Understanding the Biology of the Harmless Isolate Botrytis cinerea B459: An Approach to Bio-Targeted Toxin Identification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Vegetative Growth
2.3. Virulence Assay
2.4. Reactive Oxygen Species Production
2.5. Qualitative Analysis of Changes in the Acidification of the Culture Media
2.6. Statistical Analysis
2.7. General Experimental Procedures for Secondary Metabolite Characterization
2.8. Metabolite Production and Bio-Targeted Toxin Identification
3. Results
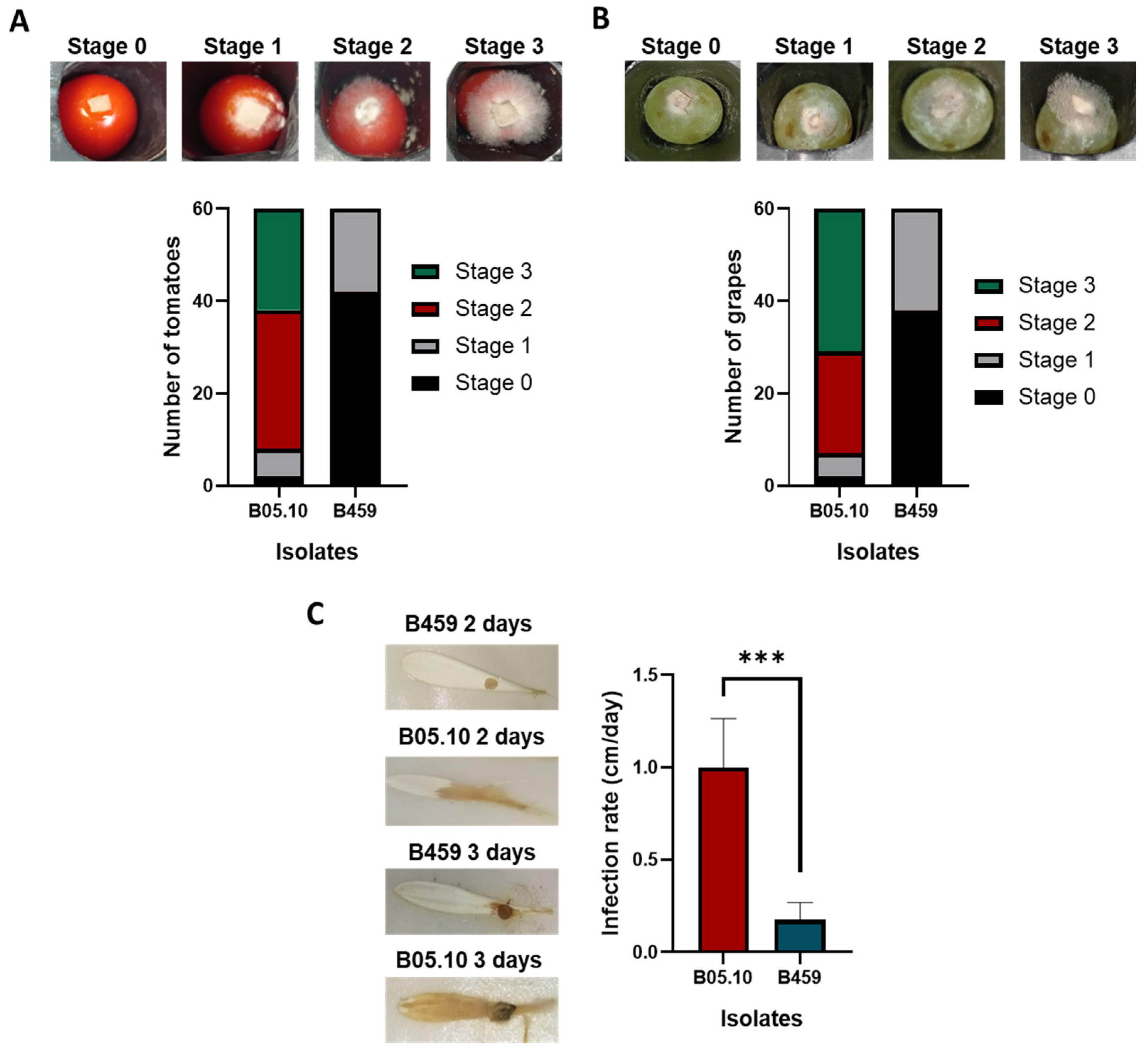
3.1. B459 Differs from B05.10 in Terms of its Biology and Infectivity
3.2. B. cinerea B459 Extracts at 7 and 14 Days Are not Phytotoxic
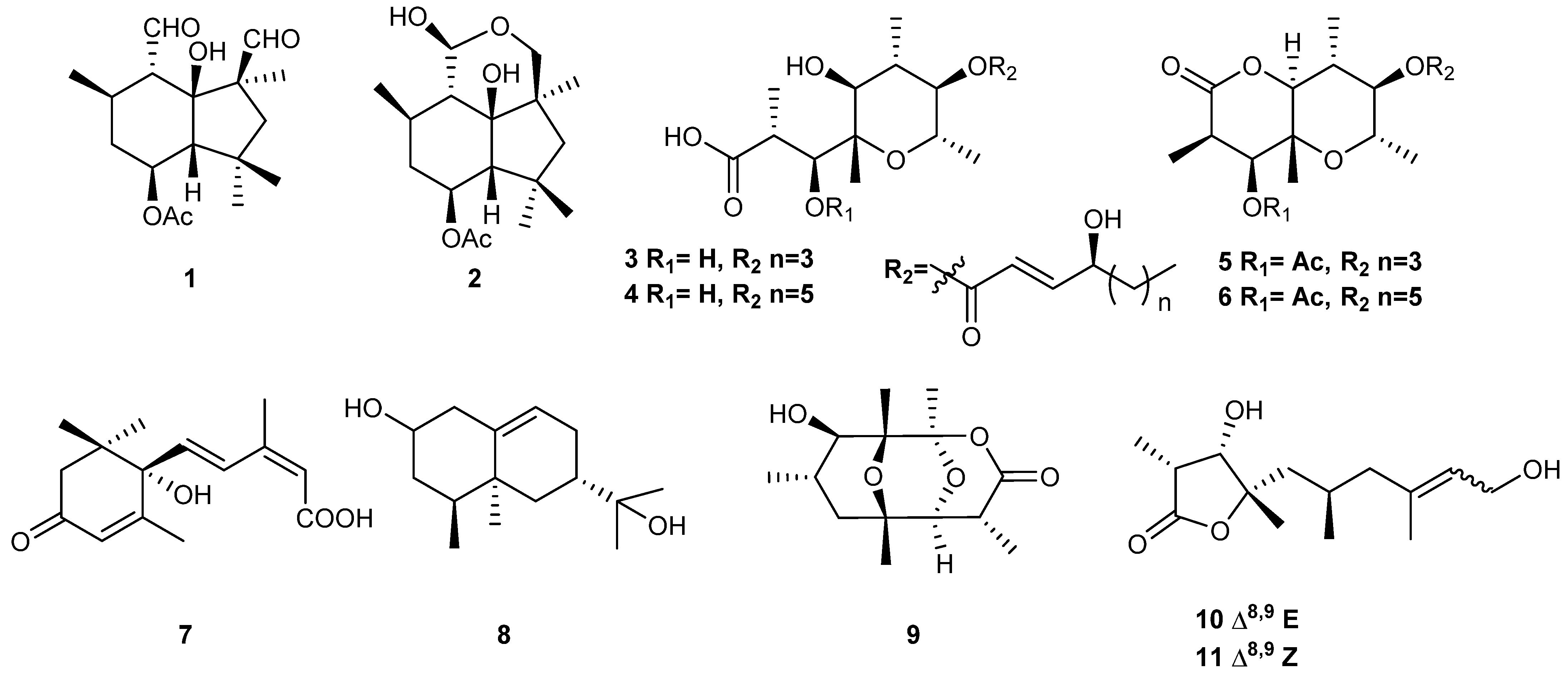
3.3. B459 Presents Differences in the Secondary Metabolism with Respect to B05.10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coley-Smith, J.R.; Verhoeff, K.; Jarvis, W.R. (Eds.) The Biology of Botrytis; Academic Press Inc. (London) Ltd.: London, UK, 1980; Volume 75, ISBN 012179850X. [Google Scholar]
- Kang, S. Plant pathology 2.0. Mol. Plant Pathol. 2014, 15, 315–318. [Google Scholar] [CrossRef]
- Plesken, C.; Weber, R.W.S.; Rupp, S.; Leroch, M.; Hahn, M. Botrytis pseudocinerea is a significant pathogen of several crop plants but susceptible to displacement by fungicide-resistant B. cinerea strains. Appl. Environ. Microbiol. 2015, 81, 7048–7056. [Google Scholar] [CrossRef]
- Xue, L.H.; Liu, Y.; Zhang, L.; Huang, X.Q.; Zhou, X.Q.; Yang, X.X.; Wu, W.X. Botrytis pseudocinerea, a new pathogen causing gray mold on Brassica napus in China. Plant Dis. 2019, 103, 367. [Google Scholar] [CrossRef]
- Walker, A.-S. Diversity within and between species of Botrytis. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; pp. 91–125. [Google Scholar]
- da Silva Ripardo-Filho, H.; Coca Ruíz, V.; Suárez, I.; Moraga, J.; Aleu, J.; Collado, I.G. From genes to molecules, secondary metabolism in Botrytis cinerea: New insights into anamorphic and teleomorphic stages. Plants 2023, 12, 553. [Google Scholar] [CrossRef]
- Marumo, S.; Katayama, M.; Komori, E.; Ozaki, Y.; Natsume, M.; Kondo, S. Microbial production of abscisic acid by Botrytis cinerea. Agric. Biol. Chem. 1982, 46, 1967–1968. [Google Scholar] [CrossRef]
- Acosta Morel, W.; Marques-Costa, T.M.; Santander-Gordón, D.; Anta Fernández, F.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Sukno, S.A.; Díaz-Mínguez, J.M.; Benito, E.P.; Marques-Costa, T.M.; et al. Physiological and population genetic analysis of Botrytis field isolates from vineyards in Castilla y León, Spain. Plant Pathol. 2019, 68, 523–536. [Google Scholar] [CrossRef]
- Acosta Morel, W.; Anta Fernández, F.; Baroncelli, R.; Becerra, S.; Thon, M.R.; van Kan, J.A.L.; Díaz-Mínguez, J.M.; Benito, E.P. A major effect gene controlling development and pathogenicity in Botrytis cinerea identified through genetic analysis of natural mycelial non-pathogenic isolates. Front. Plant Sci. 2021, 12, 663870. [Google Scholar] [CrossRef]
- Pérez-Hernández, A.; González, M.; González, C.; van Kan, J.A.L.L.; Brito, N. BcSun1, a B. cinerea Sun-family protein, is involved in virulence. Front. Microbiol. 2017, 8, 242641. [Google Scholar] [CrossRef]
- Coca-Ruiz, V.; Aleu, J.; Collado, I.G. Comparing fungal sensitivity to isothiocyanate products on different Botrytis spp. Plants 2024, 13, 756. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Aditya Srivastava, D.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS transporter from Botrytis cinerea provides tolerance to glucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Brun, S.; Malagnac, F.; Bidard, F.; Lalucque, H.; Silar, P. Functions and regulation of the Nox family in the filamentous fungus Podospora anserina: A new role in cellulose degradation. Mol. Microbiol. 2009, 74, 480–496. [Google Scholar] [CrossRef]
- Schumacher, J.; Gautier, A.; Morgant, G.; Studt, L.; Ducrot, P.-H.H.; Le Pêcheur, P.; Azeddine, S.; Fillinger, S.; Leroux, P.; Tudzynski, B.; et al. A functional bikaverin biosynthesis gene cluster in rare strains of Botrytis cinerea is positively controlled by VELVET. PLoS ONE 2013, 8, e53729. [Google Scholar] [CrossRef]
- Tkachuk, N.; Zelena, L. Onion (Allium cepa L.) as a test plant. Biota Hum. Technol. 2023, 50–59. [Google Scholar] [CrossRef]
- Varejão, E.V.V.; Demuner, A.J.; Barbosa, L.C.A.; Barreto, R.W. The search for new natural herbicides—Strategic approaches for discovering fungal phytotoxins. Crop Prot. 2013, 48, 41–50. [Google Scholar] [CrossRef]
- Çavuşoğlu, D. Powerful toxic activity of citrinin, a fungal phytotoxin, and its mode of action in onion cells. Environ. Sci. Pollut. Res. 2022, 29, 6205–6218. [Google Scholar] [CrossRef]
- Pawlowski, M.L.; Hartman, G.L. Infection mechanisms and colonization patterns of fungi associated with soybean. In Fungal Pathogenicity; InTech: London, UK, 2016. [Google Scholar]
- Shimizu, M.; Kamikubo, T.; Ogasawara, K. A new enantiocontrolled synthesis of (−)-(R)-mevalonolactone. Tetrahedron Asymmetry 1997, 8, 2519–2521. [Google Scholar] [CrossRef]
- Chruszcz-Lipska, K.; Jaworska, A.; Szczurek, E.; Baranska, M. (−)-R-mevalonolactone studied by ROA and SERS spectroscopy. Chirality 2014, 26, 453–461. [Google Scholar] [CrossRef]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and known diketopiperazines from the Caribbean Sponge, Calyx cf. podatypa. J. Nat. Prod. 1995, 58, 201–208. [Google Scholar] [CrossRef]
- Machida, K.; Matsuoka, E.; Kasahara, T.; Kikuchi, M. Studies on the constituents of Juglans species. I. Structural determination of (4S)- and (4R)-4-hydroxy-α-tetralone derivatives from the fruit of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. Chem. Pharm. Bull. 2005, 53, 934–937. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Nozoe, S.; Okuda, S.; Sato, Z. Isolation of 3,4-dihydro-3,4,8-trihydroxy-1(2H)-naphthalenone and tenuazonic acid from Pyricularia oryzae cavara. Tetrahedron Lett. 1972, 13, 13–16. [Google Scholar] [CrossRef]
- Tani, H.; Koshino, H.; Sakuno, E.; Cutler, H.G.; Nakajima, H. Botcinins E and F and botcinolide from Botrytis cinerea and structural revision of botcinolides. J. Nat. Prod. 2006, 69, 722–725. [Google Scholar] [CrossRef]
- Rolke, Y.; Liu, S.; Quidde, T.; Williamson, B.; Schouten, A.; Weltring, K.M.; Siewers, V.; Tenberge, K.B.; Tudzynski, B.; Tudzynski, P. Functional analysis of H2O2-generating systems in Botrytis cinerea: The major Cu-Zn-superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 2004, 5, 17–27. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Takács, Z.; Freynschlag, F.; Toksoy Öner, E.; Jonak, C.; Van den Ende, W. Fructans prime ROS dynamics and Botrytis cinerea resistance in Arabidopsis. Antioxidants 2020, 9, 805. [Google Scholar] [CrossRef]
- Rossi, F.R.; Krapp, A.R.; Bisaro, F.; Maiale, S.J.; Pieckenstain, F.L.; Carrillo, N. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant J. 2017, 92, 761–773. [Google Scholar] [CrossRef]
- Kunz, C.; Vandelle, E.; Rolland, S.; Poinssot, B.; Bruel, C.; Cimerman, A.; Zotti, C.; Moreau, E.; Vedel, R.; Pugin, A.; et al. Characterization of a new, nonpathogenic mutant of Botrytis cinerea with impaired plant colonization capacity. New Phytol. 2006, 170, 537–550. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Traeger, S.; Porquier, A.; Dalmais, B.; Viaud, M.; Tudzynski, B. The VELVET complex in the gray mold fungus Botrytis cinerea: Impact of BcLAE1 on differentiation, secondary metabolism, and virulence. Mol. Plant-Microbe Interact. 2015, 28, 659–674. [Google Scholar] [CrossRef]
- Müller, N.; Leroch, M.; Schumacher, J.; Zimmer, D.; Könnel, A.; Klug, K.; Leisen, T.; Scheuring, D.; Sommer, F.; Mühlhaus, T.; et al. Investigations on VELVET regulatory mutants confirm the role of host tissue acidification and secretion of proteins in the pathogenesis of Botrytis cinerea. New Phytol. 2018, 219, 1062–1074. [Google Scholar] [CrossRef]
- Rascle, C.; Dieryckx, C.; Dupuy, J.W.; Muszkieta, L.; Souibgui, E.; Droux, M.; Bruel, C.; Girard, V.; Poussereau, N. The pH regulator PacC: A host-dependent virulence factor in Botrytis cinerea. Environ. Microbiol. Rep. 2018, 10, 555–568. [Google Scholar] [CrossRef]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zong, Y.; Qin, G.; Tian, S. Exploring pathogenic mechanisms of Botrytis cinerea secretome under different ambient pH based on comparative proteomic analysis. J. Proteome Res. 2012, 11, 4249–4260. [Google Scholar] [CrossRef]
- Saldaña-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.S.; Chávez-González, M.L.; Aguilar, C.N. Trichoderma as a biological control agent: Mechanisms of action, benefits for crops and development of formulations. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-Diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef]
- Reino, J.L.; Hernández-Galán, R.; Durán-Patrón, R.; Collado, I.G. Virulence–toxin production relationship in isolates of the plant pathogenic fungus Botrytis cinerea. J. Phytopathol. 2004, 152, 563–566. [Google Scholar] [CrossRef]
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pêcheur, P.; Tudzynski, B.; Collado, I.G.; Viaud, M. The Botrytis cinerea phytotoxin botcinic acid requires two polyketide synthases for production and has a redundant role in virulence with botrydial. Mol. Plant Pathol. 2011, 12, 564–579. [Google Scholar] [CrossRef]
- Idnurm, A.; Howlett, B.J. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2001, 2, 241–255. [Google Scholar] [CrossRef]
- Sneh, B. Use of non-pathogenic or hypovirulent fungal strains to protect plants against closely related fungal pathogens. Biotechnol. Adv. 1998, 16, 1–32. [Google Scholar] [CrossRef]
- Negri, S.; Lovato, A.; Boscaini, F.; Salvetti, E.; Torriani, S.; Commisso, M.; Danzi, R.; Ugliano, M.; Polverari, A.; Tornielli, G.B.; et al. The induction of noble rot (Botrytis cinerea) infection during postharvest withering changes the metabolome of grapevine berries (Vitis vinifera L., cv. Garganega). Front. Plant Sci. 2017, 8, 1002. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coca-Ruiz, V.; Cabrera-Gomez, N.; Torres, D.S.; Casado-del Castillo, V.; Benito, E.P.; Aleu, J.; Collado, I.G. Understanding the Biology of the Harmless Isolate Botrytis cinerea B459: An Approach to Bio-Targeted Toxin Identification. Agriculture 2024, 14, 932. https://doi.org/10.3390/agriculture14060932
Coca-Ruiz V, Cabrera-Gomez N, Torres DS, Casado-del Castillo V, Benito EP, Aleu J, Collado IG. Understanding the Biology of the Harmless Isolate Botrytis cinerea B459: An Approach to Bio-Targeted Toxin Identification. Agriculture. 2024; 14(6):932. https://doi.org/10.3390/agriculture14060932
Chicago/Turabian StyleCoca-Ruiz, Víctor, Nuria Cabrera-Gomez, David Saborido Torres, Virginia Casado-del Castillo, Ernesto P. Benito, Josefina Aleu, and Isidro G. Collado. 2024. "Understanding the Biology of the Harmless Isolate Botrytis cinerea B459: An Approach to Bio-Targeted Toxin Identification" Agriculture 14, no. 6: 932. https://doi.org/10.3390/agriculture14060932
APA StyleCoca-Ruiz, V., Cabrera-Gomez, N., Torres, D. S., Casado-del Castillo, V., Benito, E. P., Aleu, J., & Collado, I. G. (2024). Understanding the Biology of the Harmless Isolate Botrytis cinerea B459: An Approach to Bio-Targeted Toxin Identification. Agriculture, 14(6), 932. https://doi.org/10.3390/agriculture14060932









