar] [CrossRef]
- Han, M.; Feng, J.; Chen, Y.; Sun, L.; Fu, L.; Zhu, B. Mycorrhizal mycelial respiration: A substantial component of soil respired CO2. Soil Biol. Biochem. 2021, 163, 108454. [Google Scholar] [CrossRef]
- Mengqi, Z.; Shi, A.; Ajmal, M.; Ye, L.; Awais, M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers. Biorefinery 2021, 13, 5445–5468. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Z.; Yang, Q.; Jian, C.; Lai, S.; Chen, Y.; Xu, B. N and P addition increase soil respiration but decrease contribution of heterotrophic respiration in semiarid grassland. Agric. Ecosyst. Environ. 2021, 318, 107493. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- De Vries, F.T.; Van Groenigen, J.W.; Hoffland, E.; Bloem, J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol. Biochem. 2011, 43, 997–1005. [Google Scholar] [CrossRef]
- Turner, B.L.; Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Gorka, S.; Dietrich, M.; Mayerhofer, W.; Gabriel, R.; Wiesenbauer, J.; Martin, V.; Zheng, Q.; Imai, B.; Prommer, J.; Weidinger, M. Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front. Microbiol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Diabate, B.; Wang, X.; Gao, Y.; Yu, P.; Wu, Z.; Zhou, D.; Yang, H. Tillage and haymaking practices speed up belowground net productivity restoration in the degraded Songnen grassland. Soil Tillage Res. 2018, 175, 62–70. [Google Scholar] [CrossRef]
- Ren, F.; Sun, N.; Xu, M.; Zhang, X.; Wu, L.; Xu, M. Changes in soil microbial biomass with manure application in cropping systems: A meta-analysis. Soil Tillage Res. 2019, 194, 104291. [Google Scholar] [CrossRef]
- Chen, S.; Zou, J.; Hu, Z.; Chen, H.; Lu, Y. Global annual soil respiration in relation to climate, soil properties and vegetation characteristics: Summary of available data. Agric. For. Meteorol. 2014, 198, 335–346. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, Z.; Zhang, Y.; Sun, H.; Song, S.; Bai, Z.; Lu, Z.; Li, C. Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Front. Plant Sci. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Keller, A.B.; Borer, E.T.; Collins, S.L.; DeLancey, L.C.; Fay, P.A.; Hofmockel, K.S.; Leakey, A.D.; Mayes, M.A.; Seabloom, E.W.; Walter, C.A. Soil carbon stocks in temperate grasslands differ strongly across sites but are insensitive to decade-long fertilization. Glob. Chang. Biol. 2022, 28, 1659–1677. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Kole, S.C. Soil organic matter and microbial role in plant productivity and soil fertility. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 2: Soil-Microbe-Plant Interaction; Springer: Berlin/Heidelberg, Germany, 2017; pp. 219–238. [Google Scholar]
- Deng, L.; Peng, C.; Zhu, G.; Chen, L.; Liu, Y.; Shangguan, Z. Positive responses of belowground C dynamics to nitrogen enrichment in China. Sci. Total Environ. 2018, 616–617, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- da Luz, F.B.; Carvalho, M.L.; Castioni, G.A.F.; de Oliveira Bordonal, R.; Cooper, M.; Carvalho, J.L.N.; Cherubin, M.R. Soil structure changes induced by tillage and reduction of machinery traffic on sugarcane—A diversity of assessment scales. Soil Tillage Res. 2022, 223, 105469. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Luo, Q.; Chen, Y.; Hu, J.; He, M.; Gao, J.; Zhou, L.; Liu, H.; Zhou, X. Interactive effects of grazing and global change factors on soil and ecosystem respiration in grassland ecosystems: A global synthesis. J. Appl. Ecol. 2019, 56, 2007–2019. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Zhu, B.; Qiang, W.; Zhang, Y.; Pang, X. Phosphorus addition decreases plant lignin but increases microbial necromass contribution to soil organic carbon in a subalpine forest. Glob. Chang. Biol. 2022, 28, 4194–4210. [Google Scholar] [CrossRef] [PubMed]
- Ernakovich, J.G.; Hopping, K.A.; Berdanier, A.B.; Simpson, R.T.; Kachergis, E.J.; Steltzer, H.; Wallenstein, M.D. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob. Chang. Biol. 2014, 20, 3256–3269. [Google Scholar] [CrossRef]
- Bacchiocchi, S.C.; Zerbe, S.; Cavieres, L.A.; Wellstein, C. Impact of ski piste management on mountain grassland ecosystems in the Southern Alps. Sci. Total Environ. 2019, 665, 959–967. [Google Scholar] [CrossRef]
- Hoque, M.M.; Inubushi, K.; Miura, S.; Kobayashi, K.; Kim, H.-Y.; Okada, M.; Yabashi, S. Biological dinitrogen fixation and soil microbial biomass carbon as influenced by free-air carbon dioxide enrichment (FACE) at three levels of nitrogen fertilization in a paddy field. Biol. Fertil. Soils 2001, 34, 453–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, X.; Qiu, Q.; Yu, R.; Yao, Y.; Li, H.; Shao, M.; Wei, X. Soil and water conservation measures reduce erosion but result in carbon and nitrogen accumulation of red soil in Southern China. Agric. Ecosyst. Environ. 2023, 346, 108346. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Z.; Xu, X.; Liu, S.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.; Ge, T. Carbon and nitrogen recycling from microbial necromass to cope with C: N stoichiometric imbalance by priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, G.G.; Tang, C.; Fang, H.; Duan, J.; Yu, X. Effects of one-year simulated nitrogen and acid deposition on soil respiration in a subtropical plantation in China. Forests 2020, 11, 235. [Google Scholar] [CrossRef]




| 2021 | 2022 | |
|---|---|---|
| Planting | 15 August | 4 August |
| N Fertilizing | 25 August, 15 September, 5 October | 15 August, 1 September, 1 October |
| Manually weeding | 15 August to 11 October | 4 August to 7 October |
| Harvesting | 11 October | 7 October |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Yan, X.; Liu, S.; Fu, L.; Gao, X.; Huang, D. Nitrogen Addition Decreased Respiration and Heterotrophic Respiration but Increased Autotrophic Respiration in a Cabbage (Brassica pekinensis Rupr) Experiment in the Northeast Plains. Agriculture 2024, 14, 596. https://doi.org/10.3390/agriculture14040596
Jiang X, Yan X, Liu S, Fu L, Gao X, Huang D. Nitrogen Addition Decreased Respiration and Heterotrophic Respiration but Increased Autotrophic Respiration in a Cabbage (Brassica pekinensis Rupr) Experiment in the Northeast Plains. Agriculture. 2024; 14(4):596. https://doi.org/10.3390/agriculture14040596
Chicago/Turabian StyleJiang, Xinming, Xu Yan, Shuyan Liu, Lili Fu, Xiaomei Gao, and Dongyan Huang. 2024. "Nitrogen Addition Decreased Respiration and Heterotrophic Respiration but Increased Autotrophic Respiration in a Cabbage (Brassica pekinensis Rupr) Experiment in the Northeast Plains" Agriculture 14, no. 4: 596. https://doi.org/10.3390/agriculture14040596
APA StyleJiang, X., Yan, X., Liu, S., Fu, L., Gao, X., & Huang, D. (2024). Nitrogen Addition Decreased Respiration and Heterotrophic Respiration but Increased Autotrophic Respiration in a Cabbage (Brassica pekinensis Rupr) Experiment in the Northeast Plains. Agriculture, 14(4), 596. https://doi.org/10.3390/agriculture14040596





