Comparative Effects of No-dig and Conventional Cultivation with Vermicompost Fertilization on Earthworm Community Parameters and Soil Physicochemical Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Producing Vermicompost from Waste Sugar Beet Pulp
2.2. Experimental Design
- NDG—no-dig + vermicompost;
- DG—conventional digging + vermicompost;
- MW—perennial hay meadow (comparative site with no intervention).
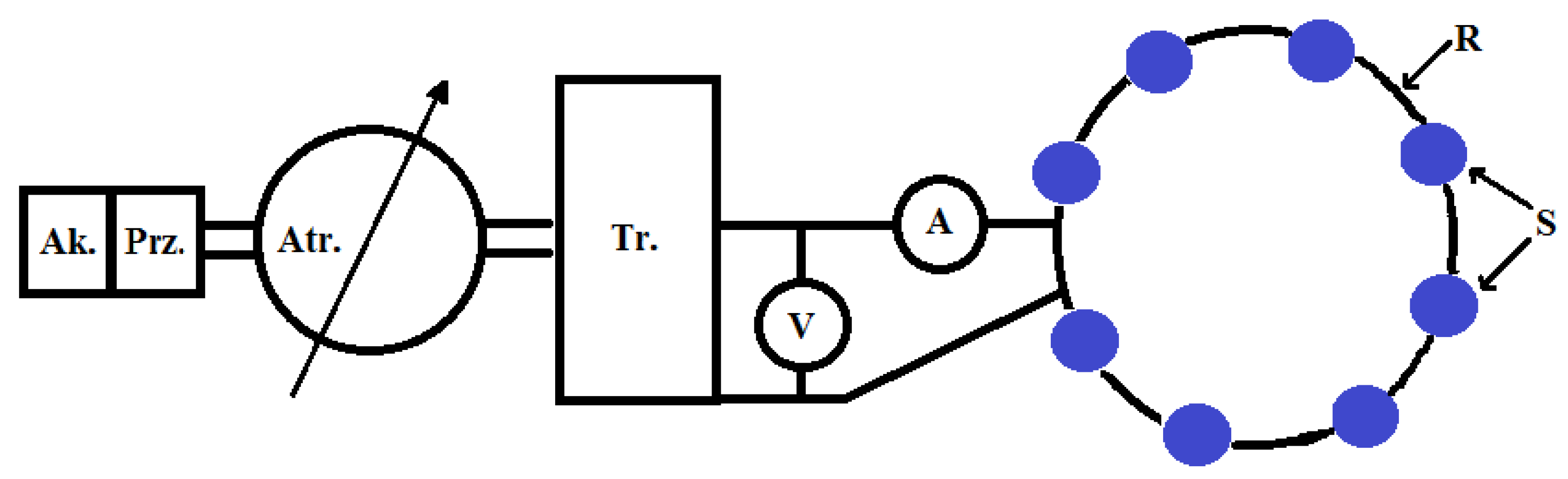
2.3. Earthworm and Soil Sampling
2.4. Physicochemical Analysis of Soil
2.5. Data Analysis
3. Results and Discussion
3.1. Earthworm Species in the Study Area
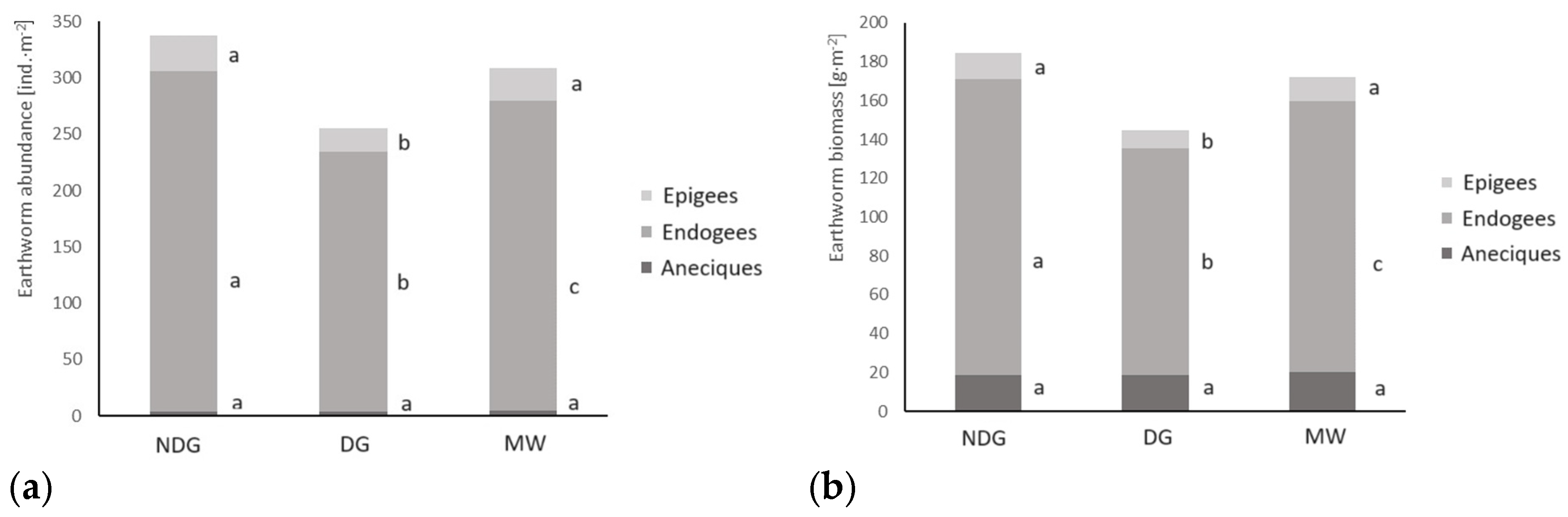
3.2. Effect of Applied Tillage Technologies on Earthworm Abundance and Biomass
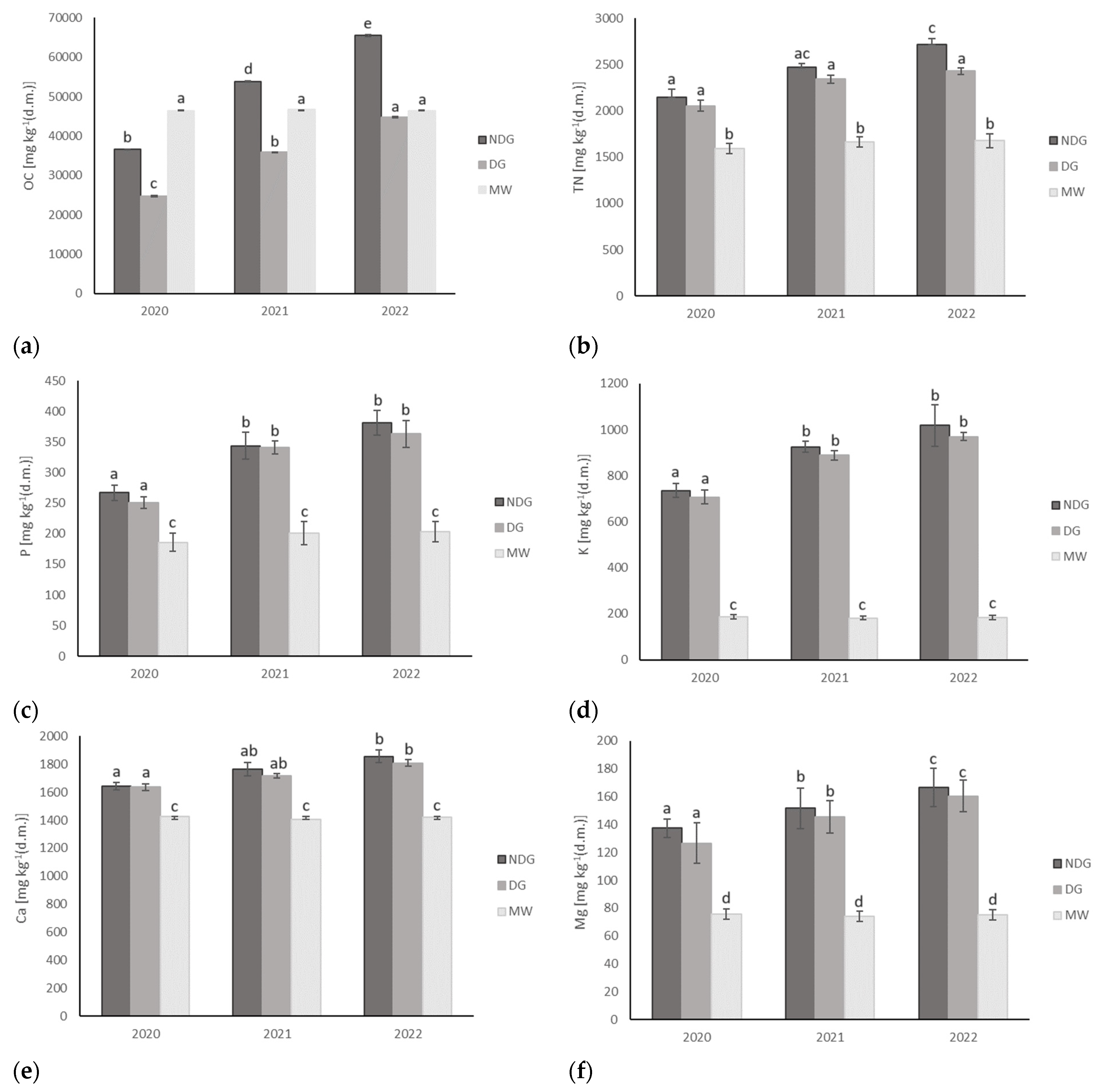
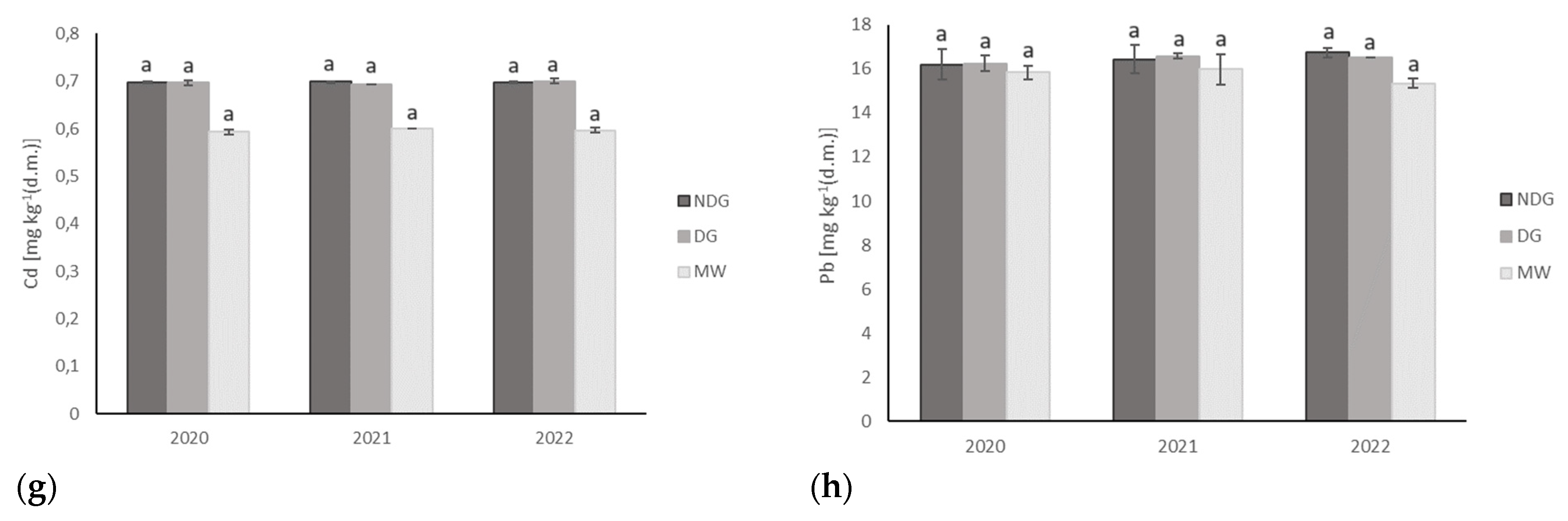
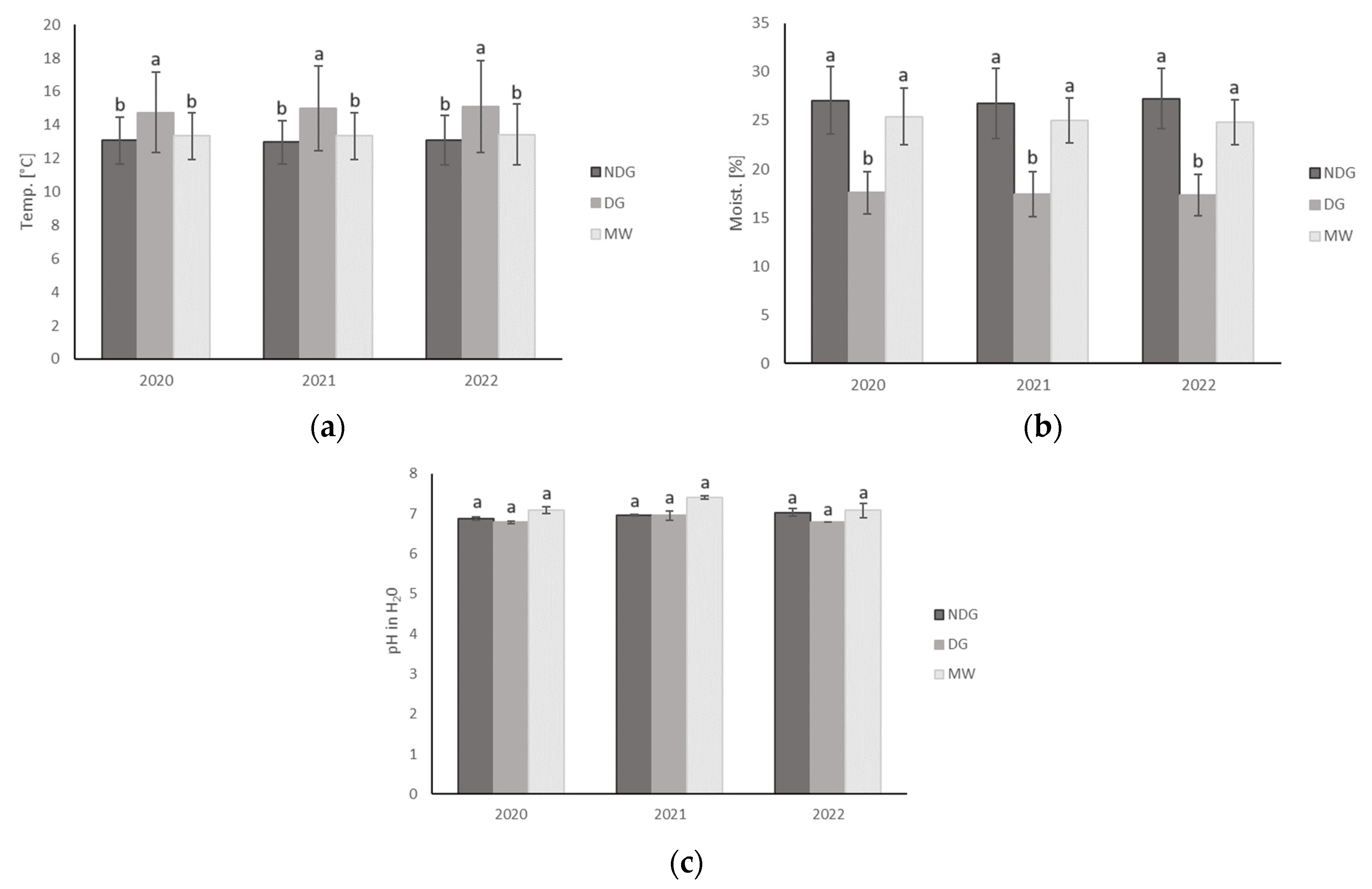
3.3. Effect of Tillage Technologies on Selected Soil Physicochemical Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, A. Soil degradation: A challenge to sustainable agriculture. Int. J. Sci. Res. Agric. Sci. 2014, 1, 50–55. [Google Scholar] [CrossRef]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Robinson, D.; Emmett, B.; Reynolds, B.; Rowe, E.; Spurgeon, D.; Keith, A.; Lebron, I.; Hockley, N.; Hester, R.; Harrison, R. Soil natural capital and ecosystem service delivery in a world of global soil change. In Soils and Food Security; Hester, R.E., Harrison, R.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2012; Volume 19, pp. 41–68. [Google Scholar] [CrossRef]
- Singer, M.J.; Warkentin, B.P. Soil in an environmental context: An American perspective. Catena 1996, 27, 179–189. [Google Scholar] [CrossRef]
- Montanarella, L. Trends in land degradation in Europe. In Climate and land Degradation; Sivakumar, M.V.K., Ndiangui, N., Eds.; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Jamatia, S.K.S.; Chaudhuri, P.S. Earthworm community structure under tea plantations (Camellia sinensis) of Tripura (India). Trop. Ecol. 2017, 58, 105–113. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J.; Linden, D.R.; Subler, S. Earthworms in agroecosystems. In Earthworm Biology and Biogeography in North America; Hendrix, P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 185–220. ISBN 978-156-670-053-5. [Google Scholar]
- Blouin, M.; Hodson, M.E.; Aranda Delgado, E.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Pérès, G.; Tondoh, J.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Scullion, J. Interactions between earthworms and residues of differing quality affecting aggregate stability and microbial dynamics. Appl. Soil Ecol. 2013, 64, 56–62. [Google Scholar] [CrossRef]
- Bossuyt, H.; Six, J.; Hendrix, P.F. Protection of soil carbon by microaggregates within earthworm casts. Soil Biol. Biochem. 2005, 37, 251–258. [Google Scholar] [CrossRef]
- Van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-induced changes in the functional diversity of soil biota—A review with a focus on German data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Hendrix, P.F.; Mueller, B.R.; Bruce, R.R.; Langdale, G.W.; Parmelee, R.W. Abundance and distribution of earthworms in relation to landscape factors on the Georgia Piedmont, U.S.A. Soil Biol. Biochem. 1992, 24, 1357–1361. [Google Scholar] [CrossRef]
- Curry, J.P. Factors affecting the abundance of earthworms in soils. In Earthworm Ecology, 2nd ed.; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 91–113. ISBN 978-042-912-904-9. [Google Scholar]
- Boström, U. Earthworm populations (Lumbricidae) in ploughed and undisturbed leys. Soil Till. Res. 1995, 35, 125–133. [Google Scholar] [CrossRef]
- Brown, G.G.; Benito, N.P.; Pasini, A.; Sautter, K.D.; de Guimaraes, M.F.; Torres, E. No-tillage greatly increases earthworm populations in Parana’ state, Brazil. Pedobiologia 2003, 47, 764–771. [Google Scholar] [CrossRef]
- Johnson-Maynard, J.L.; Umiker, K.J.; Guy, S.O. Earthworm dynamics and soil physical properties in the first three years of no-till management. Soil Till. Res. 2007, 94, 338–345. [Google Scholar] [CrossRef]
- Kladivko, E.J.; Akhouri, N.M.; Weesies, G. Earthworm populations and species distributions under no-till and conventional tillage in Indiana and Illinois. Soil Biol. Biochem. 1997, 29, 613–615. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm ecology in cultivated soils. In Earthworm Ecology: From Darwin to Vermiculture; Satchell, J.E., Ed.; Chapman and Hall: London, UK, 1983; pp. 123–137. [Google Scholar]
- Holland, J.M. The environmental consequences of adopting conservation tillage in Europe: Reviewing the evidence. Agric. Ecosyst. Environ. 2004, 103, 1–25. [Google Scholar] [CrossRef]
- Chan, K.Y. An overview of some tillage impacts on earthworm population abundance and diversity—Implications for functioning in soils. Soil Till. Res. 2001, 57, 179–191. [Google Scholar] [CrossRef]
- Yusnaini, S.; Niswati, A.; Arif, M.A.S.; Komalasari, Y.; Kaneko, N. Earthworm population under different soil tillage and herbicide application at integrated field laboratory agriculture faculty, University of Lampung. IOP Conf. Ser. Earth Environ. Sci. 2015, 215, 012015. [Google Scholar] [CrossRef]
- Peigne, J.; Ball, B.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. B 2008, 363, 477–494. [Google Scholar] [CrossRef]
- Strudley, M.W.; Green, T.R.; Ascough, J.C., II. Tillage effects on soil hydraulic properties in space and time: State of the science. Soil Till. Res. 2008, 99, 4–48. [Google Scholar] [CrossRef]
- Pączka, G.; Mazur-Pączka, A.; Garczyńska, M.; Kostecka, J.; Butt, K.R. Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts. Agriculture 2020, 10, 481. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Thieleman, U. Elektrischer regenwurmfang mit der oktett-methode. Pedobiologia 1986, 29, 296–302. [Google Scholar] [CrossRef]
- Mazur-Pączka, A.; Pączka, G.; Kostecka, J.; Podolak, A.; Garczyńska, M. Effectiveness of Lumbricidae extracting with an environmentally friendly method. J. Ecol. Eng. 2020, 21, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, K.; Soil, O., III. The Family of Earthworms (Lumbricidae), the Keys to Indicate the Invertebrates of Poland; PWN: Warsaw, Poland, 1986; 187p. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. The Soil and Plants Method of Analysis and Evaluation; IOŚ Publishing: Warsaw, Poland, 1991; 334p. [Google Scholar]
- PN ISO 11465:1999; Soil Quality—Determination of Dry Matter Content of Soil and Water in Soil in Terms of Dry Mass—Weight Method. Polish Standardization Committee: Warsaw, Poland, 1999; pp. 1–3.
- Southwood, T.R.E. Ecological Methods, 2nd ed.; Chapman and Hall: London, UK, 1978; ISBN 978-0-412-30710-2. [Google Scholar]
- Kasprzak, K.; Niedbała, W. Biocenotic indices used for data organisation and analysis in the quantitative studies. In Methods Used in Soil Zoology; Górny, M., Grüm, L., Eds.; PWN: Warsaw, Poland, 1981; pp. 397–416. [Google Scholar]
- Górny, M.; Grüm, L. Methods Used in Soil Zoology; PWN: Warsaw, Poland, 1981; 483p. [Google Scholar]
- Dumnicka, E.; Kostecka, J. Overview of oligochaeta (Oligochaeta) and leeches (Hirudinea) in the Bieszczady Mountains. Bieszczadzkie Monogr. 2004, 7, 15–28. [Google Scholar]
- Mazur-Pączka, A.; Pączka, G.; Kostecka, J.; Butt, K.R.; Jaromin, M.; Garczyńska, M.; Podolak, A. Community structure of Lumbricidae in beech woodland of the Bieszczady National Park (Carpathian mountains, SE Poland). Pedosphere 2021, 31, 391–397. [Google Scholar] [CrossRef]
- Bouche, M.B. Strategies lombriciennes. In Soil Organisms as Components of Ecosystems; Lohm, U., Persson, T., Eds.; Ecological Bulletins: Stockholm, Sweden, 1977; Volume 25, pp. 122–132. [Google Scholar]
- Mazur-Pączka, A.; Pączka, G.; Kostecka, J.; Garczyńska, M.; Podolak, A.; Szura, R. Community structure of Lumbricidae in permanent grassland and arable land. J. Ecol. Eng. 2019, 20, 1–6. [Google Scholar] [CrossRef]
- Mazur-Pączka, A.; Pączka, G.; Garczyńska, M.; Jaromin, M.; Hajduk, E.; Kostecka, J.; Butt, K.R. Effects of energy crop monocultures and sewage sludge fertiliser on soils and earthworm community attributes. Agriculture 2023, 13, 323. [Google Scholar] [CrossRef]
- Mazur-Pączka, A.; Butt, K.R.; Garczyńska, M.; Kostecka, J.; Pączka, G. Effects of selected annual and perennial energy crops on Lumbricidae community assemblages. J. Ecol. Eng. 2023, 24, 287–293. [Google Scholar] [CrossRef]
- Rodríguez, M.P.; Domínguez, A.; Ferroni, M.M.; Wall, L.G.; Bedano, J.C. The diversification and intensification of crop rotations under no-till promote earthworm abundance and biomass. Agronomy 2020, 10, 919. [Google Scholar] [CrossRef]
- Peigne, J.; Cannavaciuolo, M.; Gautronneau, Y.; Aveline, A.; Giteau, J.L.; Cluzeau, D. Earthworm populations under different tillage systems in organic farming. Soil Till. Res. 2009, 104, 207–214. [Google Scholar] [CrossRef]
- Doube, B.M.; Buckerfield, J.C.; Kirkegaard, J.A. Short-term effects of tillage and stubble man-agement on earthworm populations in cropping systems in southern New South Wales. Aust. J. Agric. Res. 1994, 45, 1587–1600. [Google Scholar] [CrossRef]
- Berry, E.C.; Karlen, D.L. Comparison of alternative farming systems. II. Earthworm population density and species diversity. Am. J. Altern. Agric. 1993, 8, 21–26. [Google Scholar] [CrossRef]
- Capowiez, Y.; Cadoux, S.; Bouchant, P.; Ruy, S.; Roger-Estrade, J.; Richard, G.; Biozard, H. The effect of tillage type and cropping system on earthworm communities, macroporosity and water infiltration. Soil Till. Res. 2009, 105, 209–216. [Google Scholar] [CrossRef]
- Tripathi, G.; Bhardwaj, P. Earthworm diversity and habitat preferences in arid regions of Rajasthan. Zoos Print J. 2004, 19, 1515–1519. [Google Scholar] [CrossRef]
- Adigun, M.O.; Akinbola, G.E.; Olaleye, A.O.; Obuh, J. Variability of soil properties across planted fallows under earthworm casts on an alfisols in South Western Nigeria. World J. Agric. Sci. 2008, 4, 435–441. [Google Scholar]
- Whalen, J.; Parmelee, R.; Edwards, C. Population dynamics of earthworm communities in corn agroecosystems receiving organic or inorganic fertilizer amendments. Biol. Fertil. Soils. 1998, 27, 400–407. [Google Scholar] [CrossRef]
- Gwenzi, W.; Gotosa, J.; Chakanetsa, S.; Mutema, Z. Effects of tillage systems on soil organic carbon dynamics, structural stability and crop yields in irrigated wheat (Triticum aestivum L.)–cotton (Gossypium hirsutum L.) rotation in semiarid Zimbabwe. Nutr. Cycl. Agroecosys. 2009, 83, 211–221. [Google Scholar] [CrossRef]
- Smith, P. Carbon sequestration in croplands: The potential in Europe and the global context. Eur. J. Agron. 2004, 20, 229–236. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Haque, M.; Biswas, J.C. Emission factors and global warming potential as influenced by fertilizer management for the cultivation of rice under varied growing seasons. Environ. Res. 2021, 197, 111156. [Google Scholar] [CrossRef]
- Jat, R.S.; Ahlawat, I.P.S. Direct and residual effect of vermicompost, biofertilizers and phosphorus on soil nutrient dynamics and productivity of chickpea-fodder maize sequence. J. Sustain. Agric. 2006, 28, 41–54. [Google Scholar] [CrossRef]
- Sakar, S.; Singh, S.R. Interactive effect of tillage depth and mulch on soil temperature, productivity and water use pattern of rainfed barley (Hordium vulgare L.). Soil Till. Res. 2007, 92, 79–86. [Google Scholar] [CrossRef]
- Guan, D.; Zhang, Y.; Mahdi, M.; Kaisi, A.; Wang, Q.; Zhang, M.; Li, Z. Tillage practices effect on root distribution and water use efficiency of winter wheat under rain-fed condition in the North China Plain. Soil Till. Res. 2015, 146, 286–295. [Google Scholar] [CrossRef]
- Matula, S. The influence of tillage treatments on water infiltration. Plant Soil Environ. 2003, 49, 298–306. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. Interactions between earthworms, microorganisms, and other invertebrates. In Biology and Ecology of Earthworms; Springer: New York, NY, USA, 1995; pp. 275–301. [Google Scholar] [CrossRef]
- Neilson, R.; Boag, B. Feeding preferences of some earthworm species common to upland pastures in Scotland. Pedobiologia 2003, 47, 1–8. [Google Scholar] [CrossRef]
- Cooke, A.; Luxton, M. Effect of microbes on food selection by Lumbricus terrestris L. Rev. Ecol. Biol. Sol. 1980, 17, 365–370. [Google Scholar]
- Brown, G.G. How do earthworms affect microfloral and faunal community diversity? Plant Soil 1995, 170, 209–231. [Google Scholar] [CrossRef]

| Substrate Characteristics | ||||||
|---|---|---|---|---|---|---|
| Parameter | Units | Vermicompost | SBAT | NDG | DG | MW |
| OC | mg kg−1 (d.m.) | 98153.3 ± 1722.4 | 13855.6 ± 159.6 a | 51996.6. ± 12584.9 b | 35149.5 ± 8687.8 c | 46527.7 ± 204.8 b |
| TN | 4911.4 ± 47.1 | 1245.5 ± 166.3 a | 2445.6 ± 255.8 b | 2276.7 ± 174.5 b | 1646.3 ± 65.5 c | |
| P | 274.2 ± 19.8 | 239.7 ± 21.5 a | 330.8 ± 52.9 b | 318.7 ± 53.2 b | 196.6 ± 16.6 a | |
| K | 3563.3 ± 41.9 | 214.5 ± 19.7 a | 892.3 ± 134.2 b | 855.1 ± 118.2 b | 183.2 ± 4.2 a | |
| Ca | 1989.5 ± 53.4 | 1522.3 ± 76.4 a | 1755.2 ± 98.7 b | 1719.9 ± 77.5 b | 1416.9 ± 12.3 a | |
| Mg | 288.1 ± 25.7 | 88.7 ± 11.5 a | 151.7 ± 16.4 b | 144.1 ± 18.2 b | 75.1 ± 2.8 a | |
| Cd | 0.8 ± 0.1 | 0.6 ± 0.0 a | 0.7 ± 0.0 a | 0.7 ± 0.0 a | 0.6 ± 0.0 a | |
| Pb | 0.7 ± 0.1 | 16.2 ± 0.6 a | 16.5 ± 0.5 a | 16.4 ± 0.2 a | 15.7 ± 0.6 a | |
| C/N ratio | - | 19.98 ± 1.5 | 11.13 ± 0.5 a | 20.99 ± 3.1 b | 15.27 ± 2.8 c | 28.30 ± 1.0 d |
| pH in H 02 | - | 6.33 ± 0.2 | 7.13 ± 0.3 a | 6.95 ± 0.1 a | 6.84 ± 0.1 a | 7.19 ± 0.2 a |
| Electrical conductivity | mS-cm−1 | 2.39 ± 0.09 | 0.27 ± 0.03 a | 0.62 ± 0.04 b | 0.47 ± 0.05 c | 0.25 ± 0.03 a |
| Temp. | °C | - | - | 13.0 ± 1.2 a | 14.9 ± 2.2 b | 13.4 ± 1.3 a |
| Moisture | % | - | - | 27.0 ± 2.9 a | 17.4 ± 1.9 b | 25.1 ± 2.2 a |
| Species/Ecological Group * | Features | Cultivation Treatments ** | ||
|---|---|---|---|---|
| NDG | DG | MW | ||
| Epigees | ||||
| Dendrodrilus rubidus | Abundance | 9.9 ± 2.4 a | 5.8 ± 1.0 b | 9.2 ± 2.2 a |
| Biomass | 1.24 ± 0.31 a | 0.72 ± 0.13 b | 1.17 ± 0.28 a | |
| Dominance % | 2.93 | 2.27 | 2.98 | |
| Lumbricus rubellus | Abundance | 21.7 ± 3.2 a | 15.5 ± 2.5 b | 19.9 ± 2.6 a |
| Biomass | 12.45 ± 2.02 a | 8.84 ± 1.49 b | 11.40 ± 1.55 a | |
| Dominance % | 6.43 | 6.07 | 6.45 | |
| Endogees | ||||
| Aporrectodea caliginosa | Abundance | 168.5 ± 15.3 a | 132.7 ± 15.4 b | 147.7 ± 13.2 c |
| Biomass | 81.29 ± 8.79 a | 64.18 ± 7.24 b | 71.77 ± 6.62 c | |
| Dominance % | 49.96 | 51.94 | 47.88 | |
| Aporrectodea rosea | Abundance | 109.2 ± 16.4 a | 79.7 ± 12.6 b | 104.3 ± 18.3 a |
| Biomass | 58.22 ± 9.22 a | 43.18 ± 7.40 b | 55.89 ± 10.20 a | |
| Dominance % | 32.37 | 31.19 | 33.81 | |
| Octolasion lacteum | Abundance | 23.8 ± 2.8 a | 17.6 ± 2.3 b | 22.9 ± 1.5 a |
| Biomass | 12.52 ± 1.57 a | 9.21 ± 1.22 b | 12.09 ± 1.47 a | |
| Dominance % | 7.06 | 6.89 | 7.42 | |
| Aneciques | ||||
| Lumbricus terrestris | Abundance | 4.2 ± 1.3 a | 4.2 ± 1.4 a | 4.5 ± 1.3 a |
| Biomass | 18.80 ± 5.89 a | 18.68 ± 6.22 a | 19.92 ± 5.79 a | |
| Dominance % | 1.25 | 1.64 | 1.46 | |
| Features | NDG | DG | MW |
|---|---|---|---|
| Abundance | 337.3 b | 255.5 a | 308.5 b |
| Biomass | 184.5 b | 144.8 a | 172.3 b |
| H′ index | 1.23 a | 1.21 b | 1.25 c |
| Species/Ecological Group | Number of Damaged Individuals [Mean ± sd. ind. m−2] | Percentage in Relation to Abundance |
|---|---|---|
| Epigees | ||
| Dendrodrilus rubidus | 0.09 ± 0.03 a | 1.55 |
| Lumbricus rubellus | 0.13 ± 0.08 b | 0.84 |
| Endogees | ||
| Aporrectodea caliginosa | 0.27 ± 0.18 c | 0.20 |
| Aporrectodea rosea | 0.21 ± 0.11 d | 0.26 |
| Octolasion lacteum | 0.11 ± 0.06 b | 0.63 |
| Aneciques | ||
| Lumbricus terrestris | 0.04 ± 0.02 e | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Pączka, A.; Butt, K.R.; Garczyńska, M.; Jaromin, M.; Hajduk, E.; Kostecka, J.; Pączka, G. Comparative Effects of No-dig and Conventional Cultivation with Vermicompost Fertilization on Earthworm Community Parameters and Soil Physicochemical Condition. Agriculture 2024, 14, 870. https://doi.org/10.3390/agriculture14060870
Mazur-Pączka A, Butt KR, Garczyńska M, Jaromin M, Hajduk E, Kostecka J, Pączka G. Comparative Effects of No-dig and Conventional Cultivation with Vermicompost Fertilization on Earthworm Community Parameters and Soil Physicochemical Condition. Agriculture. 2024; 14(6):870. https://doi.org/10.3390/agriculture14060870
Chicago/Turabian StyleMazur-Pączka, Anna, Kevin R. Butt, Mariola Garczyńska, Marcin Jaromin, Edmund Hajduk, Joanna Kostecka, and Grzegorz Pączka. 2024. "Comparative Effects of No-dig and Conventional Cultivation with Vermicompost Fertilization on Earthworm Community Parameters and Soil Physicochemical Condition" Agriculture 14, no. 6: 870. https://doi.org/10.3390/agriculture14060870
APA StyleMazur-Pączka, A., Butt, K. R., Garczyńska, M., Jaromin, M., Hajduk, E., Kostecka, J., & Pączka, G. (2024). Comparative Effects of No-dig and Conventional Cultivation with Vermicompost Fertilization on Earthworm Community Parameters and Soil Physicochemical Condition. Agriculture, 14(6), 870. https://doi.org/10.3390/agriculture14060870






