Abstract
The goal of this study is to assess the impacts of ripening stage of four Solanum fruit species: (Solanum melanocerasum, Solanum nigrum, Solanum villosum, and Solanum retroflexum) on the content of amino acids and protein. Our objective is to enhance comprehension of the maturity process, with a particular focus on Solanum fruits, in order to determine the most advantageous time for harvesting. Amino acids play a crucial role in human nutrition by serving as building blocks for various primary and secondary metabolites. They are either a source of nutraceutical substances or important dietary components. The studied Solanum fruit’s amino acid profiles were found essential and nonessential amino acids. Our findings showed that dominant amino acids were nonessential amino acids. Depending on the ripening stage, the nonessential amino acid content of the Solanum melanocerasum fruits varied from 8.22 to 9.25 g 100 g−1, Solanum villosum from 5.34 to 6.60 g 100 g−1, Solanum nigrum from 6.12 to 8.73 g 100 g−1, and Solanum retroflexum from 8.27 to 9.75 g 100 g−1. A differentiated level of total protein is found in Solanum fruits at different ripening stages. The interval was from 10.62 to 28.06 g 100 g−1 depending on species or ripening stages.
1. Introduction
Solanum, which contains over 2000 species, is one of the most extensive taxa within the Solanaceae family. Its distribution primarily encompasses tropical and subtropical regions of Africa, Australia, and select areas of Asia, including China, India, and Japan, extending across the Himalayan mountain range and into portions of the southern and eastern regions of India. The genus comprises vegetation that are native to Brazil, spanning from the northern to the southern regions. Solanum species possess bioactive phyto-constituents, including steroidal saponins, steroidal alkaloids, terpenes, flavonoids, lignans, sterols, polyphenols, fatty acids, vitamin C, coumarins, and mineral nutrients, which contribute to their nutraceutical and pharmaceutical value [1,2,3,4,5].
Amino acids are essential molecules that play a crucial role in protein synthesis, metabolism, and the immunological response. Moreover, they serve as crucial flavor-enhancing substances employed as pharmacological constituents to control diverse physiological functions and even mitigate and cure diseases [6]. Amino acids serve as a primary source of organic nitrogen in plants. The majority of proteinogenic amino acids are synthesized in the plastids of mesophyll cells. However, they can also be produced in other cellular compartments, including mitochondria, peroxisomes, and the cytosol [7,8]. Amino acids serve as both the building blocks and components of proteins, and they have a significant impact on metabolism and growth [9,10]. Assimilation of absorbed inorganic nitrogen into amino acids can occur in the roots and/or leaves. Primary transport of amino acids from roots to source foliage occurs via the xylem transpiration stream. Phloem is utilized by developing sink organs to transport amino acids synthesized in leaves or derived from roots, which are the primary transporters of organic nitrogen in the majority of plant species. As a result, the distribution and transportation of amino acids within plants are vital to their development, seed germination, and growth [11].
Amino acids are essential for metabolic activities and the transportation and storage of nutrients, including carbohydrates, proteins, vitamins, minerals, water, and lipids. Metabolic abnormalities are responsible for the development of various illnesses, including diabetes, sleeplessness, obesity, and arthritis. Optimal arrangement of amino acids has the potential to rectify these metabolic deficits [12]. Over 60% of the proteins necessary for human growth and development are derived from plant sources. Amino acids exhibit antioxidant properties. Free amino acids are crucial for the secondary metabolism of plants and the creation of chemicals like phenolics and glucosinolates. These molecules have a vital role in the interaction between the environment and human health, either directly or indirectly [13].
The synthesis and accumulation of metabolic products in Solanum spp. fruits may be primarily influenced by the meteorological conditions and management models that possess distinct local characteristics. In previous studies, an evaluation was conducted on the accumulation of organic and amino acids in the two Solanum species (Solanum nigrum and Solanum torvum) as a result of Cd stress [14] S. nigrum is also a rich source of amino acids like arginine, aspartic acid, alanine, isoleucine, l-proline, serine, and valine [15,16]. However, there remains a lack of systematic research pertaining to the composition and content diversity of amino acids in Solanum spp. The present study is focused on the occurrence of total protein and amino acids in four species of Solanum spp.: S. retroflexum, S. melanocerasum, S. nigrum, and S. villosum.
2. Materials and Methods
2.1. Field Experiment
The displayed findings represent the mean values obtained from two consecutive years (2021–2022) of harvesting. A two-factor experiment was conducted. Factor A is obtained from four species of Solanum spp.: Solanum retroflexum (SR), Solanum melanocerasum (SM), Solanum nigrum (SN), and Solanum villosum (SV) (Figure 1). Factor B comprises three discrete phases of fruit maturation: In the first stage of ripening, the fruit is green and is 30% mature, which corresponds to BBCH stage 81. In the second stage of ripening, the fruit is 40–60% purplish–violet or yellow–orange in color and is 60% mature. The inside of the fruit is not fully ripe yet, corresponding to BBCH stage 85. In the third stage of ripening, the fruit is 100% velvety black–blue or orange in color. The inside of the fruit is fully ripe, indicating 100% maturity, corresponding to BBCH stage 89 [17,18].

Figure 1.
Fruit ripening stages of Solanum sp. fruit (photos by J. Staveckienė). 1—S. retroflexum, 2—S. melanocerasum, 3—S. nigrum, 4—S. vilosum.
The two-factor experiment was carried out in four replications, with the method of arranging the variants being randomized blocks of replications. In each replicate block, 4 seedlings of each species were planted in each field. A total of 16 plants were planted per replicate block, with a length of 7.5 m and a width of 1.5 m. The width of the rows was 0.80 m and the spacing of the plants in a row was 0.80 m. A buffer zone of 1.5 m was left on each side. The total area of the experimental site was 148 m2.
In the field experiment, seedlings were planted in the soil. March saw the sowing of seeds in nurseries, with the strongest seedlings being sent to the field during the third ten-day stretch of May. A black agro film was applied on the soil surface prior to planting, and holes were made in the film for the placement of seedlings. Under the agrofilm, a drip irrigation system was set up; the watering rate was one liter per hour, depending on the weather. Fruits were collected from the block and a pooled sample was taken according to species and ripening stage.
2.2. Sample Preparation
Following the collection of the fruits for analysis, a composite sample weighing 1.5 kg was generated for each individual species and stage of ripening. The fruits were subjected to a process of purification using tap water, followed by drying, and then stored at a temperature of 34 °C. The samples underwent lyophilization for a period of 24 h using a Freeze-Drying Plant Sublimator 304 05 (ZIRBUS Technology GmbH, Bad Grund, Germany). After undergoing freeze-drying, the fruits were pulverized into a fine powder using a Grindomix GM 200 machine manufactured by Retsch GmbH in Haan, Germany. The powder was subsequently stored in a lightless environment at a temperature of 5 °C until it was analyzed.
2.3. Determination of the Total Protein Content
Total protein content (TP) was determined using the spectrophotometric method [19]. Plant material was ground with liquid nitrogen and extracted with 50 mM phosphate buffer containing 1 mM EDTA and 1 mM dithiothreitol. The extract was centrifuged for 10 min at 4000 rpm, and then 100 µL of supernatant was mixed with 1.5 mL Bradford reagent. The protein calibration curve used bovine albumin as a standard. Absorbance was read at 595 nm (Shimadzu UV-1280, Kyoto, Japan). Data are presented as the mean of three analytical samples in mg g−1 DW.
2.4. Determination of Free Amino Acids by HPLC
The free amino acid contents were analyzed by high-performance liquid chromatography (HPLC) method with UV/Vis detection (Shimadzu, Japan) according to Steed [20]. injection program, including derivatization steps with OPA and FMOC; injected mixture contained 0.5 µL sample. Amino acids were analyzed using HPLC ZORBAX Eclipse-AAA (3.5 µm; 150 × 4.6 mm) column with 40 mM phosphate buffer pH 7.8 as the mobile phase solution A and methanol/acetonitrile/water at 45/45/10 (v/v/v) as the mobile phase solution B. The flow rate was 2 mL min−1 and the following gradient was used: held at 0% B for 1.9 min, gradient from 0% to 57% B at 18.1 min, then to 100% B at 18.6 min, held at 100% B until 22.3 min, gradient from 100% to 0% B at 23.2 min, held at 0% B until 26 min. Derivatized amino acids were detected at 338 nm and 262 nm. The results are presented as g 100 g−1.
2.5. Statistical Analysis
A statistical analysis was performed using Microsoft Excel 2016 and Addinsoft XLSTAT 2022 statistical and data analysis (Long Island, NY, USA). A two-way analysis of variance followed by Tukey’s honest significant difference test at p < 0.05 for multiple comparisons was used to evaluate the differences between the means of the measurements. The principal component analysis (PCA) was performed at a 95% significance level. The results presented in PCA biplots indicate distinct effects of ripening stage and cultivar on levels of amino acids and the correlation circles (based on Pearson’s correlation matrix) that summarize relationships between investigated amino acids in different ripening stage Solanum fruits.
3. Results and Discussion
3.1. Content of Essential and Nonessential Amino Acids
Amino acids are fundamental components of proteins and are classified into essential and nonessential categories based on their production in humans. Plants exclusively synthesize the essential amino acids (leucine, isoleucine, methionine, phenylalanine, arginine, histidine, tryptophan, valine, threonine, and lysine), while both plants and humans synthesize the nonessential amino acids (alanine, β-alanine, asparagine, cysteine, glutamine, aspartic acid, glycine, proline, serine, and tyrosine) [21]. Amino acids play a crucial role in human nutrition by serving as building blocks for various primary and secondary metabolites. They are either a source of nutraceutical substances or important dietary components [22]. The quantity of amino acids differs among different plants, contingent upon their metabolic activities [23]. The transmission of substances can readily occur through the root hairs and thereafter pass through the plant’s vessels. Amino acids are crucial in several biological processes, whether they exist independently or as part of proteins. As a result, their significance and efficacy are evident in the different stages of plant growth. They enhance the cell’s ability to absorb water and nutrients from the growth media, thereby promoting vegetative growth. Additionally, they stimulate the synthesis of proteins that play various roles in plant metabolism and improve the rate at which carbon is assimilated, resulting in increased total dry matter production and ultimately higher yield [24,25].
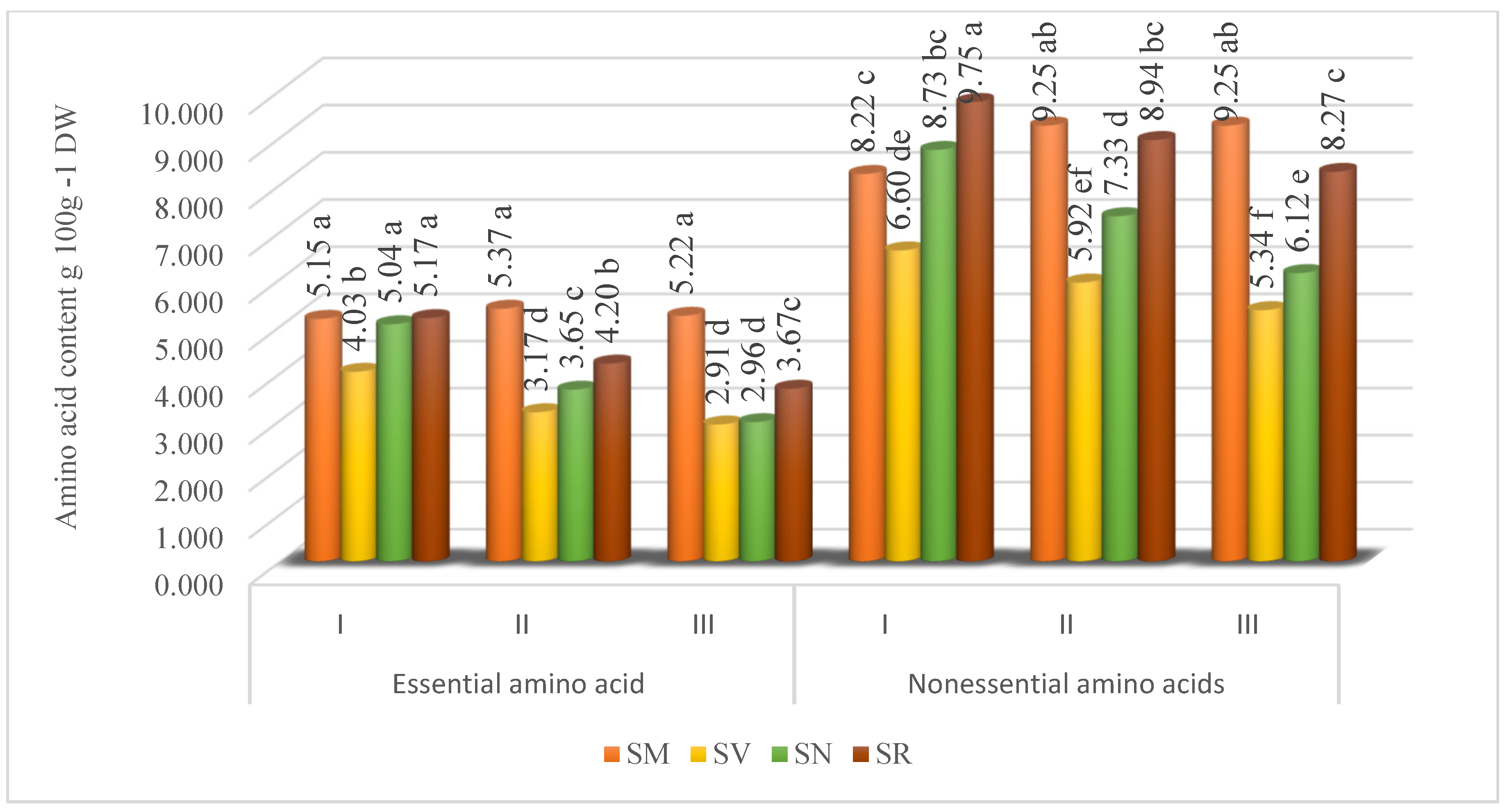
The studied Solanum fruit’s amino acid profiles were found to contain essential and nonessential amino acids. The results showed that dominant amino acids were nonessential amino acids. Depending on the ripening stage, the nonessential amino acid content of the SM fruits varied from 8.22 to 9.25 g 100 g−1, SV from 5.34 to 6.60 g 100 g−1, SN from 6.12 to 8.73 g 100 g−1, and SR from 8.27 to 9.75 g 100 g−1 (Figure 2).
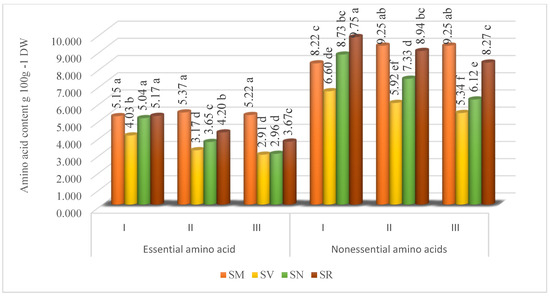
Figure 2.
Total essential and nonessential amino acid content (g 100 g−1 DW) in Solanum fruits at different ripening stages. Different small letters (a–f) represent significant differences between the means (p < 0.05); S. retroflexum—(SR), S. melanocerasum—(SM), S. nigrum—(SN), S. villosum—(SV); Ripening Stages I, II, and III.
The study revealed that the highest content of total nonessential amino acids was found in SM fruits collected at Ripening Stage II and III (9.25 and 9.25 g 100 g−1, respectively), whereas SV, SN, and SR fruits picked at Ripening Stage I contained 6.60, 8.37, and 9.75 g 100 g−1, respectively. The findings from two experimental years indicated that the total content of essential amino acids in Solanum fruits is influenced by the species and ripening stage. Depending on the maturity, the essential amino acid content of the SM varied from 5.15 to 5.37 g 100 g−1, SV from 2.96 to 4.03 g 100 g−1, SN from 2.96 to 5.04 g 100 g−1, and SR from 3.67 to 5.17 g 100 g−1. Our study results demonstrated that the harvest time did not significantly affect the content of total essential amino acids fruits of SM. The SV, SN, and SR fruits at the Ripening Stage I had the highest contents of total essential amino acids of 4.03, 5.04, and 5.17 g 100 g−1, respectively.
The investigation determined nine essential amino acids in the Solanum fruits: glutamic acid, aspartic acid, proline, arginine, alanine, serine, glycine, tyrosine, and cysteine. Our study revealed that the dominant nonessential amino acid was glutamic acid, whose content varied from 1.67 to 2.65 g 100 g−1 (Table 1). Oms-Oliu [26] indicated the comparative response ratios of amino acids in tomato fruit during the stages of growth, maturation, and ripening. The findings indicated that the levels of amino acids fluctuate depending on the various stages of maturation and ripening. Noticeable elevations in glutamic and aspartic acid, methionine, phenylalanine, and threonine are observed, while, concomitantly, a decrease in levels of β-alanine, glycine, and valine is evident during the process of maturity and ripening. At the breaker phases, the levels of other amino acids, such as asparagine, glutamine, leucine, isoleucine, and serine, increase, but they drop at later postharvest stages.

Table 1.
Nonessential amino acids content (g 100 g−1 DW) in Solanum fruits at different ripening stages.
Amino acids, such as proline and arginine, have a significant function in withstanding unfavorable environments such salt, dryness, and extreme temperatures. Proline, specifically, is involved in the creation of proteins. It has been noted that the level of proline in a plant is directly related to the intensity of stress it experiences, whether from living organisms or environmental factors. Proline tends to accumulate in the plant portions that are exposed to the stress [27].
The investigation determined nine essential amino acids in the Solanum fruits: glutamic acid, aspartic acid, proline, arginine, alanine, serine, glycine, tyrosine, and cysteine. Our study revealed that the dominant nonessential amino acid was glutamic acid, whose content varied from 1.67 to 2.65 g 100 g−1 (Table 1).
In fruits of SM, the content of this amino acid increases during ripening, and the highest content was determined at Ripening Stage III (2.62 g 100 g−1). In contrast, the highest content of this acid in SV, SN, and SR was determined at the beginning of the experiment, Ripening Stage I (1.82, 2.45, and 2.65 g 100 g−1, respectively) (Table 1). Ali et al. [28] also reported that in tomatoes glutamic acid (10.13 g 100 g−1) was the most abundant nonessential amino acid, while cysteine (0.21 g 100 g−1) was the least abundant.
Aspartic acid was identified as the second dominant nonessential amino acid in Solanum fruits (Table 1). The results indicated that this amino acid content in the studied fruits varied from 1.01 to 1.69 g 100 g−1. The highest aspartic acid contents in all studied fruits were determined at Ripening Stage I (1.69, 1.21, 1.59, and 1.64 g 100 g−1, respectively), and in maturity, content decreased (Table 1). Ali et al. [28] determined a similar content of aspartic acid in fully ripe tomatoes, g 100 g−1. Zhang et al. [29] conducted studies on kiwifruit of different varieties and found that the content of this amino acid varied from 0.05 to 0.24 g 100 g−1.
The highest contents of proline were detected in the fruits of SM and SV at Ripening Stage I (1.25 and 0.65 g 100 g−1, respectively), while in fruits of SN and SR at Ripening Stage II (0.84 and 1.200 g 100 g−1, respectively) (Table 1). Proline plays a role in plants’ defense mechanisms against biotic and biotic stressors, such as lack of water, high salt levels, intense light and UV radiation, toxic metals, and harmful microorganisms [30]. Ali et al. [28] reported that in tomatoes, proline content was 1.53 g 100 g−1.
Solanum fruit species and the ripening stage had a significant effect on the contents of arginine (Table 1). The significantly highest content of SM fruits was determined at Ripening Stages II and III (1.02 and 1.11 g 100 g−1, respectively); in contrast, the highest content of this amino acid in SV, SN, and SR fruits was at the beginning of the experiment, Ripening Stage I (0.77, 0.98, and 1.12 g 100 g−1, respectively) (Table 1). Zhang et al. [29] reported that this amino acid in different species of kiwifruit varied from 0.04 to 0.32 g 100 g−1. Pęksa et al. [31] found that arginine acid in different cultivars of potatoes varied from 0.40 to 1.98 g 100 g−1. Plants store nitrogen as arginine when nitrogen is abundant due to the high nitrogen-to-carbon ratio of arginine. The buildup of arginine is accomplished by alleviating the feedback inhibition of the N-acetylglutamate kinase (NAGK) gene, which is involved in arginine production. This process relies on the regulatory protein PII, which can detect the nitrogen and carbon levels in the cell to maximize the activity of arginine biosynthesis [32].
The highest content of nonessential amino acid alanine in SM fruits was found at Ripening Stage II (0.75 g 100 g−1); however, significant differences were not found throughout the ripening period. The highest content of this amino acid in SV, SN, and SR fruits was determined at Ripening Stage I (0.55, 0.71, and 0.75 g 100 g−1, respectively). The study by Zhang et al. [29] determined that this amino acid content of fifteen kiwifruits varied from 0.05 to 0.10 g 100 g−1.
In the investigation, results indicated that the content of amino acid serine varied from 0.42 to 0.75 g 100 g−1. In the fruits of SM, the highest content of this amino acid was determined at Ripening Stages II and III (0.72 and 0.70 g 100 g−1), while in the SV, SN, and SR, the highest amount was established at the beginning of the experiment. In these fruit species, serine content regularly decreased during the ripening period. Pęksa et al. [31] studied six different potato cultivars and determined that serine varied from 0.23 to 0.80 g 100 g−1.
The glycine acid content increased during ripening in SM fruits (0.66–0.68 g 100 g−1), while in fruits of SV, SN, and SR, it regularly decreased in maturity and it was significantly higher at Ripening Stage I (0.52, 0.64, and 0.68 g 100 g−1, respectively). Other authors who have studied kiwifruits have reported that glycine acid contents range from 0.02 to 0.07 g 100 g−1 [33]. It is known that glycine plays a crucial role in the synthesis of chlorophyll in plants by enhancing chlorophyll concentration, hence promoting optimal photosynthetic activity. Certain amino acids are distinguished by their sulfur content, which plays a significant role in numerous plant proteins. Sulfur is responsible for maintaining the three-dimensional structure of these proteins and generating the active sites of enzymes. The sulfuric amino acids that contain sulfur in their side chain include cysteine and methionine [34,35].
The content of amino acid tyrosine increased irregularly in fruits of SM, and the highest content was determined at Ripening Stage II—0.44 g 100 g−1; in contrast, in fruits of SV, SN, and SR, this amino acid decreased in maturity, and the highest content was determined at Ripening Stage I (0.33, 0.42, and 0.45 g 100 g−1, respectively).
The results indicated that the content of cysteine increased during ripening in SM fruits, and the highest content of this acid ranged from 0.28 to 0.34 g/100 g DW, respectively), and in SV, SN, and SR fruits, cysteine significantly decreased during the ripening period. Ali et al. [28] reported that amino acid tyrosine and cysteine in fully ripe tomatoes was 1.82 and 0.21 g 100 g−1, respectively.
The evaluated Solanum fruits contained eight essential amino acids: leucine, lysine, valine, phenylalanine, isoleucine, threonine, histidine, and methionine. Ali et al. [28] also found nine essential amino acids in tomatoes. Leucine is the most abundant necessary amino acid found in tomato, whereas methionine is the least abundant.
The study showed that leucine was the main essential amino acid, whose content ranged from 0.60 to 1.13 g 100 g−1 (Table 2). In the fruits of SM was found the significantly highest amount of this acid at Ripening Stage II (1.13 g 100 g−1, respectively) in SV and SN, and SR leucine was significantly higher at Ripening Stage I (0.86, 1.05, and 1.11 g 100 g−1, respectively). Ali et al. [28] reported that the content of leucine acid in fully ripe tomato fruits was 2.80 g 100 g−1.

Table 2.
Essential amino acid content (g 100 g−1 DW) in Solanum fruits at different ripening stages.
The second dominant essential amino acid in Solanum fruits was the lysine which ranged from 0.43 to 0.96 g 100 g−1 (Table 2). The highest content of lysine was found in I ripening stage in all species. The significantly highest amount of this amino acid determined SM fruits (0.96 g 100 g−1, respectively). Other authors determined 9.6 times lower content of this amino acid in fully ripe tomatoes [36].
The next established essential amino acid was valine. A study showed that the content of this amino acid in the Solanum fruits ranged from 0.40 to 0.78 g 100 g−1 (Table 2). The significantly highest amount was found in fruits of SM at Ripening Stages I and II (0.77 and 0.78 g 100 g−1, respectively). According to the other author’s studies in potatoes, valine content was determined at 1.28, kiwifruits 0.06, and tomatoes 2.49 g 100 g−1 [30,31].
The content of amino acid phenylalanine in Solanum fruits varied depending on the ripening stage and species. The highest amount of this acid was determined by SR and SM fruits at Ripening Stage I (0.76 and 0.72 g 100 g−1, respectively) while in SM in Ripening Stage II and III (0.72 and 0.75 g 100 g−1, respectively). According to Lopez et al. [36] fully ripe tomatoes were determined 2,3 times higher content of this amino acid.
The isoleucine acid content in fruits of SV, SN, and SR also depends on the ripening stage and species. The highest content of this amino acid was established at Ripening Stage I, while the ripening stage had no significant effect on this essential amino acid amount in Solanum fruits of SM and content varied from 0.56 to 0.62 g 100 g−1 during the experiment.
Essential amino acid threonine increased with ripening stages Solanum fruit of SM, the highest content of this amino acid was established at Ripening Stages II and III (0.62 and 0.59 g 100 g−1, respectively), while the threonine acid significantly decreased with maturity in fruits of SV, SN, and SR species.
The histidine acid in SM species fruits also increased with maturity (Table 2). The highest content of this acid was at Ripening Stages II and III (0.33 and 0.31 g 100 g−1, respectively). In SV, SN, and SR, fruit species are highest at Ripening Stage I (0.26, 0.31, and 0.29 g 100 g−1, respectively). Pęksa et al. [31] reported similar content of threonine and histidine acids in potatoes 0.56 and 0.44 g 100 g−1, respectively.
In the fruits of SV, SN, and SR the highest content of methionine acid was determined at Ripening Stage I (0.16, 0.21, and 0.21 g 100 g−1, respectively), while in the fruits of SM at Ripening Stage III (0.22 g 100 g−1)
Various factors before and after harvesting can impact the physical and chemical properties, nutritional value, and active components in fruits and vegetables. Studies have demonstrated that the nutritional composition, physical characteristics, and biochemical properties of fruits and vegetables are primarily influenced by their maturity and ripening stages [37]. This holds for hawthorns as well. In addition, a significant amount of unripe fruits, such as those that are thinned or dropped due to natural factors like drought, pests, or physiological diseases, are discarded in orchards every year. These fruits have potential worth for utilization and development, making their wastage a considerable loss of resources.
3.2. Content of Total Protein
Proteins are chains of amino acids that are involved in almost every process in the body. The main role of proteins is to provide the body with the amino acids needed to synthesize new proteins [38]. According to the European Union Protein Strategy [39], meat and other animal products are the main source of protein for the majority of adults in the European Union, but plant-based protein sources are also gaining popularity.
Protein is the dominant nutrient in human nutrition that is caused by the need to ensure the optimal level of essential amino acids directly affecting vital functions; since the body cannot store protein, it is supplied to the body exclusively through nutrition [38].
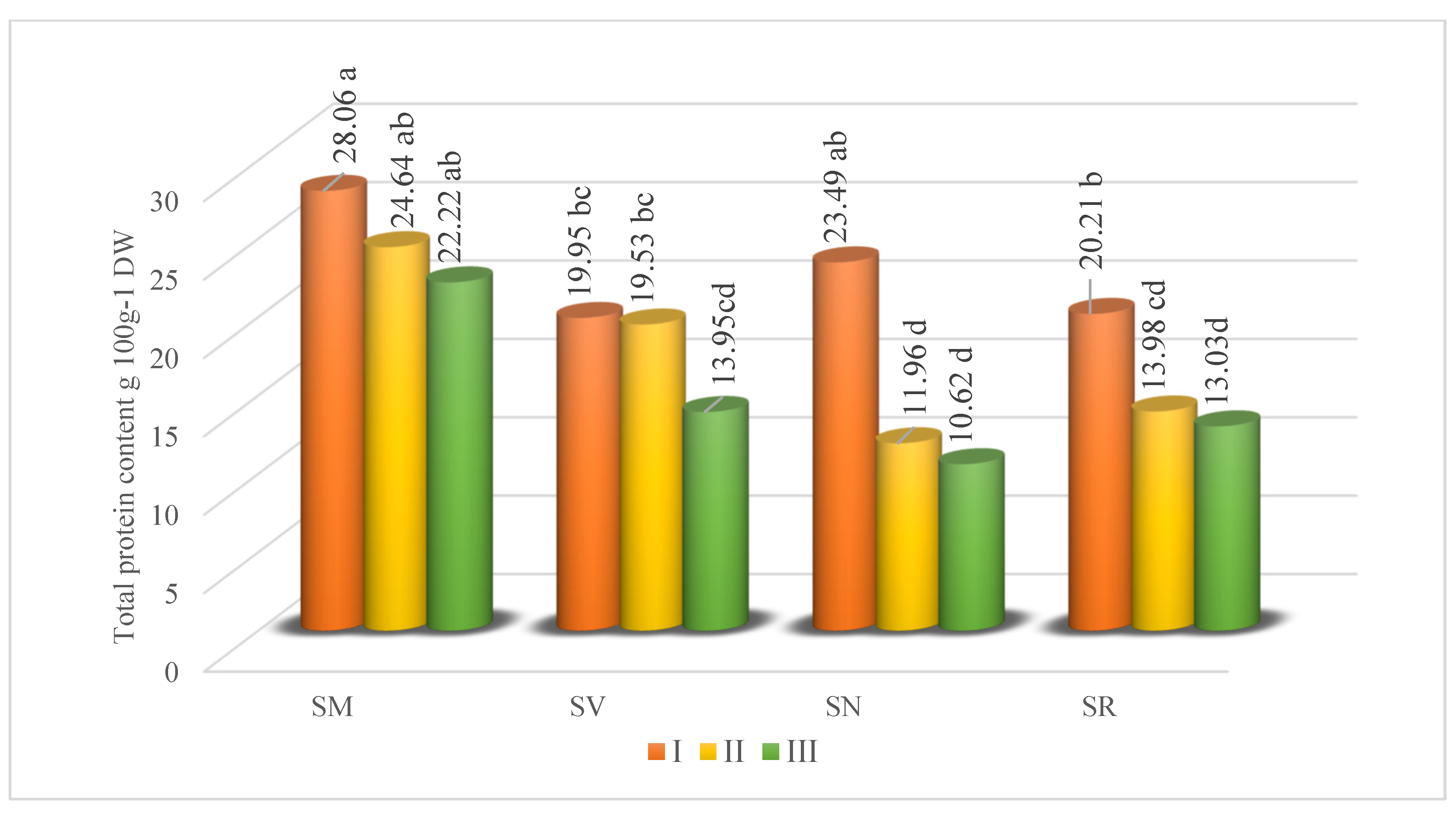
A differentiated level of total protein is found in Solanum fruits at different ripening stages. The interval was from 10.62 to 28.06 g 100 g−1 depending on species or ripening stages (Figure 3). Unripe Solanum fruit was characterized by higher protein content than ripe fruit, confirming that protein content in fruit decreases during ripening. The synthesis of protein took place for the most part during the early stage of ripening before marked physical changes became apparent in the tissue (like color). The highest amount of total protein was determined in SM fruits in the I stage of ripening. Tlili et al. [40] monitored the variation of protein content in Rhus tripartitum fruit and found that protein content grew from 4.81% (immature) to 9.37% (intermediate) in Ain Jalloula species, and from 6.16% (immature) to 10.5% (intermediate) in Dkhila species. The rise in protein levels during the process of ripening may be attributed to the activation of certain enzymes, such as cellulase and polygalacturonase, which have important functions in the softening of the fruit. Furthermore, the protein level in R. tripartitum reduced to 6.75% and 7.88% in Ain Jalloula and Dkhila, respectively. This drop exceeded 25%. According to Vendramini and Trugo [41], protein content can drop by 30% as a result of biochemical degradation and the potential formation of secondary metabolites throughout the maturation process. This reduction would not impact the nutritional value, but it could potentially influence the flavor attributes of the fruits.
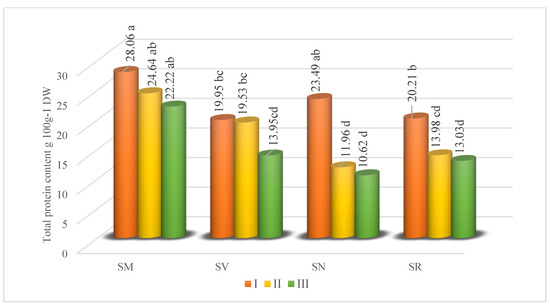
Figure 3.
Total protein content (g 100 g−1 DW in Solanum fruits at different ripening stages. Different small letters (a–d) represent significant differences between the means (p < 0.05); S. retroflexum—(SR), S. melanocerasum—(SM), S. nigrum—(SN), S. villosum—(SV); Ripening Stages I, II, and III.
3.3. Principal Component Analysis
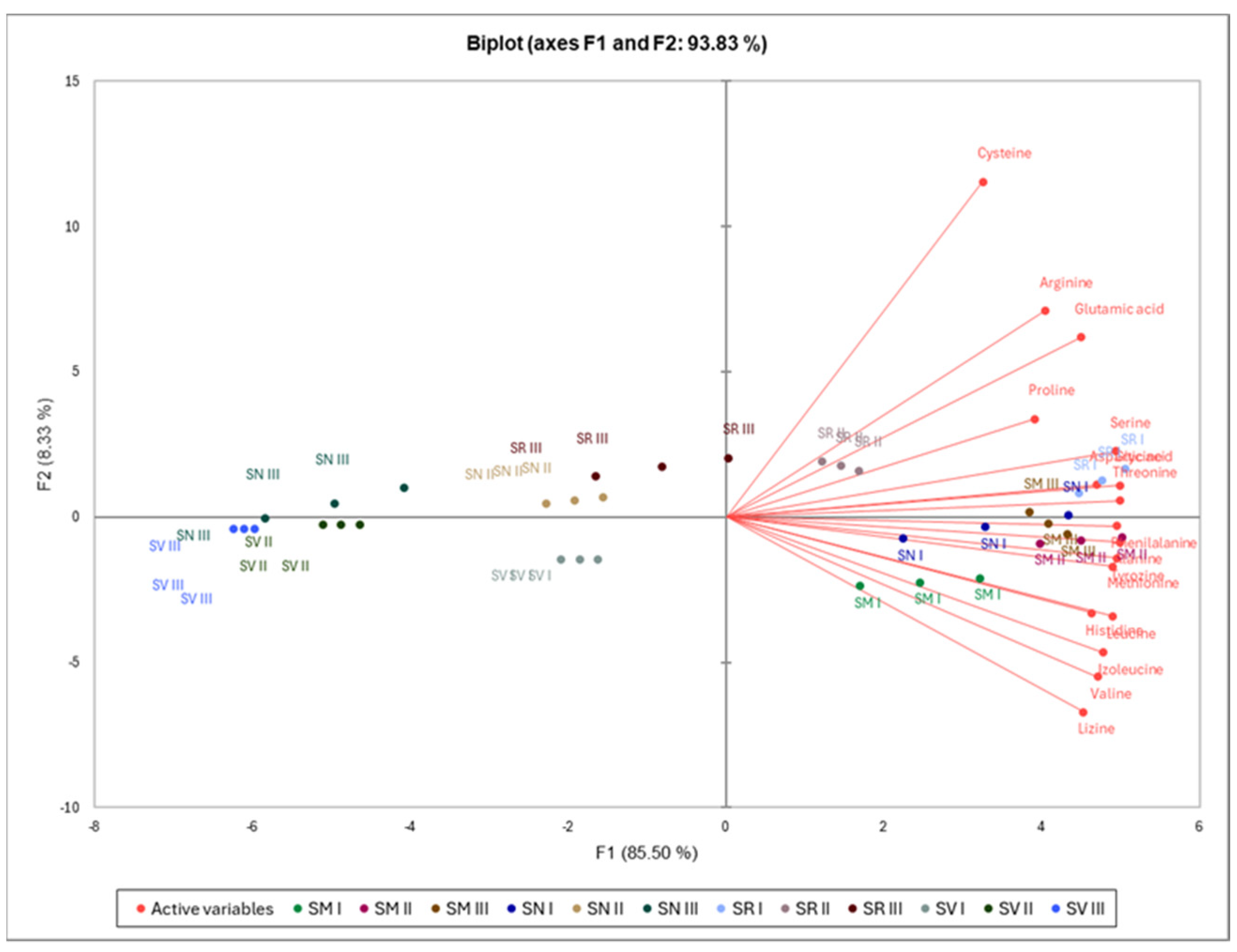
The first two PCAs extracted from the components amounted to 93.83% of the total data (Figure 4). The PCA indicates that histidine, alanine, tyrosine, valine, methionine, lysine, isoleucine, leucine, and phenylalanine were associated with SM I–III and SN I, which had positive and negative score along with F1 and F2, respectively. Aspartic acid, glutamic acid, serine, glycine, threonine, arginine, proline, and cysteine were associated with SR I–II, which had a positive score along with F1 and F2. There were no associations between F1 and F2 scores of the SV I–III, SN II–III, and SR at Ripening Stage II with individual free amino acids.
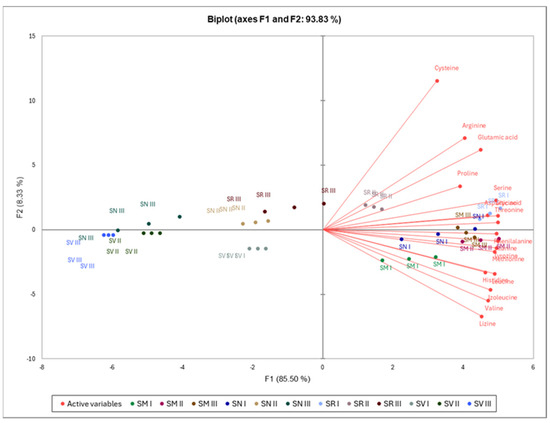
Figure 4.
Principal component analysis of free amino acids in Solanum species fruits. Note: S. retroflexum—(SR), S. melanocerasum—(SM), S. nigrum—(SN), S. villosum—(SV); Ripening Stages I, II, and III.
4. Conclusions
The results of this study provide additional and valuable insights into the beneficial properties of Solanum fruits, which depend on the accumulation of amino acids and protein content in fruits. The study revealed that the highest content of total nonessential amino acids was found in SM fruits collected at Ripening Stages II and III. Therefore, we can state that the maturity of fruits did not significantly affect the content of total essential amino acids in fruits of SM. The SV, SN, and SR fruits at Ripening Stage I had the highest contents of total essential amino acids of 4.03, 5.04, and 5.17 g 100 g−1, respectively.
Unripe Solanum fruit was characterized by higher protein content, and a tendency was found that the protein content decreased as the fruit ripens.
Author Contributions
Conceptualization, J.S., V.V.-K., J.K., B.M. and E.J.; methodology, J.S. and V.V.-K.; software, J.S. and V.V.-K.; validation, J.S., V.V.-K., J.K., B.M. and E.J.; formal analysis, J.S. and V.V.-K.; investigation, J.S. and V.V.-K.; resources, J.S. and V.V.-K.; data curation, J.S., V.V.-K., J.K. and B.M.; writing—original draft preparation, J.S., V.V.-K., J.K., B.M. and E.J.; writing—review and editing, J.S., J.K., B.M. and E.J.; visualization, J.S.; supervision, J.S. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Patel, S. Rose Hip as an Underutilized Functional Food: Evidence-Based Review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Patel, P.; Prasad, A.; Srivastava, K.; Singh, S.S.; Chakrabarty, D.; Misra, P. Updates on Steroidal Alkaloids and Glycoalkaloids in Solanum Spp.: Biosynthesis, in Vitro Production and Pharmacological Values. Stud. Nat. Prod. Chem. 2021, 69, 99–127. [Google Scholar] [CrossRef]
- Staveckienė, J.; Medveckienė, B.; Jarienė, E.; Kulaitienė, J. Effects of Different Ripening Stages on the Content of the Mineral Elements and Vitamin C of the Fruit Extracts of Solanum Species: S. melanocerasum, S. nigrum, S. villosum, and S. retroflexum. Plants 2024, 13, 343. [Google Scholar] [CrossRef]
- Staveckienė, J.; Kulaitienė, J.; Levickienė, D.; Vaitkevičienė, N. Changes in Fatty Acid Content in Solanum Spp. Fruits during Ripening. Plants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Staveckienė, J.; Kulaitienė, J.; Levickienė, D.; Vaitkevičienė, N.; Vaštakaitė-Kairienė, V. The Effect of Ripening Stages on the Accumulation of Polyphenols and Antioxidant Activity of the Fruit Extracts of Solanum Species. Plants 2023, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, B.; Luo, Z.; Yuan, Y.; Zhao, Z.; Liu, M. Composition Analysis and Nutritional Value Evaluation of Amino Acids in the Fruit of 161 Jujube Cultivars. Plants 2023, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Rentsch, D. Uptake and Partitioning of Amino Acids and Peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Schmidt, S.; Tegeder, M. Transporters for Uptake and Allocation of Organic Nitrogen Compounds in Plants. FEBS Lett. 2007, 581, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.B.; Teixeira, A.R.N. Amino acids, Metabolism. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 197–206. [Google Scholar] [CrossRef]
- Lea, P.J.; Azevedo, R.A. Amino Acids. In Encyclopedia of Applied Plant Sciences; Academic Press: Cambridge, MA, USA, 2016; Volume 2, pp. 56–66. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and Sink Mechanisms of Nitrogen Transport and Use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S. Amino acid content in Rhododendron schlippenbachii Maxim. flowers of different colors. Biosci. Biotechnol. Res. Asia 2016, 13, 1285–1289. [Google Scholar] [CrossRef]
- Gomes, M.H.; Rosa, E. Free amino acid composition in primary and secondary inflorescences of 11 broccoli (Brassica oleracea var italica) cultivars and its variation between growing seasons. J. Sci. Food Agric. 2001, 81, 295–299. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative Physiological Responses of Solanum nigrum and Solanum torvum to Cadmium Stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Gupta, A.K.; Majumder, U.K.; Ghosal, S. The chemistry behind the toxicity of black nightshade, Solanum nigrum and the remedy. Pharmacologyonline 2009, 1, 705–723. [Google Scholar]
- Jabamalairaj, A.; Priatama, R.A.; Heo, J.; Park, S.J. Medicinal Metabolites with Common Biosynthetic Pathways in Solanum nigrum. Plant Biotechnol. Rep. 2019, 13, 315–327. [Google Scholar] [CrossRef]
- Kliszcz, A. Phenological Growth Stages and BBCH-Identification Keys of Jerusalem Artichoke (Helianthus tuberosus L.). Ann. Univ. Paedagog. Cracoviensis Stud. Naturae 2021, 6, 203–225. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Stinco, C.M.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J. Effect of Regulated Deficit Irrigation on Commercial Quality Parameters, Carotenoids, Phenolics and Sugars of the Black Cherry Tomato (Solanum lycopersicum L.) ‘Sunchocola’. J. Food Compos. Anal. 2022, 105, 104220. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Steed, R. Analysis of Amino Acids by HPLC Analysis of Amino Acids by HPLC Amino Acid Analysis-Agilent Restricted; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2010. [Google Scholar]
- Baqir, H.A.; Zeboon, N.H.; Al-Behadili, A.A.J. The role and importance of amino acids within plants: A review. Plant Arch. 2019, 19, 1402–1410. [Google Scholar]
- Sharma-Natu, P.; Ghildiyal, M.C. Potential targets for improving photosynthesis and crop yield. Curr. Sci. 2005, 88, 1918–1928. [Google Scholar]
- Dreccer, M.F.; Van Oijen, M.; Schapendonk, A.H.C.M.; Pot, C.S.; Rabbinge, R. (Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy. Impact on canopy photosynthesis. Ann. Bot. 2000, 86, 821–831. [Google Scholar] [CrossRef]
- Abu-Dahi, Y.M.; Al-Younis, M.A. Plant Nutrition Handbook; Ministry of Higher Education and Scientific Research of Baghdad: Baghdad, Iraq, 1988; p. 411. [Google Scholar]
- Oms-Oliu, G.; Hertog, M.L.A.T.M.; Van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.H.; Nicolai, B.M. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative Analysis of Physicochemical Characteristics, Nutritional and Functional Components and Antioxidant Capacity of Fifteen Kiwifruit (Actinidia) Cultivars—Comparative Analysis of Fifteen Kiwifruit (Actinidia) Cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, Transport, and Regulation of Amino Acids: What Is Missing in Rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Nemś, A.; Rytel, E. The Free-Amino-Acid Content in Six Potatoes Cultivars through Storage. Molecules 2021, 26, 1267. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.; Kaufman, P.B.; Warber, S.; Bolling, S.; Chang, S.C. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J. Agric. Food Chem. 2003, 51, 3973–3976. [Google Scholar] [CrossRef]
- Menz, G.; Vriesekoop, F. Physical and chemical changes during the maturation of Gordal sevillana olives (Olea europaea L., cv. Gordal sevillana). J. Agric. Food Chem. 2010, 58, 4934–4938. [Google Scholar]
- Zheng, H.Z.; Kim, Y.I.; Chung, S.K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Pollack, M.A. The end of creeping competence? EU policy-making since Maastricht. J. Common Mark. Stud. 2000, 3, 519–538. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Baum, J.I.; Børsheim, E.; Allman, B.R.; Walker, S. Health benefits of dietary protein throughout the life cycle. In The Health Benefits of Foods-Current Knowledge and Further Development; IntechOpen: London, UK, 2020. [Google Scholar]
- Pikosky, M.A.; Ragalie-Carr, J.; Miller, G.D. Recognizing the Importance of Protein Quality in an Era of Food Systems Transformation. Front. Sustain. Food Syst. 2022, 6, 1012813. [Google Scholar] [CrossRef]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “Requirements” beyond the RDA: Implications for Optimizing Health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef]
- Tlili, N.; Tir, M.; Benlajnef, H.; Khemiri, S.; Mejri, H.; Rejeb, S.; Khaldi, A. Variation in protein and oil content and fatty acid composition of Rhus tripartitum fruits collected at different maturity stages in different locations. Ind. Crops Prod. 2014, 59, 197–201. [Google Scholar] [CrossRef]
- Vendramini, A.L.; Trugo, L.C. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).