1. Introduction
Recent investigations have revealed the complexity of the
Botrytis genus, which encompasses a diverse group of pathogenic fungal species, with
B. cinerea being one of the most economically significant due to its necrotrophic behavior and its impact on various horticultural and floral crops [
1,
2]. Traditionally viewed as a single species,
B. cinerea has been found to be a species complex, including at least one cryptic species,
B. pseudocinerea [
3,
4]. Molecular genetic techniques have identified more than 30 different fungal species within the
Botrytis genus [
5].
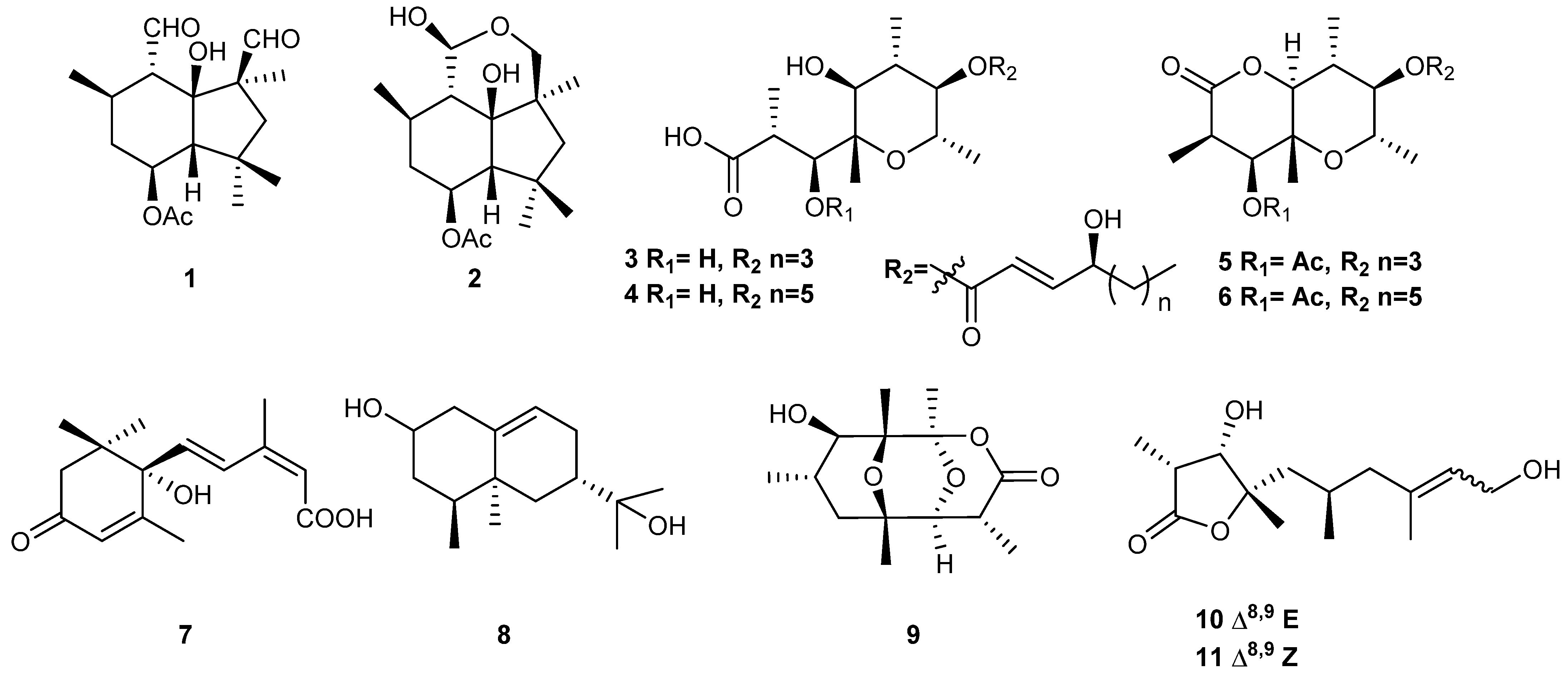
Among the arsenal of phytotoxins synthesized by
B. cinerea B05.10 strain, two families stand out: botryane-type sesquiterpenes with botrydial (
1) and dihydrobotrydial (
2) as primary constituents, along with their various analogues [
6]. Complementing this, polyketides such as botcinic and botrycineric acids (
3,
4) and their cyclic derivatives (
5,
6) add to the diversity of the toxic compounds produced (
Figure 1) [
6]. These compounds collectively induce chlorosis and cell collapse, believed to aid in the invasion and colonization of plant tissue. Notably, botrydial (
1) has been identified as a pivotal pathogenicity factor, as it is detected during plant infection. Additionally,
B. cinerea produces the sesquiterpene abscisic acid (ABA) (
7), which is involved in plant leaf abscission [
7], along with structurally related derivatives [
6]. Another family of sesquiterpenes with a (+)-4-
epi-eremophil-9-en-11-ol (
8) skeleton is synthesized by
B. cinerea and has been found to be involved in the formation of spores and infection cushions [
6]. Other notable metabolites include botrylactone (
9) [
6] and cinbotolides A and B (
10,
11), whose function is still unknown (
Figure 1) [
6]. All the secondary metabolites isolated in
B. cinerea up to 2023 were recently reported by da Silva Ripardo-Filho et al. [
6].
On the other hand, several studies have focused on the genetic variation within natural populations of
B. cinerea, revealing significant morphological and physiological diversity [
8,
9]. For instance, a study by E. Pérez Benito’s research group examined the physiological and genetic diversity of
B. cinerea populations in the vineyards of Castilla y León, Spain, identifying multiple species and reporting notable differences in physiological traits indicative of genetic diversity [
8]. Among these populations,
B. cinerea isolates predominated, with isolates belonging to
B. pseudocinerea and
B. prunorum also being identified for the first time in Spain. Additionally, two isolates closely related to
B. californica were found. Physiologically, the
B. cinerea population featured a normal distribution of aggressiveness values in
Vitis vinifera leaves, suggesting that this trait was quantitative. Several isolates unable to cause infection were identified, most of them belonging to a mycelial morphotype. Population genetic analysis revealed high genotypic diversity, with multiple infections of the same bunch of grapes by different genotypes occurring frequently. The high genotypic diversity observed, the even distribution of both mating types, and the linkage disequilibrium values support a mixed mode of reproduction with low levels of clonality. The low degree of population differentiation observed in the wine-producing area where each isolate was collected did not depend solely on geographic distances but also on the management practices used by growers and wine producer associations [
8]. B459 stands out among the isolates purified in that survey. It is representative of a group of mycelial isolates recovered from plant tissue. Noteworthy characteristics of these strains include their lack of sporulation, absence of sclerotia production, and non-infectivity on leaves of
Vitis vinifera and
Phaseolus vulgaris. Gene Bcin04g03490, a major effect gene controlling development and pathogenicity in
B. cinerea, is altered [
9]. The B459 allele generates a truncated version of the encoded protein lacking the two functional domains described in this protein. A complete loss of function for the encoded protein is expected.
This study aimed to phenotypically characterize B459, exploring its growth, reactive oxygen species production, and infectivity across different plant substrates. Additionally, metabolomic studies were used to investigate its secondary metabolism and the presence of toxins, contributing to a deeper understanding of
B. cinerea biology and its potential applications [
6]. This comprehensive approach sought to shed light on the distinctive features and interactions of
B. cinerea, particularly isolate B459, offering insights into its biology and potential applications in various contexts.
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
The CIALE (University of Salamanca, Spain) provided B. cinerea isolate B459. Fungal strains were consistently cultivated on YGG–agar medium (2% glucose, 0.5% yeast extract, and 0.3% Gamborg B5 (ref. G0209.0005, Duchefa Biochemie, Haarlem, The Netherlands) at 20 °C. Malt–agar media (ref.1038, Condalab, Madrid, Spain) was used to qualitatively analyze growth media acidification by the fungal strains. Mycelium stock suspensions were preserved in 10% glycerol at −80 °C. Plant material, i.e., Gerbera jamesonii, grape, and tomato fruits, was sourced locally. Fresh samples with the same morphology and stage ensured experimental accuracy.
2.2. Vegetative Growth
The study assessed fungal growth alterations by inoculating 0.5 cm diameter mycelium agar plugs onto YGG–agar medium and then recording daily colony radii at 20 °C over three days. Results are presented as the mean value of the growth radius in cm ± standard deviation at 72 h and are derived from 60 biological replicates (N = 60) from two independent experiments of 30 biological replicates each. Sensitivity to stress conditions was evaluated modifying YGG–agar medium with the addition of 1.4 M sorbitol (ref. S1876-1KG, Sigma-Aldrich, St. Louis, MO, USA) to evaluate osmotic stress, 5 mM H
2O
2 (ref.018556, Foret, Barcelona, Spain) to analyze oxidative stress, and 0.02% sodium dodecyl sulfate (SDS) (ref. L3771-500G, Sigma-Aldrich, St. Louis, Missouri, USA) to check the wall rigidity [
10]. In addition, B459 isolate was also evaluated using 100 µM ethanol (ref. 141086.1214, ITW Reagents Panreac, Barcelona, Spain) to use this isolate as a candidate for biotransformations and phenethylisothiocyanate (PhITC, ref. 8070280025, Sigma-Aldrich, St. Louis, Missouri, USA) to test its tolerance to thiocyanate degradation products [
11,
12]. All the Petri dishes were prepared and used on the same day as a precaution owing to the volatile nature of PhITC and EtOH used in this study. The isothiocyanates and the ethanol were introduced quickly once the medium reached 40 °C after sterilization [
11]. The radial expansion of mycelial growth (R, measured in millimeters) was assessed on the third day of growth for treatments incorporating sorbitol, H
2O
2, PhITC, and EtOH products (Rt) and controls devoid of any supplementation (Rc) in the YGG medium.
The fungitoxic effects of sorbitol, H
2O
2, SDS, ethanol, and PhITC were quantified as the percentage of growth inhibition (GI) using the following formula: GI (%) = [(Rc − Rt)/Rc] × 100 [
11,
12]. Results are presented as the mean value of the percentage of growth inhibition observed compared to basal growth of radii after 3 days from 60 biological replicates (N = 60) derived from two independent experiments of 30 biological replicates each. Fungal biomass accumulation in liquid culture was assessed by incubating 30 mL YGG medium with 0.9 cm diameter mycelium agar plugs in 90 mm diameter Petri dishes during 4 days with 40 rpm of orbital shaking. The mycelium was then harvested, washed, and air-dried for fresh weight estimation. Dry weight was determined by incubation at 50 °C until a constant weight was achieved. The experiment was conducted in 30 biological replicates (N = 30) from two independent experiments of 15 biological replicates each. Results are presented as the mean biomass values (mg) ± standard deviation from the 60 biological replicates (N = 60).
2.3. Virulence Assay
Virulence assay was performed following previous studies from Pérez-Hernández et al. in 2017 with minor modifications [
10]. Gerbera petals, tomato, and grape were inoculated with 0.3 cm diameter mycelium agar plugs on 5 µL droplets of a TGGK solution (60 mM KH
2PO
4, 10 mM glycine, 0.01% Tween 20 (ref.28320, Thermo Fisher Scientific, Waltham, Massachusetts, USA), and 100 mM glucose). These inoculated samples were then incubated under dark conditions at 20 °C with high humidity, and photographs were taken every 24 h.
Gerbera petal lesion radius was quantified using ImageJ software [
13] (ImageJ version 1.54i, Wayne Rasband, National Institutes of Health, Bethesda, MD, USA;
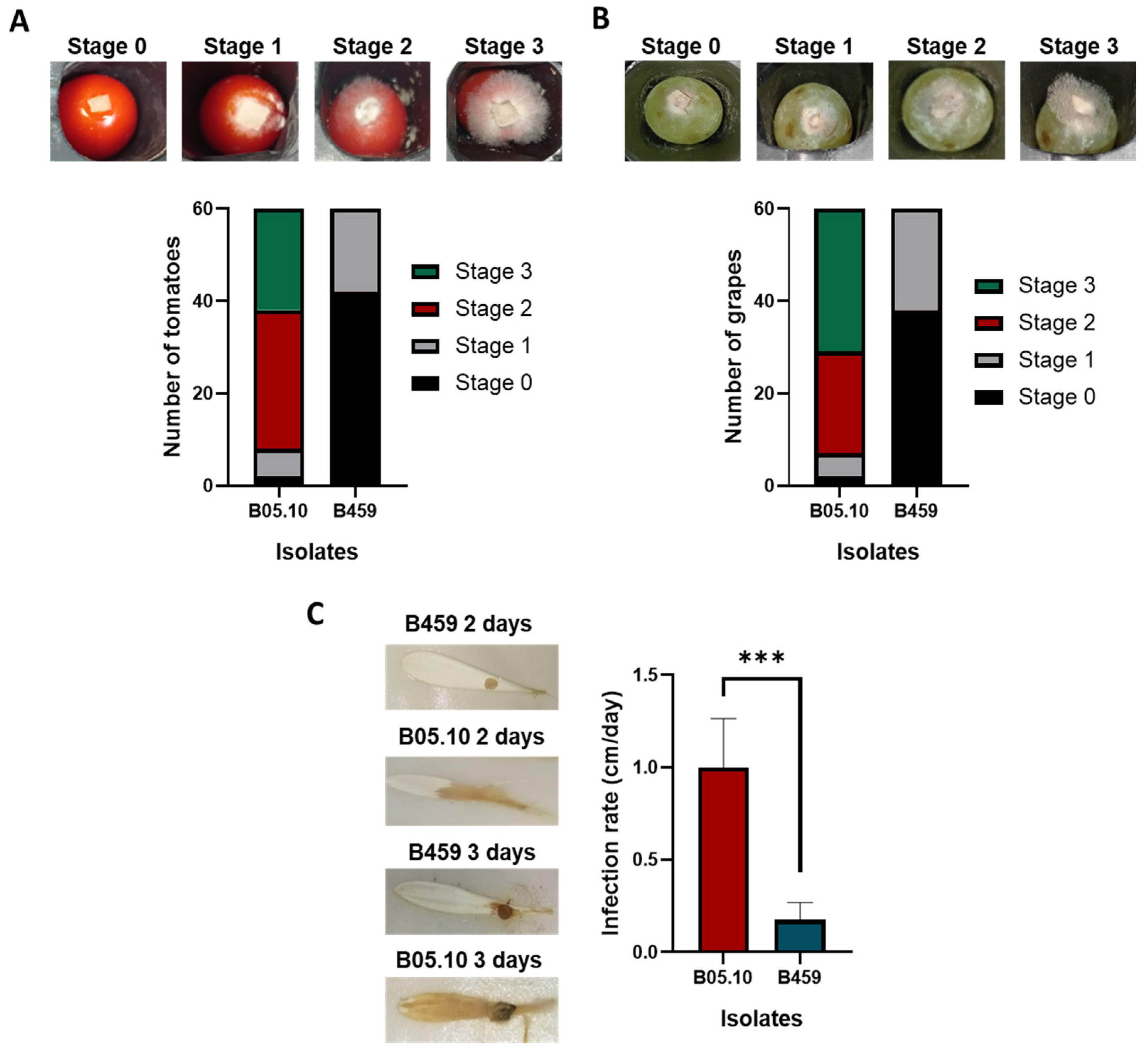
https://imagej.net/ij/ (accessed on 17 February 2024)) to calculate the growth rate (in cm/day) between days 2 and 3 of infection. Results are presented as the mean value of the growth rate (in cm/day) from 60 biological replicates (N = 60) derived from two independent experiments based on 30 biological replicates each. Fungal virulence on fruits was evaluated using a semi-quantitative scale with four infection categories ranging from stage zero (hardly any or no fungal growth on the substrate) to stage 3 (plant tissues fully colonized by the fungus). Results are presented based on the number of fruits classified by disease rating after 3 days of infection based on 60 biological replicates (N = 60) derived from two independent experiments of 30 biological replicates each after 3 days of infection.
2.4. Reactive Oxygen Species Production
Brun et al.’s (2009) [
14] methodology with minor modifications was followed for quantitative determination of H
2O
2. Thus, 24 mg of fresh mycelium was incubated with 1 mL of DAB (3,3′-diaminobenzidine) solution (0.5 mg/mL DAB in 100 mM citric acid (ref. C0759-100G, Sigma-Aldrich, St. Louis, Missouri, USA), pH 3.7) for 5 h at room temperature in darkness. After mycelium harvesting, the optical density of the supernatant was measured at 471 nm. Absorbance values were then compared to a standard curve prepared with known peroxide concentrations, and the results are reported as the mean value of nanograms of H
2O
2 per milligram of mycelium from 30 biological replicates (N = 30) from two independent experiments of 15 biological replicates each.
2.5. Qualitative Analysis of Changes in the Acidification of the Culture Media
A qualitative analysis based on the methodology of Schumacher et al. 2013 with modifications was conducted using agar–malt plates supplemented with a pH indicator, 0.01% (
w/
v) bromocresol green (ref.171759, ITW Reagents Panreac, Barcelona, Spain), to evaluate the acidification of the culture medium by fungi [
15]. A 9 mm diameter mycelial agar plug was inoculated onto each plate prepared with agar–malt medium supplemented with the pH indicator. Five plates were used for each isolate: B459 and B05.10 of
B. cinerea. These plates were incubated at room temperature in darkness for 4 and 7 days. Changes in the medium color from blue to yellow indicated acidification.
2.6. Statistical Analysis
Statistical analyses were performed using Graphpad Prism 8. Normality assessments were conducted employing either the Kolmogorov–Smirnov test (for samples exceeding 50) or the Shapiro–Wilk test (for samples less than 50). Depending on the normality outcomes, the t-test or Mann–Whitney test was then applied to compare normally distributed or nonparametric data, respectively. A p-value below 0.05 was considered statistically significant.
2.7. General Experimental Procedures for Secondary Metabolite Characterization
Secondary metabolite characterization followed a comprehensive experimental protocol. Optical rotations measurements were determined with a JASCO P-2000 polarimeter (JASCO Analytica Spain S.L., Madrid, Spain). Infrared spectra (IR) were recorded on a PerkinElmer Spectrum BX FT-IR spectrophotometer (PerkinElmer, Inc., Waltham, Massachusetts, USA) and reported as wavenumber (cm−1). Nuclear magnetic resonance (NMR) analyses, including 1H and 13C NMR, were recorded on Bruker 400, 500, and 700 MHz spectrometers (Bruker, Billerica, Massachusetts, USA) with SiMe4 as the internal reference. Chemical shifts were calibrated against CD3OD (Merck, Darmstadt, Germany; δH 3.30 ppm, δC 49.0 ppm), and assignments were made employing a combination of 1D and 2D techniques. High-resolution mass spectrometry (HRMS) was conducted on a Q-TOF mass spectrometer operating in negative-ion electrospray ionization (ESI) mode. Thin-layer chromatography (TLC) and preparative TLC were carried out on Merck silica gel 60 Å F254 plates with layer thicknesses of 0.25 mm and 1 mm, respectively. Column chromatography (CC) was performed using silica gel 60 (60−100 mesh, Merck, Darmstadt, Germany). High-performance liquid chromatography (HPLC) purification was executed on a Merck-Hitachi system (Merck, Darmstadt, Germany) equipped with a UV−vis detector (Primaide 1410) and a refractive index detector (RI-5450). Isolation experiments employed LiChroCART 250-10, LiChrospher 100 RP-18 (10 µm), and ACE 5 SIL (250 mm × 4.6 mm id) columns.
2.8. Metabolite Production and Bio-Targeted Toxin Identification
To assess metabolite production the culture media preparation was based on previous studies [
6]. Consequently,
B. cinerea isolate B459 was cultivated on modified malt–agar medium (containing 20 g/L D-glucose, 20 g/L malt extract, 1 g/L peptone, and 20 g/L agar, pH 6.5–7) in Petri dishes at 25 °C for 7 days to generate mycelium plugs. These plugs were then used to inoculate 40 Erlenmeyer flasks, each containing 200 mL of modified Czapek–Dox medium (composed of 50 g saccharose, 1 g yeast extract, 1 g K
2HPO
4, 2.5 g NaNO
3, 0.5 g MgSO
4⋅7H
2O, 0.01 g FeSO
4⋅7H2O, and 0.005 g CuSO
4⋅5H
2O per liter of water, pH 6.5–7) for metabolite production.
Each Erlenmeyer flask was inoculated with a single mycelium plug (9 mm in diameter) and then incubated for 7 and 14 days (time of incubation in which the two main toxins, botrydial (
1) and botcinins (
5,
6) (
Figure 1), are produced [
6]) at 25 °C under daylight conditions while shaken at 140 rpm. Subsequently, 8 L of culture medium from each fermentation were filtered through a nylon 200 µm mesh filter under reduced pressure to eliminate mycelium. The filtrates were then subjected to liquid–liquid extraction with ethyl acetate (EtOAc x 3) and dried over anhydrous Na
2SO
4. The solvent was then concentrated to dryness at reduced pressure, yielding yellow oil extracts at 7 (100 mg) and 14 days (330 mg). For metabolite isolation and bio-directed toxin identification, the crude extracts were initially fractionated by column chromatography on silica gel with a mixture of ethyl acetate/hexane containing increasing percentages (10−100%) of ethyl acetate, finishing with ethyl acetate/methanol (20%).
For targeted toxin identification, the presence of metabolites with phytotoxic activity was evaluated based on previous studies through plasmolysis detection in onion epidermis (
Allium cepa) [
16,
17]. Onion epidermis was placed on glass slides, and 10 μL (0.1 mg) of each fraction of increasing polarity from the culture at seven and fourteen days was added in triplicate. The extracts from the reference strain B05.10 at 7 days (high concentration of botrydial (
1)) and 14 days (high concentration of botcinins (
5,
6)) (
Figure 1,
Supplementary Table S1, Supplementary Figures S1–S5) served as a positive control, while the solvent acted as the negative control. Slides were incubated for 24 h, and events were observed under an optical microscope to detect the possible presence of toxins.
To purify the metabolites, the different fractions were chromatographed by column chromatography (CC), as previously indicated, and subsequently with HPLC with a mixture of acetone, ethyl acetate, and hexane. The isolated pure metabolites were subjected to extensive spectroscopic analysis by
1H-NMR and
13C-NMR using 1D and 2D NMR, HRMS, and IR techniques. The metabolites were analyzed and their physical and spectroscopic constants compared with authentic samples previously isolated from different strains of
B. cinerea [
6] and with data reported in the literature to afford compounds
12,
13, and
14 (
Supplementary Table S1, Supplementary Figures S6–S8).
4. Discussion
In terms of growth, isolates B05.10 and B459 exhibit notable differences (
Figure 2A), which may indicate variations in their proliferation capacity. The results suggest that isolate B05.10 has a superior growth rate and more fungal biomass, indicating increased efficiency in nutrient utilization for growth compared to B459 (
Figure 3A). The second isolate, while displaying a lower growth capacity and having a limited ability to infect any host, is more resilient to several stresses and has a greater capability to retain water in its mycelium (in terms of grams of water retained per gram of mycelium). These differences certainly rely on the genetic makeup of isolate B459 in comparison with isolate B05.10 and are the consequence of its genetic background, which includes a natural variant allele of gene Bcin04g03490 [
9]. The observed differences under stress conditions (
Figure 2B) may be attributed to genetic adaptations that enhance B459’s ability to withstand stressful environments. Additionally, the higher capacity of its mycelium to retain water could contribute to improve its survival in arid environments.
These phenotypic and genotypic differences between the two isolates support the view of
B. cinerea as a highly diverse and genetically rich species and the beneficiary of evolutionary forces, leading to adaptations in response to different factors [
25]. Further attention should be given to analyses of the differences between isolates B05.10 and B459, as they may play a pivotal role in shaping the effectiveness of these strains in various applications. Reduced robustness is assumed for isolate B459, as it does not infect or sporulate and exhibits reduced saprophytic growth capacity. However, B459 as well as other mycelial field isolates of the same phenotype are present in the field and are not eliminated by natural selection. The observations made in the course of this work on strain B459 might bring to light factors that could help to understand the behavior of these strains in the field.
The B459 strain generates less reactive oxygen species (ROS) and is less susceptible to hydrogen peroxide (H
2O
2) stress (
Figure 3C). This suggests enhanced antioxidant defenses and improved tolerance to oxidative conditions. Reactive oxygen species play a crucial role in cellular signaling and stress responses [
23]. A strain with lower ROS production may possess mechanisms for effective antioxidant defense, mitigating potential cellular damage. The strain’s ability to mitigate ROS and withstand H
2O
2 stress highlights its potential for applications in oxidative stress research and biotechnological processes requiring robust cellular resilience. Understanding these differences in ROS dynamics between strains is essential in deciphering their adaptive strategies and potential applications, particularly in contexts where oxidative stress is a significant factor, such as in interactions with host organisms or environmental niches [
26,
27,
28].
B05.10 acidifies the environment to a greater degree than B459 (
Figure 3D). This is likely a consequence of a higher production of acidic metabolites by isolate B05.10. This difference is suggestive of a differential impact of both strains on their surroundings environments, which can potentially influence both fungal pathogenicity and plant responses. Moreover, the secretion of oxalic acid by
B. cinerea has been linked to medium acidification [
29,
30] and its production has been qualitatively evaluated by methods based on the utilization of pH indicators such as bromothymol blue [
15]. However, recent analyses of organic acids released by
B. cinerea showed the production of other acid compounds such as citric acid as one of the predominant acids, followed by malic acid, with minimal levels of fumaric and succinic acids [
31]. Previous studies have also explored how
B. cinerea adjusts ambient pH during host interaction by secreting either acids or ammonia [
32]. Specifically, the
BcpacC knock-out mutant from the Pal/Pac pathway (a fungal pH-responsive signaling mechanism) revealed pH-dependent alterations in fungal growth and virulence [
32]. Consequently, the mutant’s ability to acidify its environment, particularly oxalic acid production and reactive oxygen species generation, were affected [
32]. Manteau et al. in 2003 provided evidence that the ambient pH plays a role in regulating the synthesis of pathogenicity factors in
Botrytis [
33]. These authors suggested that ambient pH could serve as a regulatory factor, aiding the fungus in adjusting its virulence mechanisms to match the composition of the host tissue [
31,
33,
34]. Our study attributes the lower infectivity of isolate B459 to its decreased capacity to acidify the medium, supporting the relationship between these two parameters in previous descriptions of non-aggressive mutants of
B. cinerea. Furthermore, this lower acidification of the medium by B459 is accompanied by lower reactive oxygen species production (
Figure 3C,D). Further analyses are necessary to evaluate the differences in the nature and kinetics of production and secretion of the metabolites produced by the two strains that could account for their different abilities to acidify the medium.
B459 isolate shows a reduced ability to infect tomato and grape fruit and gerbera petals compared to B05.10 (
Figure 4). This study shows that B459 has difficulty infecting plant substrates different from the ones described by Acosta Morel et al. in 2019 [
8]. It also provides valuable insights into the complex interactions between microbial pathogens and their plant hosts, informing strategies for disease management and crop protection [
35]. This observation is accompanied by the inability to produce toxins at 7 (for botrydial (
1)) and 14 days (botcinins (
5,
6)), typical of
B. cinerea B05.10 (
Figure 1 and
Figure 6) [
6]. Moreover, only compounds
12 and
13 were isolated from B459, which was previously isolated from isolate B05.10 [
6]. Compound
14 is described here for the first time from a
B. cinerea isolate [
36], although four cyclodipeptides have been isolated from a marine strain
Botryotinia fuckeliana MCCC 03A00494 [
6]. In conclusion, isolate B459 displays low metabolic production, which may affect its ability to adapt to different environmental conditions.
These results show that strain B459 has impaired its secondary metabolism, inhibiting the biosynthesis of the characteristic sesquiterpenes produced by these fungal species, such as botryanes, and derived from the main toxin involved in pathogenicity, such as botrydial (
1), or eremophilenes (
8), sesquiterpenes involved in sporogenesis, and abscisic acid (
7) and derivatives (
Figure 1) [
6]. This fact would explain the accumulation and isolation of mevalonolactone (
12), a key intermediate in terpene biosynthesis. Also, the absence of the second family of toxins, the polyketide botcinins (
5,
6), is striking since strains or mutants that do not produce botrydial (
1) overproduce botcinins (
5,
6), thus maintaining infectivity [
37,
38]. These results would appear to indicate a down-regulation of secondary metabolism, including melanin biosynthesis, which would explain the white color of B459 mycelium and the isolation of isosclerone (
13), an intermediate in melanin biosynthesis [
6].
In conclusion, this study demonstrates that B459 is an interesting isolate for biotechnological research on the behavior of the phytopathogen
B. cinerea, with potential implications for agricultural advancements. Further investigation into the stress-response mechanisms of this subject could provide valuable insights into biotechnological and agricultural progress. Variations in ROS production highlight microbial adaptability in fields such as agriculture, medicine, and biotechnology. Recognizing these biological differences is crucial for effectively utilizing these strains. Understanding infection patterns helps to assess pathogenicity, host specificity, and agricultural impacts. Therefore, comprehending these nuances is pivotal for advancing biotechnological and agricultural practices sustainably. Pathogenicity-deficient strains of pathogenic microorganisms are excellent systems to investigate potential virulence factors by applying a wide arsenal of molecular genetic tools [
39]. Another use of non-pathogenic fungi is to protect plants from closely related fungal pathogens by competing within the same habitat [
40]. There is a close relationship between
Botrytis and wine production. Depending on the environment,
Botrytis could develop grey mold or noble rot, which is a process used in vineyards to produce certain types of wine. The use of non-pathogenic isolates for this purpose is an area offering many prospects to explore [
41].