The Effects of Dietary Resveratrol and β-Hydroxy-β-Methylbutyric Acid Supplementation at Two Protein Levels on the Ruminal Microbiome and Metabolome of Tibetan Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Processing
2.3. Determination of Digestive Enzyme Activity, Antioxidant Capacity, and (LPS) Concentration
2.4. DNA Extraction and 16S rDNA Gene Sequencing
2.5. Metabolite Extraction and LC-MS/MS Analysis
2.6. Correlation Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Dietary Supplementation with RES and HMB at Different Protein Levels on Digestive Enzyme Activity
3.2. Effect of Dietary Supplementation with RES and HMB at Different Protein Levels on the Antioxidant Capacity
3.3. Effect of Dietary Supplementation with RES and HMB at Different Protein Levels on LPS Concentration
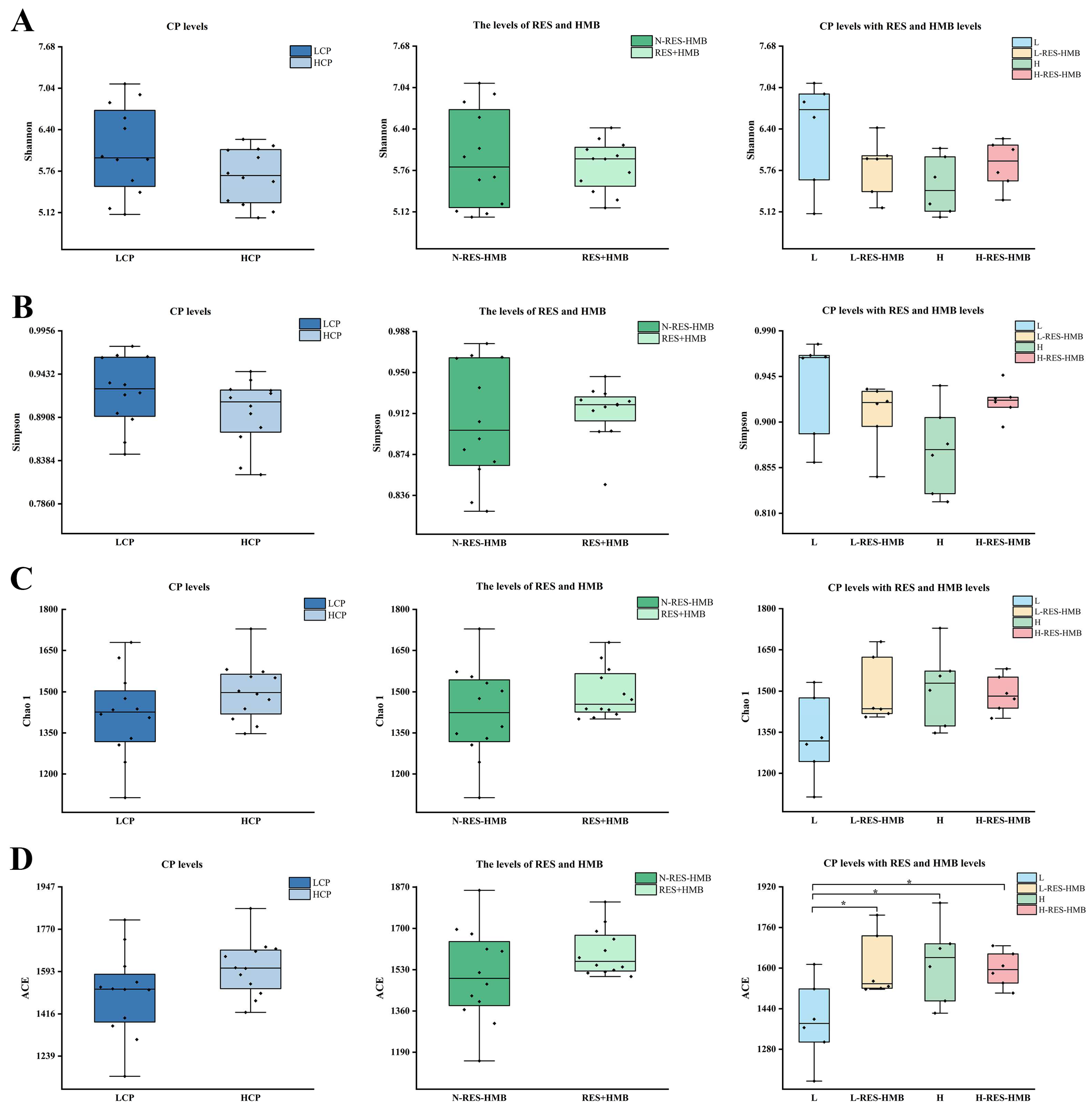
3.4. Differences in the Diversity in Rumen Microbial Communities
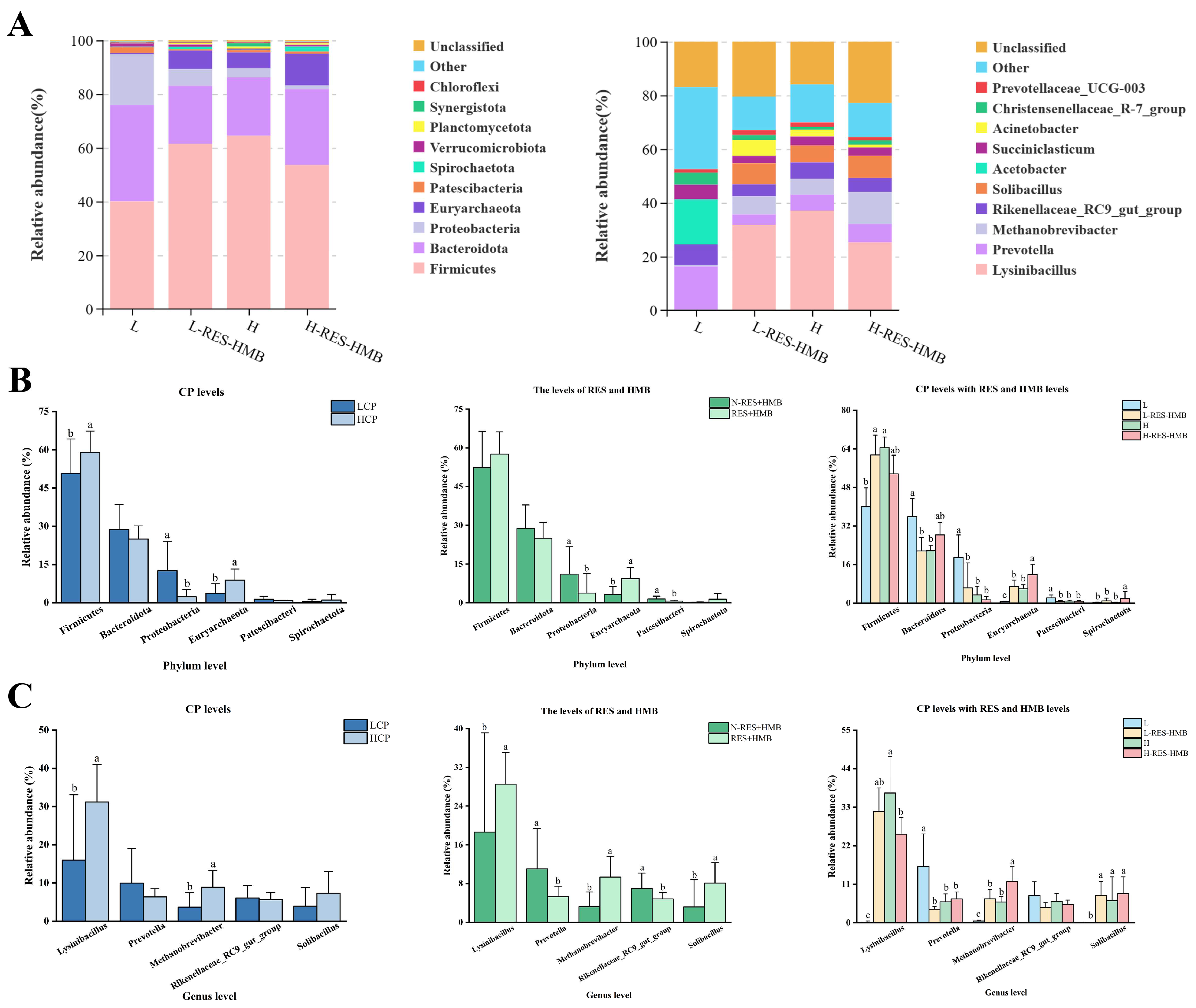
3.5. Differences in Rumen Microbial Compositions
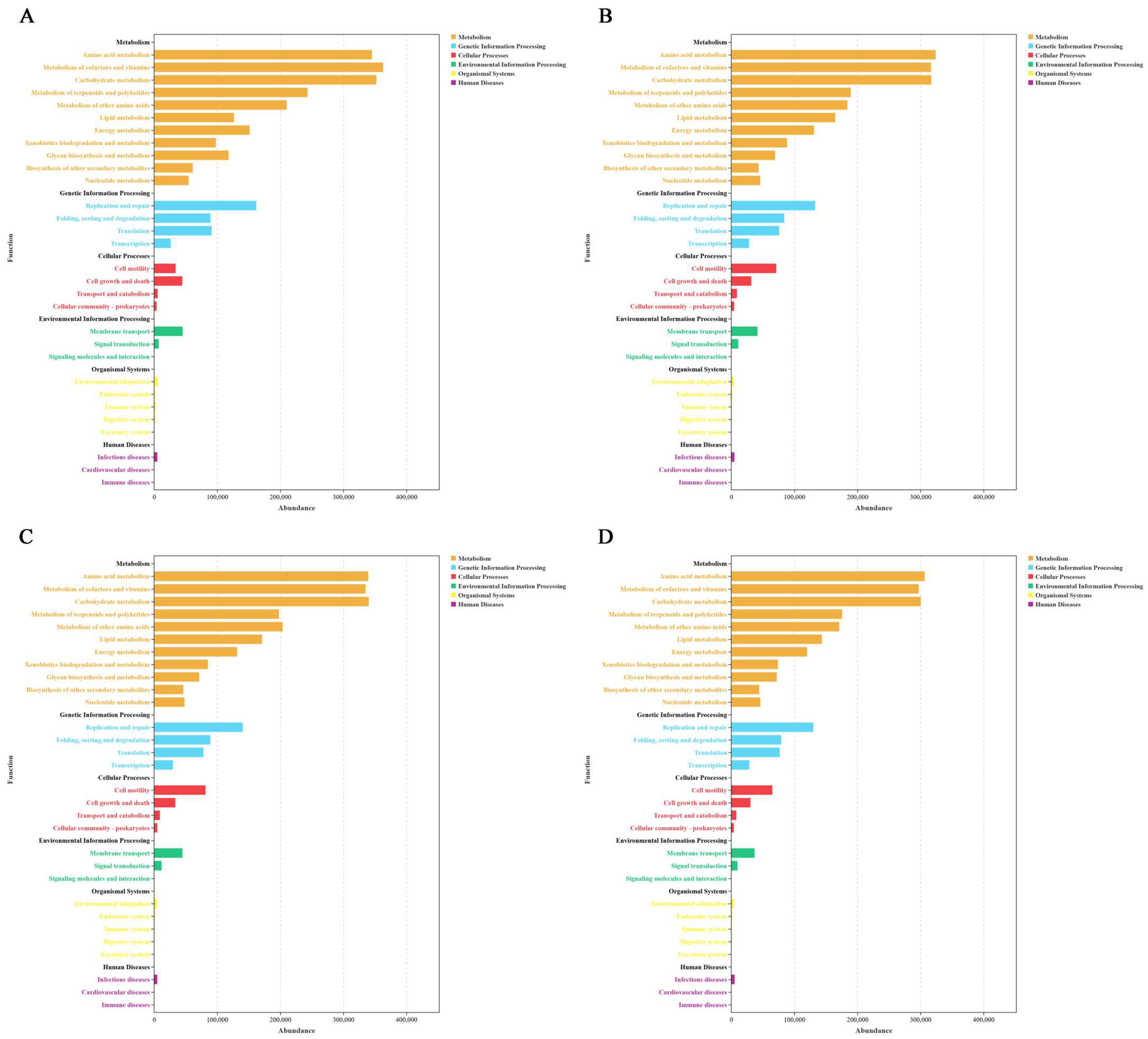
3.6. Differences in Rumen Microbial Functions in Tibetan Sheep
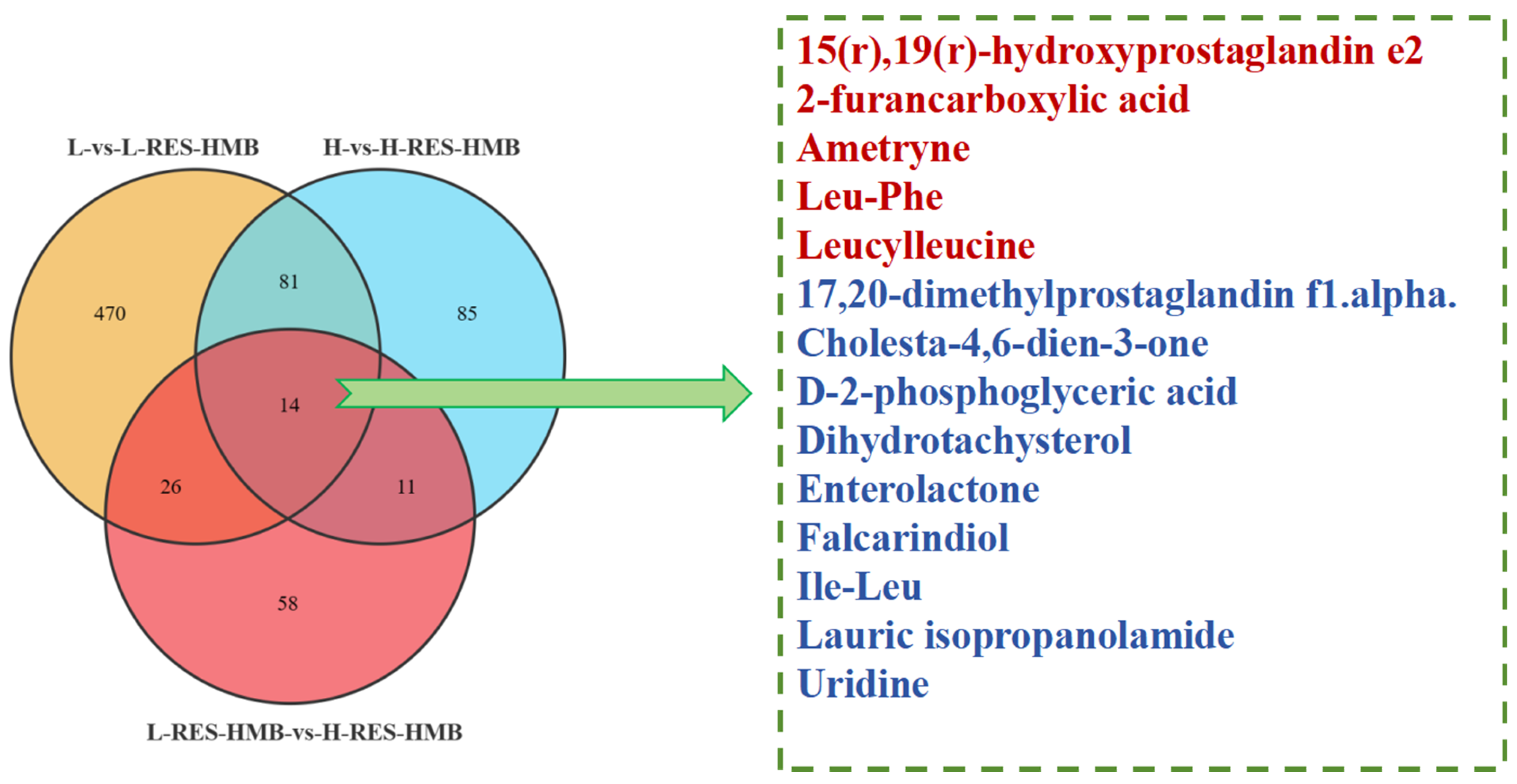
3.7. Metabolomic Analysis of the Rumen of Tibetan Sheep
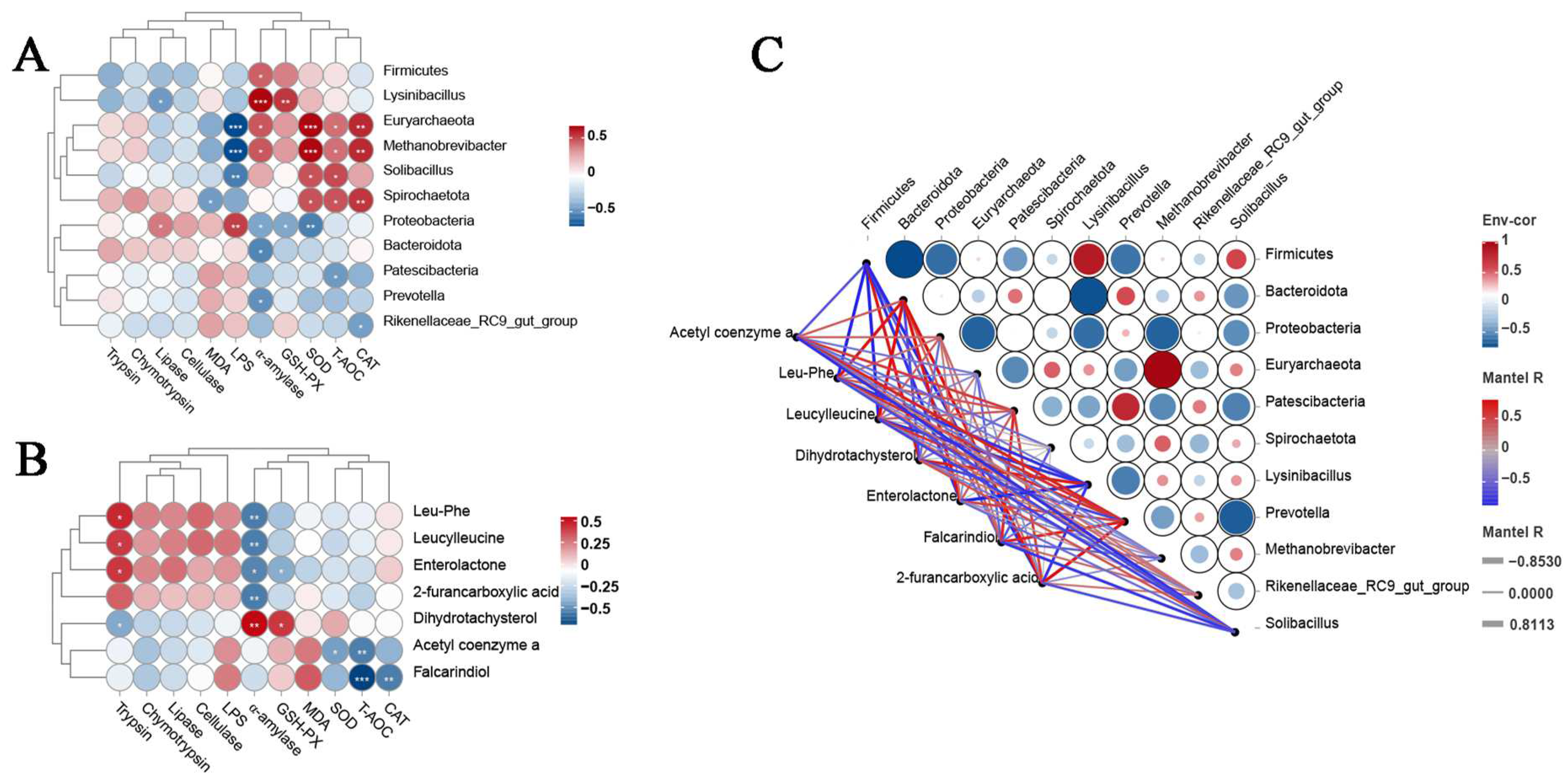
3.8. Correlation Analysis of Digestive Enzyme Activity, Antioxidant Capacity, LPS Concentration, Microbial Communities, and Metabolites in Rumen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, K.; Wang, S.; Gaowa, N.; Huang, S.; Li, S.; Shao, W. Identification of the molecular mechanisms underlying brisket disease in Holstein heifers via microbiota and metabolome analyses. AMB Express 2021, 11, 86. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Gui, L.; Raza, S.H.A.; Hou, S.; Yang, B.; Wang, Z.; Ma, Y.; Makhlof, R.T.M.; Alhuwaymil, Z.; et al. Metabolome and microbiome analysis revealed the effect mechanism of different feeding modes on the meat quality of Black Tibetan sheep. Front. Microbiol. 2022, 13, 1076675. [Google Scholar] [CrossRef]
- da Silva, P.C.G.; Brandão Ferreira Ítavo, C.C.; Vinhas Ítavo, L.C.; de Nadai Bonin Gomes, M.; Dias Feijó, G.L.; Monteiro Ferelli, K.L.S.; da Silva Heimbach, N.; da Silva, J.A.; de Melo, G.K.A.; Filgueira Pereira, M.W. Carcass traits and meat quality of Texel lambs raised in Brachiaria pasture and feedlot systems. Anim. Sci. J. Nihon Chikusan Gakkaiho 2020, 91, e13394. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Ma, S.K.; Peng, W.; Fang, Y.G.; Duo, H.R.; Fu, H.Y.; Jia, G.X. Genetic diversity and population structure of Tibetan sheep breeds determined by whole genome resequencing. Trop. Anim. Health Prod. 2021, 53, 174. [Google Scholar] [CrossRef]
- Yue, Z.B.; Li, W.W.; Yu, H.Q. Application of rumen microorganisms for anaerobic bioconversion of lignocellulosic biomass. Bioresour. Technol. 2013, 128, 738–744. [Google Scholar] [CrossRef]
- Connor, E.E.; Baldwin, R.L.t.; Li, C.J.; Li, R.W.; Chung, H. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genom. 2013, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, Q.; Jiang, Y.; Wang, K.; Lin, Z.; Li, Z.; Bibi, F.; Yang, Y.; Wang, J.; Nie, W.; et al. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science 2019, 364, eaav6202. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Oddy, V.H.; Archibald, A.L.; Vercoe, P.E.; Dalrymple, B.P. Epithelial, metabolic and innate immunity transcriptomic signatures differentiating the rumen from other sheep and mammalian gastrointestinal tract tissues. PeerJ 2016, 4, e1762. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Zheng, H.; Dewhurst, R.; Roehe, R. A Knowledge-Driven Network-Based Analytical Framework for the Identification of Rumen Metabolites. IEEE Trans. Nanobioscience 2020, 19, 518–526. [Google Scholar] [CrossRef]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Zhang, C.; Feng, Y.; Zhang, X.; Wang, X.; Wu, G. Dynamics and stabilization of the rumen microbiome in yearling Tibetan sheep. Sci. Rep. 2019, 9, 19620. [Google Scholar] [CrossRef]
- Khazaei, M.; Rahmati, S.; Khazaei, M.R.; Rezakhani, L. Accelerated wound healing with resveratrol-loaded decellularized pericardium in mice model. Cell Tissue Bank. 2024, 25, 245–253. [Google Scholar] [CrossRef]
- Meng, Q.; Li, J.; Wang, C.; Shan, A. Biological function of resveratrol and its application in animal production: A review. J. Anim. Sci. Biotechnol. 2023, 14, 25. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.B.; Bi, Y.L.; Tu, Y.; Ma, T.; Dong, L.F.; Du, H.C.; Diao, Q.Y. Sanguinarine and resveratrol affected rumen fermentation parameters and bacterial community in calves. Anim. Feed Sci. Technol. 2019, 251, 64–75. [Google Scholar] [CrossRef]
- Ma, T.; Chen, D.D.; Tu, Y.; Zhang, N.F.; Si, B.W.; Deng, K.D.; Diao, Q.Y. Effect of dietary supplementation with resveratrol on nutrient digestibility, methanogenesis and ruminal microbial flora in sheep. J. Anim. Physiol. Anim. Nutr. 2015, 99, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Wang, Y.; Wang, C.; Liu, J. Effects of addition of Aspergillus oryzae culture and 2-hydroxyl-4-(methylthio) butanoic acid on milk performance and rumen fermentation of dairy cows. Anim. Sci. J. Nihon Chikusan Gakkaiho 2017, 88, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Mirande, C.; Morgavi, D.P.; Forano, E.; Devillard, E.; Mosoni, P. Methionine analogues HMB and HMBi increase the abundance of cellulolytic bacterial representatives in the rumen of cattle with no direct effects on fibre degradation. Anim. Feed Sci. Technol. 2013, 182, 16–24. [Google Scholar] [CrossRef]
- Noftsger, S.M.; St-Pierre, N.R.; Karnati, S.K.; Firkins, J.L. Effects of 2-hydroxy-4-(methylthio) butanoic acid (HMB) on microbial growth in continuous culture. J. Dairy Sci. 2003, 86, 2629–2636. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 722557. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, W.; Feng, L.; Zhang, C.; Yan, C.; Zhang, J.; Lai, J.; Yan, T.; He, Z.; Du, X.; et al. Resveratrol Improves the Digestive Ability and the Intestinal Health of Siberian Sturgeon. Int. J. Mol. Sci. 2022, 23, 11977. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Debnath, D.; Sahu, N.P.; Pal, A.K.; Jain, K.K.; Baruah, K. Dietary protein enhances non-specific immunity, anti-oxidative capability and resistance to Aeromonas hydrophila in Labeo rohita fingerlings pre-exposed to short feed deprivation stress. Fish Shellfish. Immunol. 2016, 59, 439–446. [Google Scholar] [CrossRef]
- Diaz-Medina, L.K.; Colín-Navarro, V.; Arriaga-Jordán, C.M.; Brunett-Pérez, L.; Vázquez-de-Aldana, B.R.; Estrada-Flores, J.G. In vitro nutritional quality and antioxidant activity of three weed species as feed additives for sheep in the Central Highlands of Mexico. Trop. Anim. Health Prod. 2021, 53, 394. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Pereira, S.L.; Das, T. Efficacy of Nutrients in Reducing the Symptoms of Radiation Induced Oral Mucositis in a Hamster Model. Nutr. Cancer 2022, 74, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiang, L.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Effects of dietary leucine on antioxidant activity and expression of antioxidant and mitochondrial-related genes in longissimus dorsi muscle and liver of piglets. Anim. Sci. J. Nihon Chikusan Gakkaiho 2019, 90, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Szabolcs, A.; Varga, I.S.; Varga, C.; Berkó, A.; Kaszaki, J.; Letoha, T.; Tiszlavicz, L.; Sári, R.; Lonovics, J.; Takács, T. Beneficial effect of resveratrol on cholecystokinin-induced experimental pancreatitis. Eur. J. Pharmacol. 2006, 532, 187–193. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Chen, H.; Wei, S.; Yao, K.; Sun, X.; Yang, G.; Jiang, L.; Zhang, C.; Wang, N.; et al. Resveratrol protected acrolein-induced ferroptosis and insulin secretion dysfunction via ER-stress-related PERK pathway in MIN6 cells. Toxicology 2022, 465, 153048. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Z.; Huang, Z.; Chen, D.; He, J.; Chen, H.; Yu, B.; Yu, J.; Luo, J.; Luo, Y.; et al. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim. Biotechnol. 2021, 32, 240–245. [Google Scholar] [CrossRef]
- Deminice, R.; Portari, G.V.; Marchini, J.S.; Vannucchi, H.; Jordao, A.A. Effects of a low-protein diet on plasma amino acid and homocysteine levels and oxidative status in rats. Ann. Nutr. Metab. 2009, 54, 202–207. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, C.; Zhong, Y.; Song, B.; Yan, Z.; Kong, X.; Deng, J.; Li, F.; Yin, Y. Beta-hydroxy beta-methyl butyrate decreases muscle protein degradation via increased Akt/FoxO3a signaling and mitochondrial biogenesis in weanling piglets after lipopolysaccharide challenge. Food Funct. 2019, 10, 5152–5165. [Google Scholar] [CrossRef]
- Zheng, C.; Song, B.; Duan, Y.; Zhong, Y.; Yan, Z.; Zhang, S.; Li, F. Dietary β-hydroxy-β-methylbutyrate improves intestinal function in weaned piglets after lipopolysaccharide challenge. Nutrition 2020, 78, 110839. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Zhou, J.; Wu, H.A.; Mbazor, E.; Song, G.; Balick, M.; DeVoti, J.A.; Redenti, S.; Castellanos, M.R. A novel cancer preventative botanical mixture, TriCurin, inhibits viral transcripts and the growth of W12 cervical cells harbouring extrachromosomal or integrated HPV16 DNA. Br. J. Cancer 2021, 124, 901–913. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Aguirre, L.; Crujeiras, A.B.; Irles, E.; Segues, N.; Bujanda, L.; Portillo, M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants 2020, 9, 1042. [Google Scholar] [CrossRef]
- Mota, M.; Porrini, V.; Parrella, E.; Benarese, M.; Bellucci, A.; Rhein, S.; Schwaninger, M.; Pizzi, M. Neuroprotective epi-drugs quench the inflammatory response and microglial/macrophage activation in a mouse model of permanent brain ischemia. J. Neuroinflammation 2020, 17, 361. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J.; Liu, G. Regulatory Effect of Resveratrol on Inflammation Induced by Lipopolysaccharides via Reprograming Intestinal Microbes and Ameliorating Serum Metabolism Profiles. Front. Immunol. 2021, 12, 777159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Protective effects of leucine against lipopolysaccharide-induced inflammatory response in Labeo rohita fingerlings. Fish Shellfish. Immunol. 2016, 52, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Han, D.; Meng, J.; Wang, H.; Cao, G. L-lysine ameliorates sepsis-induced acute lung injury in a lipopolysaccharide-induced mouse model. Biomed. Pharmacother. 2019, 118, 109307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yin, L.; Li, J.Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y.; et al. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish. Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef]
- Lanza, V.F.; Tedim, A.P.; Martínez, J.L.; Baquero, F.; Coque, T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015, 3, 379–419. [Google Scholar] [CrossRef]
- Luo, Z.; Li, C.; Cheng, Y.; Hang, S.; Zhu, W. Effects of low dietary protein on the metabolites and microbial communities in the caecal digesta of piglets. Arch. Anim. Nutr. 2015, 69, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea Are Interactive Components of Complex Microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, J.L.; González, J.; Carro, M.D. Influence of protein fermentation and carbohydrate source on in vitro methane production. J. Anim. Physiol. Anim. Nutr. 2017, 101, e288–e296. [Google Scholar] [CrossRef] [PubMed]
- Hurley, A.M.; López-Villalobos, N.; McParland, S.; Kennedy, E.; Lewis, E.; O’Donovan, M.; Burke, J.L.; Berry, D.P. Inter-relationships among alternative definitions of feed efficiency in grazing lactating dairy cows. J. Dairy Sci. 2016, 99, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.R.; Lam, A.C. Polymorphic proteins of Chlamydia spp.--autotransporters beyond the Proteobacteria. Trends Microbiol. 2001, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, W.; Gong, S.; Li, Y.; Xia, S.; Zhang, B.; Ma, J. Effects of dietary protein on gut development, microbial compositions and mucin expressions in mice. J. Appl. Microbiol. 2022, 132, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shu, Q. Novel primers to identify a wider diversity of butyrate-producing bacteria. World J. Microbiol. Biotechnol. 2024, 40, 76. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, L.; Peng, S.; Liu, T.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Zhao, X.; Liang, X.; et al. Resveratrol regulates intestinal barrier function in cyclophosphamide-induced immunosuppressed mice. J. Sci. Food Agric. 2022, 102, 1205–1215. [Google Scholar] [CrossRef]
- Duan, Y.; Zhong, Y.; Xiao, H.; Zheng, C.; Song, B.; Wang, W.; Guo, Q.; Li, Y.; Han, H.; Gao, J.; et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 10019–10033. [Google Scholar] [CrossRef]
- Zhao, R.; Coker, O.O.; Wu, J.; Zhou, Y.; Zhao, L.; Nakatsu, G.; Bian, X.; Wei, H.; Chan, A.W.H.; Sung, J.J.Y.; et al. Aspirin Reduces Colorectal Tumor Development in Mice and Gut Microbes Reduce its Bioavailability and Chemopreventive Effects. Gastroenterology 2020, 159, 969–983.e964. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Kim, G.; Morales, W.; Sung, J.; Rooks, E.; Pokkunuri, V.; Weitsman, S.; Barlow, G.M.; Chang, C.; Pimentel, M. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity 2013, 21, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Funayama, H.; Mayanagi, H.; Takada, H.; Endo, Y. Elevation of histidine decarboxylase activity in the mandible of mice by Prevotella intermedia lipopolysaccharide and its augmentation by an aminobisphosphonate. Arch. Oral Biol. 2000, 45, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell Host Microbe 2024, 32, 63–78.e67. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Liu, T.; Xie, P.; Jiang, S.; Yi, W.; Dai, P.; Guo, X. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci. 2020, 258, 118160. [Google Scholar] [CrossRef]
- Wang, J.; Wen, Y.; Zhao, W.; Zhang, Y.; Lin, F.; Ouyang, C.; Wang, H.; Yao, L.; Ma, H.; Zhuo, Y.; et al. Hepatic conversion of acetyl-CoA to acetate plays crucial roles in energy stress. eLife 2023, 12, RP87419. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.L.; Schroeder, N.J.; Calverley, M.J.; Burrin, J.M.; Makin, H.L.; Cunningham, J. Potent suppression of the parathyroid glands by hydroxylated metabolites of dihydrotachysterol(2). Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2000, 15, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.E.; Bell, P.A. Metabolism of dihydrotachysterol and 5,6-trans-cholecalciferol in the chick and the rat. Biochem. J. 1974, 142, 37–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.; Liu, J.; Wang, S.; Zeng, Z.; Li, T.; Liu, Y.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; et al. Enterolactone has stronger effects than enterodiol on ovarian cancer. J. Ovarian Res. 2017, 10, 49. [Google Scholar] [CrossRef]
- Jin, H.R.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.Z.; Huang, W.H.; Li, S.P.; He, T.C.; Yuan, C.S.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef]
- Parker, R.A.; Kariya, T.; Grisar, J.M.; Petrow, V. 5-(Tetradecyloxy)-2-furancarboxylic acid and related hypolipidemic fatty acid-like alkyloxyarylcarboxylic acids. J. Med. Chem. 1977, 20, 781–791. [Google Scholar] [CrossRef] [PubMed]



| L-CP | H-CP | |
|---|---|---|
| Ingredient (%) | ||
| Corn | 58.30 | 51.50 |
| Soybean meal | 1.00 | 2.00 |
| Rapeseed meal | 7.00 | 12.80 |
| Cottonseed meal | 2.00 | 2.00 |
| Palm meal | 25.00 | 25.00 |
| NaCl | 1.00 | 1.00 |
| Limestone | 1.00 | 1.00 |
| Baking soda | 0.10 | 0.10 |
| Premix (1) | 4.60 | 4.60 |
| Total | 100 | 100 |
| Nutrient levels (2) | ||
| Digestible energy (MJ·kg−1) | 12.71 | 12.84 |
| Crude protein (%) | 14.27 | 12.13 |
| Ether extract (%) | 3.29 | 3.44 |
| Crude fiber (%) | 11.64 | 11.05 |
| Neutral detergent fiber (%) | 26.70 | 26.04 |
| Acid detergent fiber (%) | 19.97 | 19.11 |
| Ca (%) | 0.86 | 0.80 |
| P (%) | 0.40 | 0.40 |
| Trypsin (ng/mL) | Chymotrypsin (ng/L) | Lipase (ng/L) | Cellulase (ng/L) | α-Amylase (μmol/L) | ||
|---|---|---|---|---|---|---|
| Groups | L | 32.79 ± 1.50 a | 738.42 ± 55.10 a | 278.08 ± 61.63 a | 308.35 ± 19.29 | 101.16 ± 11.20 |
| L-RES-HMB | 32.49 ± 1.42 a | 761.33 ± 35.20 a | 289.62 ± 11.54 a | 314.11 ± 21.92 | 112.50 ± 5.44 | |
| H | 23.23 ± 3.38 b | 539.67 ± 12.61 b | 180.38 ± 7.12 b | 280.07 ± 10.76 | 123.33 ± 13.54 | |
| H-RES-HMB | 32.90 ± 2.88 a | 764.67 ± 60.92 a | 231.28 ± 10.59 a | 294.04 ± 10.43 | 118.82 ± 31.08 | |
| p-value | CP level | <0.001 | <0.001 | <0.001 | 0.054 | 0.080 |
| RES-HMB | <0.001 | <0.001 | 0.001 | 0.190 | 0.659 | |
| RES-HMB × CP level | <0.001 | <0.001 | 0.013 | 0.577 | 0.312 |
| GSH-PX (pmol/mL) | SOD (pg/mL) | T-AOC U-(u/mL) | CAT (ng/L) | MDA (pg/mL) | ||
|---|---|---|---|---|---|---|
| Groups | L | 26.61 ± 5.82 | 167.18 ± 9.52 | 3.06 ± 0.28 | 128.04 ± 10.10 | 1.57 ± 0.59 |
| L-RES-HMB | 28.33 ± 6.41 | 174.70 ± 7.29 | 3.55 ± 0.73 | 140.65 ± 4.26 | 1.24 ± 0.14 | |
| H | 38.64 ± 2.62 | 174.76 ± 4.25 | 3.06 ± 0.30 | 119.78 ± 13.38 | 1.59 ± 0.33 | |
| H-RES-HMB | 31.55 ± 0.87 | 182.77 ± 11.41 | 3.97 ± 0.63 | 143.36 ± 6.05 | 0.99 ± 0.50 | |
| p-value | CP Level | 0.016 | 0.093 | 0.476 | 0.544 | 0.570 |
| RES-HMB | 0.338 | 0.095 | 0.029 | 0.001 | 0.032 | |
| RES-HMB × CP level | 0.128 | 0.956 | 0.463 | 0.238 | 0.513 |
| LPS (ng/L) | ||
|---|---|---|
| Groups | L | 279.05 ± 18.70 a |
| L-RES + HMB | 268.25 ± 10.74 a | |
| H | 226.19 ± 13.11 b | |
| H-RES + HMB | 200.40 ± 15.61 c | |
| p-value | CP level | <0.001 |
| RES-HMB | 0.024 | |
| RES-HMB × CP Level | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Zhang, Y.; Zhang, F.; Wu, Z.; Su, Q.; Hou, S.; Gui, L. The Effects of Dietary Resveratrol and β-Hydroxy-β-Methylbutyric Acid Supplementation at Two Protein Levels on the Ruminal Microbiome and Metabolome of Tibetan Sheep. Agriculture 2024, 14, 936. https://doi.org/10.3390/agriculture14060936
Zhu K, Zhang Y, Zhang F, Wu Z, Su Q, Hou S, Gui L. The Effects of Dietary Resveratrol and β-Hydroxy-β-Methylbutyric Acid Supplementation at Two Protein Levels on the Ruminal Microbiome and Metabolome of Tibetan Sheep. Agriculture. 2024; 14(6):936. https://doi.org/10.3390/agriculture14060936
Chicago/Turabian StyleZhu, Kaina, Yu Zhang, Fengshuo Zhang, Zhenling Wu, Quyangangmao Su, Shengzhen Hou, and Linsheng Gui. 2024. "The Effects of Dietary Resveratrol and β-Hydroxy-β-Methylbutyric Acid Supplementation at Two Protein Levels on the Ruminal Microbiome and Metabolome of Tibetan Sheep" Agriculture 14, no. 6: 936. https://doi.org/10.3390/agriculture14060936
APA StyleZhu, K., Zhang, Y., Zhang, F., Wu, Z., Su, Q., Hou, S., & Gui, L. (2024). The Effects of Dietary Resveratrol and β-Hydroxy-β-Methylbutyric Acid Supplementation at Two Protein Levels on the Ruminal Microbiome and Metabolome of Tibetan Sheep. Agriculture, 14(6), 936. https://doi.org/10.3390/agriculture14060936





