Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change
Abstract
1. Introduction
2. Climate Change Exacerbates Crop Fungal Diseases
2.1. Drought Exacerbates Crop Fungal Diseases
2.2. Flooding and High Humidity Exacerbates Crop Fungal Diseases
2.3. High Temperatures Exacerbate Crop Fungal Diseases
3. Impact of Climate Change on Crop Fungal Diseases with Different Routes of Transmission
3.1. Soil-Borne Fungal Diseases
3.2. Air-Borne Fungal Diseases
3.3. Seed-Borne Fungal Diseases
4. Control of Fungal Diseases of Crops
4.1. Disease Detection
4.1.1. Methods for Diagnosing Fungal Diseases of Crops
Direct Detection Methods
Indirect Detection Methods
- (1)
- Spectroscopy. Spectroscopy is used to assess the health of a crop by irradiating light of a specific wavelength onto the crop tissue and measuring the intensity of the light wavelengths reflected back. Examples of types of spectroscopy include fluorescence spectroscopy, visible spectroscopy, infrared spectroscopy, and nuclear magnetic resonance spectroscopy. Near-infrared spectroscopy can detect maize spot disease and olive leaf spot [101,102]. However, spectroscopic methods cannot detect diseases before they develop in crops and cannot detect multiple diseases occurring at the same time.
- (2)
- Biosensors. A sensor is an analytical device that converts chemical, physical, or biological information into a useful analytical signal. A biosensor is “an integrated receptor–transducer device capable of providing selective quantitative or semiquantitative analytical information using biometric elements” [103]. In recent years, biosensors have attracted much attention due to their good results in detecting, classifying, diagnosing, and quantifying crop diseases. Biosensors can be categorized into optical biosensors, volatile biosensors, electrochemical biosensors, and mass-sensitive biosensors. Despite the ability of biosensors to rapidly and accurately detect disease-causing fungal pathogens, only a few devices are commercially available for detecting crop fungal pathogens in crops [104].
- (3)
- Gas chromatography coupled with mass spectrometry (GC–MS). GC–MS is a technique for analyzing volatiles produced by fungal infections in crops [105]. It has the advantages of not destroying the crop and enabling continuous monitoring for a long time [106], but it has the disadvantages of environmental factors such as humidity potentially interfering with the sensor readings [107] and the need to collect volatile organic compound samples prior to the GC–MS analysis, which limits its application in the field.
- (4)
- Imaging technology. Imaging technologies include thermal, hyperspectral, and red–green–blue (RGB) and fluorescence imaging, coupled with unmanned aerial vehicles (UAVs) that can monitor large farms. Using such technologies, diseases are diagnosed indirectly by detecting changes in the color, texture, and temperature of crop leaves. In this context, intelligent agricultural machines and robots can detect crop diseases at an early stage and monitor disease development remotely [108,109]. Meanwhile, aerial remote sensing (RS) using UAVs or unmanned aerial systems with intelligent vision systems is an efficient and inexpensive way of detecting diseases in crops [108,109,110,111,112,113]. It is reported that the integration of RGB, multispectral, hyperspectral, fluorescence, and thermal infrared imaging sensors, coupled with efficient algorithms on unmanned aerial vehicles (UAVs), enables effective detection, differentiation, and quantification of the severity of symptoms induced by diverse pathogens under field conditions [114,115]. It has been shown that these methods can achieve reliable diagnosis in cereal crops, such as rice [116], corn [117], and wheat [118,119]; vegetables, such as grapevines [120], potatoes [121], soybeans [122], and tomatoes [123]; and forests, such as pine forests [108]. The combination of imaging obtained by drones with artificial intelligence algorithms, such as machine learning, is a promising new approach that not only enables early and accurate diagnosis of crop diseases but also improves crop yields while reducing the cost of disease treatment [124]. Utilizing RS technology to retrieve and gather data, encompassing meteorological information as well as the properties of the earth’s surface and soil, and offering timely feedback, holds significant promise as an effective approach for diagnosing fungal crop diseases in the context of climate change [125] (Table 3).
4.2. Prevention and Control of Crop Fungal Diseases
4.2.1. Breeding Measures for Disease Resistance
4.2.2. Agronomic Measures
4.2.3. Chemical Control
4.2.4. Biological Control
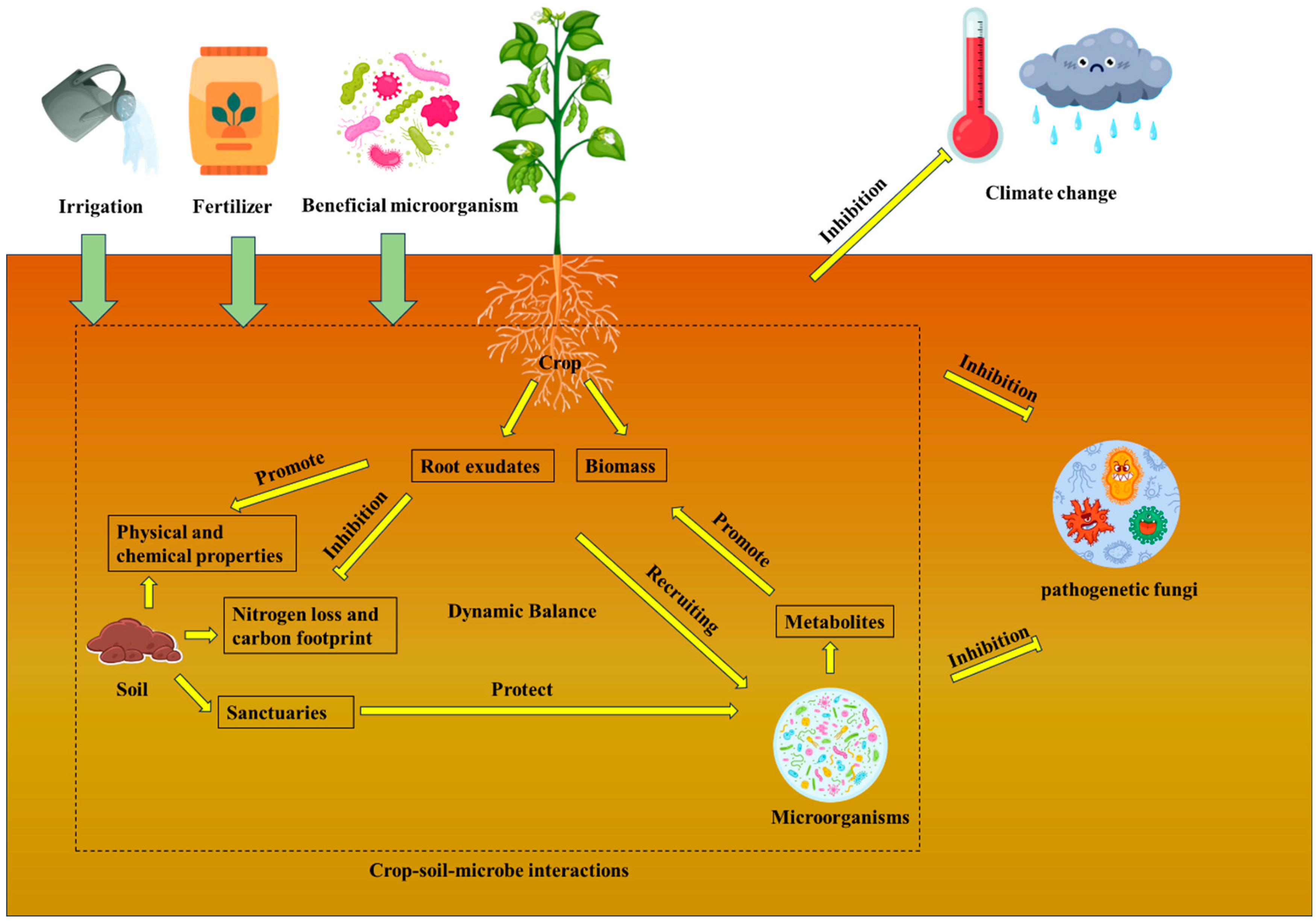
4.2.5. Ecological Regulation
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The Proportion of Soil-Borne Pathogens Increases with Warming at the Global Scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Li, E.; Ling, J.; Wang, G.; Xiao, J.; Yang, Y.; Mao, Z.; Wang, X.; Xie, B. Comparative Proteomics Analyses of Two Races of Fusarium oxysporum f. sp. Conglutinans that Differ in Pathogenicity. Sci. Rep. 2015, 5, 13663. [Google Scholar] [CrossRef]
- Narayanasamy, P. Microbial Plant Pathogens-Detection and Disease Diagnosis; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago Maydis as a Pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef]
- Nourozian, J.; Etebarian, H.R.; Khodakaramian, G. Biological Control of Fusarium graminearum on Wheat by Antagonistic Bacteria. Songklanakarin J. Sci. Technol. 2006, 28 (Suppl. S1), 29–38. [Google Scholar]
- Tamura, T.; Shinzato, N.; Ito, M.; Ueno, M. Microbial Secondary Metabolite Induction of Abnormal Appressoria Formation Mediates Control of Rice Blast Disease Caused Bymagnaporthe Oryzae. J. Phytopathol. 2019, 167, 156–162. [Google Scholar] [CrossRef]
- Karelov, A.; Kozub, N.; Sozinova, O.; Pirko, Y.; Sozinov, I.; Yemets, A.; Blume, Y. Wheat Genes Associated with Different Types of Resistance against Stem Rust (Puccinia graminis Pers.). Pathogens 2022, 11, 1157. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice Blast: A Disease with Implications for Global Food Security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef]
- Su, J.; Zhao, J.; Zhao, S.; Li, M.; Pang, S.; Kang, Z.; Zhen, W.; Chen, S.; Chen, F.; Wang, X. Genetics of Resistance to Common Root Rot (Spot Blotch), Fusarium Crown Rot, and Sharp Eyespot in Wheat. Front. Genet. 2021, 12, 699342. [Google Scholar] [CrossRef]
- Pennisi, E. Armed and Dangerous. Science 2010, 327, 804–805. [Google Scholar] [CrossRef]
- Lobell, D.; Burke, M.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.; Naylor, R.L. Prioritizing Climate Change Adaptation Needs for Food Security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- Challinor, A.J.; Watson, J.E.M.; Lobell, D.; Howden, S.M.; Smith, D.R.; Chhetri, N. A Meta-Analysis of Crop Yield under Climate Change and Adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Hijmans, R.J. The Effect of Climate Change on Global Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Reisinger, A.; Kitching, R.; Chiew, F.; Hughes, L.; Newton, P.; Schuster, S.; Tait, A.; Whetton, P.; Barnett, J.; Becken, S.; et al. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Working Group II Contribution to the Fifth Assessment Report of the IPCC: Australasia Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Rogelj, J.; den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement Climate Proposals Need a Boost to Keep Warming Well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Anand, G.; Kapoor, R. Incidence and Severity of Fungal Diseases of Safflower in India. Crop Prot. 2019, 125, 104905. [Google Scholar] [CrossRef]
- Salunkhe, V.N.; Gedam, P.; Pradhan, A.; Gaikwad, B.; Kale, R.; Gawande, S. Concurrent Waterlogging and Anthracnose-Twister Disease in Rainy-Season Onions (Allium cepa): Impact and Management. Front. Microbiol. 2022, 13, 1063472. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Cazzato, S.; Walder, F.; Vogelgsang, S.; Bender, S.F.; van der Heijden, M.G.A. Humidity and High Temperature Are Important for Predicting Fungal Disease Outbreaks Worldwide. New Phytol. 2022, 234, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Haddoudi, I.; Mhadhbi, H.; Gargouri, M.; Barhoumi, F.; Romdhane, S.B.; Mrabet, M. Occurrence of Fungal Diseases in Faba Bean (Vicia faba L.) under Salt and Drought Stress. Eur. J. Plant Pathol. 2021, 159, 385–398. [Google Scholar] [CrossRef]
- Feulefack, J.; Khan, A.; Forastiere, F.; Sergi, C.M. Parental Pesticide Exposure and Childhood Brain Cancer: A Systematic Review and Meta-Analysis Confirming the IARC/WHO Monographs on Some Organophosphate Insecticides and Herbicides. Children 2021, 8, 1096. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Wubet, T. Chronic Exposure to Pesticides Disrupts the Bacterial and Fungal Co-Existence and the Cross-Kingdom Network Characteristics of Honey Bee Gut Microbiome. Sci. Total Environ. 2024, 906, 167530. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, B.; Langridge, P.; Budak, H. Abiotic Stress Mirnomes in the Triticeae. Funct. Integr. Genom. 2017, 17, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C. Abiotic Stress-Induced Anthocyanins in Plants: Their Role in Tolerance to Abiotic Stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, R.P.; Tarara, J.M.; Smithyman, R.P. Deficit Irrigation Promotes Arbuscular Colonization of Fine Roots by Mycorrhizal Fungi in Grapevines (Vitis vinifera L.) In an Arid Climate. Mycorrhiza 2007, 17, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Chilakala, A.R.; Mali, K.V.; Irulappan, V.; Patil, B.S.; Pandey, P.; Rangappa, K.; Ramegowda, V.; Kumar, M.N.; Puli, C.O.R.; Mohan-Raju, B.; et al. Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Front. Plant Sci. 2022, 13, 890551. [Google Scholar] [CrossRef] [PubMed]
- Bidzinski, P.; Ballini, E.; Ducasse, A.; Michel, C.; Zuluaga, P.; Genga, A.; Chiozzotto, R.; Morel, J.B. Transcriptional Basis of Drought-Induced Susceptibility to the Rice Blast Fungus Magnaporthe oryzae. Front. Plant Sci. 2016, 7, 1558. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-K.; Iandolino, A.; Silva, F.; Cook, D. Water Deficit Modulates the Response of Vitis vinifera to the Pierce’s Disease Pathogen Xylella fastidiosa. Mol. Plant-Microbe Interact. MPMI 2013, 26, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Jat, R.A.; Wani, S.P.; Sahrawat, K.L. Conservation Agriculture in the Semi-Arid Tropics: Prospects and Problems. Adv. Agron. 2012, 117, 191–273. [Google Scholar]
- Radzikowski, P.; Jończyk, K.; Feledyn-Szewczyk, B.; Jóźwicki, T. Assessment of Resistance of Different Varieties of Winter Wheat to Leaf Fungal Diseases in Organic Farming. Agriculture 2023, 13, 875. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Meraj, S.; Nafisa, N.; Khan, K.; Javaid, A. Influence of Salinity and Fusarium Oxysporum as the Stress Factors on Morpho-Physiological and Yield Attributes in Onion. Physiol. Mol. Biol. Plants 2018, 24, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Chojak-Koźniewska, J.; Linkiewicz, A.M.; Sowa, S.; Radzioch, M.A.; Kuźniak, E. Interactive Effects of Salt Stress and Pseudomonas syringae pv. lachrymans Infection in Cucumber: Involvement of Antioxidant Enzymes, Abscisic acid and Salicylic Acid. Environ. Exp. Bot. 2017, 136, 9–20. [Google Scholar]
- Al-Sadi, A.M.; Al-masoudi, R.S.; Al-Habsi, N.; Al-Said, F.A.-J.; Al-Rawahy, S.A.; Ahmed, M.; Deadman, M.L. Effect of Salinity on Pythium Damping-Off of Cucumber and on the Tolerance of Pythium aphanidermatum. Plant Pathol. 2010, 59, 112–120. [Google Scholar] [CrossRef]
- Allard, S.M.; Ottesen, A.R.; Micallef, S.A. Rain Induces Temporary Shifts in Epiphytic Bacterial Communities of Cucumber and Tomato Fruit. Sci. Rep. 2020, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, D.; Czubacka, A.; Agacka-Mołdoch, M.; Trojak-Goluch, A.; Księżak, J. The Occurrence of Fungal Diseases in Maize in Organic Farming Versus an Integrated Management System. Agronomy 2022, 12, 558. [Google Scholar] [CrossRef]
- Chávez-Arias, C.C.; Gómez-Caro, S.; Restrepo-Díaz, H. Physiological, Biochemical and Chlorophyll Fluorescence Parameters of Physalis peruviana L. Seedlings Exposed to Different Short-Term Waterlogging Periods and Fusarium Wilt Infection. Agronomy 2019, 9, 213. [Google Scholar] [CrossRef]
- Iliff, G.J.; Mukherjee, R.; Gruszewski, H.A.; Schmale Iii, D.G.; Jung, S.; Boreyko, J.B. Phase-Change-Mediated Transport and Agglomeration of Fungal Spores on Wheat Awns. J. R. Soc. Interface 2022, 19, 20210872. [Google Scholar] [CrossRef] [PubMed]
- Gopi, R.; Chandran, K.; Ramesh Sundar, A.; Nisha, M.; Mahendran, B.; Keerthana; Jayaraman, S.; Viswanathan, R. Occurrence of False Floral Smut in Sugarcane Inflorescence and Associated Weather Variables. SugarTech 2023, 25, 1411–1418. [Google Scholar] [CrossRef]
- Nene, W.; Kapinga, F.; Shomari, S.; Assenga, B. Cashew Leaf and Nut Blight Disease Outbreaks under Unimodal Rainfall Pattern in Tanzania. J. Plant Pathol. 2022, 104, 929–938. [Google Scholar] [CrossRef]
- Khaliq, I.; Moore, K.; Sparks, A.H. The Relationship between Natural Rain Intensity and Ascochyta Blight in Chickpea Development. Eur. J. Plant Pathol. 2022, 164, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rollano-Peñaloza, O.M.; Palma-Encinas, V.; Widell, S.; Mollinedo, P.; Rasmusson, A.G. The Disease Progression and Molecular Defense Response in Chenopodium Quinoa Infected with Peronospora Variabilis, the Causal Agent of Quinoa Downy Mildew. Plants 2022, 11, 2946. [Google Scholar] [CrossRef] [PubMed]
- Barro, M.; Kassankogno, A.I.; Wonni, I.; Sérémé, D.; Somda, I.; Kaboré, H.K.; Béna, G.; Brugidou, C.; Tharreau, D.; Tollenaere, C. Spatiotemporal Survey of Multiple Rice Diseases in Irrigated Areas Compared to Rainfed Lowlands in the Western Burkina Faso. Plant Dis. 2021, 105, 3889–3899. [Google Scholar] [CrossRef] [PubMed]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight Hard or Die Trying: When Plants Face Pathogens under Heat Stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Salotti, I.; Altieri, V.; Li, M.; Rossi, V. Temperature-Dependent Growth and Spore Germination of Fungi Causing Grapevine Trunk Diseases: Quantitative Analysis of Literature Data. Plant Dis. 2023, 107, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine Trunk Diseases under Thermal and Water Stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Barakat, R.; Kaur, J.; Epstein, L. The Effect of Temperature on Disease Severity and Growth of Fusarium oxysporum f. sp. Apii Races 2 and 4 in Celery. Phytopathology 2022, 112, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Nelson, B.D. Effect of Temperature on Fusarium solani and F. Tricinctum Growth and Disease Development in Soybean. Can. J. Plant Pathol. 2020, 42, 527–537. [Google Scholar] [CrossRef]
- Shakya, S.K.; Goss, E.M.; Dufault, N.S.; van Bruggen, A.H. Potential Effects of Diurnal Temperature Oscillations on Potato Late Blight with Special Reference to Climate Change. Phytopathology 2015, 105, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Shafiekhani, S.; Wilson, S.A.; Atungulu, G.G. Impacts of Storage Temperature and Rice Moisture Content on Color Characteristics of Rice from Fields with Different Disease Management Practices. J. Stored Prod. Res. 2018, 78, 89–97. [Google Scholar] [CrossRef]
- Odebode, A.C.; Unachukwu, N.E. Effect of Storage Environment on Carrot Root Rots and Biochemical Changes During Storage. Z. Für Leb. Und-Forsch. A 1997, 205, 277–281. [Google Scholar] [CrossRef]
- Casagrande, E.; Génard, M.; Lurol, S.; Charles, F.; Bevacqua, D.; Martinetti, D.; Lescourret, F. Brown Rot Disease in Stored Nectarines: Modeling the Combined Effects of Preharvest and Storage Conditions. Phytopathology 2022, 112, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Meshram, S.; Adhikari, T.B. Microbiome-Mediated Strategies to Manage Major Soil-Borne Diseases of Tomato. Plants 2024, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Raaijmakers, J.M.; de Hollander, M.; Mendes, R.; Tsai, S.M. Influence of Resistance Breeding in Common Bean on Rhizosphere Microbiome Composition and Function. ISME J. 2018, 12, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Adolf, B.; Andrade-Piedra, J.; Bittara Molina, F.; Przetakiewicz, J.; Hausladen, H.; Kromann, P.; Lees, A.; Lindqvist-Kreuze, H.; Perez, W.; Secor, G.A. Fungal, Oomycete, and Plasmodiophorid Diseases of Potato. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 307–350. [Google Scholar] [CrossRef]
- Guan, Z.; Biswas, T.; Wu, F. The U.S. Tomato Industry: An Overview of Production and Trade. EDIS 2018, 2018. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification Caused by Excessive Application of Nitrogen Fertilizer Aggravates Soil-Borne Diseases: Evidence from Literature Review and Field Trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Schauberger, B.; Archontoulis, S.; Arneth, A.; Balkovic, J.; Ciais, P.; Deryng, D.; Elliott, J.; Folberth, C.; Khabarov, N.; Müller, C.; et al. Consistent Negative Response of US Crops to High Temperatures in Observations and Crop Models. Nat. Commun. 2017, 8, 13931. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lin, M.; Qiu, M.; Kong, L.; Xu, Y.; Li, Y.; Wang, Y.; Ye, W.; Dong, S.; He, S.; et al. Chitin Synthase Is Involved in Vegetative Growth, Asexual Reproduction and Pathogenesis of Phytophthora capsici and Phytophthora sojae. Environ. Microbiol. 2019, 21, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- You, M.P.; Lamichhane, J.R.; Aubertot, J.-N.; Barbetti, M.J. Understanding Why Effective Fungicides Against Individual Soilborne Pathogens Are Ineffective with Soilborne Pathogen Complexes. Plant Dis. 2019, 104, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, M.J.; Khan, T.N.; Pritchard, I.; Lamichhane, J.R.; Aubertot, J.N.; Corrales, D.C.; You, M.P. Challenges With Managing Disease Complexes During Application of Different Measures Against Foliar Diseases of Field Pea. Plant Dis. 2021, 105, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Haydock, P.; Jenkinson, P. Disease Complexes Involving Plant Parasitic Nematodes and Soilborne Pathogens. Plant Pathol. 2002, 51, 683–697. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mirakhorli, N.; Erginbas-Orakci, G.; Ansari, O.; Braun, H.-J.; Paulitz, T.C.; Dababat, A. Interactions among Cereal cyst Nematode Heterodera filipjevi, Dryland crown rot Fusarium culmorum, and Drought on Grain Yield Components and Disease Severity in Bread wheat. Can. J. Plant Pathol. 2021, 44, 415–431. [Google Scholar] [CrossRef]
- You, M.P.; Rensing, K.; Renton, M.; Barbetti, M.J. Modeling Effects of Temperature, Soil, Moisture, Nutrition and Variety As Determinants of Severity of Pythium Damping-Off and Root Disease in Subterranean Clover. Front. Microbiol. 2017, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Gibert, S.; Edel-Hermann, V.; Gautheron, E.; Gautheron, N.; Bernaud, E.; Sol, J.M.; Capelle, G.P.J.; Galland, R.; Bardon-Debats, A.; Lambert, C.; et al. Identification, Pathogenicity and Community Dynamics of Fungi and Oomycetes Associated with Pea Root Rot in Northern France. Plant Pathol. 2022, 71, 1550–1569. [Google Scholar] [CrossRef]
- Haas, D.; Ilieva, M.; Fritz, T.; Galler, H.; Habib, J.; Kriso, A.; Kropsch, M.; Ofner-Kopeinig, P.; Reinthaler, F.F.; Strasser, A.; et al. Background Concentrations of Airborne, Culturable Fungi and Dust Particles in Urban, Rural and Mountain Regions. Sci. Total Environ. 2023, 892, 164700. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.; Pautasso, M.; Jeger, M.; Haines-Young, R. Evolution of the International Regulation of Plant Pest and Challenges for Future Plant Health. Food Secur. 2010, 2, 49–70. [Google Scholar] [CrossRef]
- Wellings, C.; Mcintosh, R.; Walker, J. Puccinia striiformis f.sp. Tritici in Eastern Australia Possible Means of Entry and Implications for Plant Quarantine. Plant Pathol. 2007, 36, 239–241. [Google Scholar] [CrossRef]
- Ding, Y.; Cuddy, W.S.; Wellings, C.R.; Zhang, P.; Thach, T.; Hovmøller, M.S.; Qutob, D.; Brar, G.S.; Kutcher, H.R.; Park, R.F. Incursions of Divergent Genotypes, Evolution of Virulence and Host Jumps Shape a Continental Clonal Population of the Stripe Rust Pathogen Puccinia striiformis. Mol. Ecol. 2021, 30, 6566–6584. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Meyer, M.; Park, R.F.; Gilligan, C.A.; Burgin, L.E.; Hort, M.C.; Hodson, D.P.; Pretorius, Z.A. Microsatellite Analysis and Urediniospore Dispersal Simulations Support the Movement of Puccinia graminis f. sp. Tritici from Southern Africa to Australia. Phytopathology 2019, 109, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Poudyal, D.; Chen, X.M.; Wan, A.M.; Zhan, G.M.; Kang, Z.S.; Cao, S.Q.; Jin, S.L.; Morgounov, A.; Akin, B.; Mert, Z.; et al. Virulence Characterization of International Collections of the Wheat Stripe Rust Pathogen, Puccinia striiformis f. sp. Tritici. Plant Dis 2013, 97, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Gladieux, P.; Leconte, M.; Gautier, A.; Justesen, A.F.; Hovmøller, M.S.; Enjalbert, J.; de Vallavieille-Pope, C. Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen Puccinia striiformis f. sp. Tritici. PLoS Pathog. 2014, 10, e1003903. [Google Scholar] [CrossRef] [PubMed]
- Picornell, A.; Rojo, J.; Trigo, M.M.; Ruiz-Mata, R.; Lara, B.; Romero-Morte, J.; Serrano-García, A.; Pérez-Badia, R.; Gutiérrez-Bustillo, M.; Cervigón-Morales, P.; et al. Environmental Drivers of the Seasonal Exposure to Airborne Alternaria spores in Spain. Sci. Total Environ. 2022, 823, 153596. [Google Scholar] [CrossRef] [PubMed]
- Sindt, C.; Besancenot, J.-P.; Thibaudon, M. Airborne Cladosporium Fungal Spores and Climate Change in France. Aerobiologia 2016, 32, 53–68. [Google Scholar] [CrossRef]
- Estève, R.S.; Baisnée, D.; Guinot, B.; Sodeau, J.; O’Connor, D.J.; Belmonte, J.; Besancenot, J.-P.; Petit, J.-E.; Thibaudon, M.; Oliver, G.; et al. Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sens. 2019, 11, 1671. [Google Scholar] [CrossRef]
- Urban, J.; Lebeda, A. Variation of Fungicide Resistance in Czech Populations of Pseudoperonospora cubensis. J. Phytopathol. 2007, 155, 143–151. [Google Scholar] [CrossRef]
- Zhanbin, S.; Yu, S.; Hu, Y.; Wen, Y. Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae 2022, 8, 410. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Bischoff-Schaefer, M.; Bluemel, S.; Dachbrodt-Saaydeh, S.; Dreux, L.; Jansen, J.P.; Kiss, J.; Köhl, J.; Kudsk, P.; Malausa, T.; et al. Identifying Obstacles and Ranking Common Biological Control Research Priorities for Europe to Manage Most Economically Important Pests in Arable, Vegetable and Perennial Crops. Pest Manag. Sci. 2017, 73, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia Inaequalis: The Causal Agent of Apple Scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Armengol, J.; Rossi, V. Biology and Epidemiology of Venturia Species Affecting Fruit Crops: A Review. Front. Plant Sci. 2017, 8, 1496. [Google Scholar] [CrossRef]
- Jha, G.; Thakur, K.; Thakur, P. The Venturia Apple Pathosystem: Pathogenicity Mechanisms and Plant Defense Responses. J. Biomed. Biotechnol. 2009, 2009, 680160. [Google Scholar] [CrossRef] [PubMed]
- Abuley, I.K.; Nielsen, B.J. Evaluation of Models to Control Potato Early Blight (Alternaria solani) in Denmark. Crop Prot. 2017, 102, 118–128. [Google Scholar] [CrossRef]
- Meno, L.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Modification of the TOMCAST Model with Aerobiological Data for Management of Potato Early Blight. Agronomy 2020, 10, 1872. [Google Scholar] [CrossRef]
- Singh, V.; Shrivastava, A.; Jadon, S.; Wahi, N.; Singh, A.; Sharma, N. Alternaria Diseases of Vegetable Crops and Its Management Control to Reduce the Low Production. Int. J. Agric. Biol. 2015, 7, 834. [Google Scholar]
- Campos, H.; Ortiz, O. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind. In The Potato Crop; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Escuredo, O.; Seijo-Rodríguez, A.; Meno, L.; Rodríguez-Flores, M.S.; Seijo, M.C. Seasonal Dynamics of Alternaria during the Potato Growing Cycle and the Influence of Weather on the Early Blight Disease in North-West Spain. Am. J. Potato Res. 2019, 96, 532–540. [Google Scholar] [CrossRef]
- Sarita; Buts, A.K.; Singh, R. Seed Borne Mycoflora of Mung Bean (Phaseolus aureus Roxb.) and Its Control by Fungicides. Adv. Appl. Sci. Res. 2014, 5, 8–10. [Google Scholar]
- Howell, C.R. Cotton Seedling Preemergence Damping-Off Incited by Rhizopus oryzae and Pythium spp. and Its Biological Control with Trichoderma spp. Phytopathology 2002, 92, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Pieczul, K.; Perek, A.; Kubiak, K. Detection of Tilletia caries, Tilletia laevis and Tilletia controversa Wheat Grain Contamination Using Loop-Mediated Isothermal DNA Amplification (LAMP). J. Microbiol. Methods 2018, 154, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, L.; Mhango, W.; Saka, V.W.; Juma, S.; Goodman, J.; Brandenburg, R.L.; Jordan, D.L. Influence of Plant Population and Harvest Date on Peanut (Arachis hypogaea) Yield and Aflatoxin Contamination. Peanut Sci. 2021, 48, 33–39. [Google Scholar] [CrossRef]
- Pramunadipta, S.; Widiastuti, A.; Wibowo, A.; Priyatmojo, A. Rep-PCR Analysis of Fusarium proliferatum Causing Sheath Rot Disease and Its Relationship to Light, PH, Temperature and Rice Varieties. Arch. Phytopathol. Plant Prot. 2022, 55, 973–990. [Google Scholar] [CrossRef]
- Rao, P.J.M.; Pallavi, M.; Bharathi, Y.; Priya, P.B.; Sujatha, P.; Prabhavathi, K. Insights into Mechanisms of Seed Longevity in Soybean: A Review. Front. Plant Sci. 2023, 14, 1206318. [Google Scholar] [CrossRef] [PubMed]
- Farber, C.; Mahnke, M.; Sanchez, L.; Kurouski, D. Advanced Spectroscopic Techniques for Plant Disease Diagnostics. A Review. TrAC Trends Anal. Chem. 2019, 118, 43–49. [Google Scholar] [CrossRef]
- Genaev, M.A.; Skolotneva, E.S.; Gultyaeva, E.I.; Orlova, E.A.; Bechtold, N.P.; Afonnikov, D.A. Image-Based Wheat Fungi Diseases Identification by Deep Learning. Plants 2021, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kindo, A.J.; Tupaki-Sreepurna, A.; Yuvaraj, M. Banana Peel Culture as an Indigenous Medium for Easy Identification of Late-Sporulation Human Fungal Pathogens. Indian J. Med. Microbiol. 2016, 34, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.; de la Escosura-Muñiz, A.; Merkoçi, A. Biosensors for Plant Pathogen Detection. Biosens. Bioelectron. 2017, 93, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Farber, C.; Bryan, R.; Paetzold, L.; Rush, C.; Kurouski, D. Non-Invasive Characterization of Single-, Double- and Triple-Viral Diseases of Wheat with a Hand-Held Raman Spectrometer. Front. Plant Sci. 2020, 11, 01300. [Google Scholar] [CrossRef] [PubMed]
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.A.; Ford, R. Biosensor Technologies for Early Detection and Quantification of Plant Pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khalaf, N.; Salman, M. Detecting Plant Diseases Using Visible/Near Infrared Spectroscopy. NIR News 2013, 24, 12–25. [Google Scholar] [CrossRef]
- Larsolle, A.; Hamid Muhammed, H. Measuring Crop Status Using Multivariate Analysis of Hyperspectral Field Reflectance with Application to Disease Severity and Plant Density. Precis. Agric. 2007, 8, 37–47. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Neethirajan, S.; Ragavan, K.V.; Weng, X. Agro-Defense: Biosensors for Food from Healthy Crops and Animals. Trends Food Sci. Technol. 2018, 73, 25–44. [Google Scholar] [CrossRef]
- Wilson, A.D. Noninvasive Early Disease Diagnosis by Electronic-Nose and Related VOC-Detection Devices. Biosensors 2020, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Guo, M.; Qi, D.; Zhou, X.; Li, F.; Wu, J. Three Highly Sensitive Monoclonal Antibody-Based Serological Assays for the Detection of Tomato Mottle Mosaic Virus. Phytopathol. Res. 2021, 3, 23. [Google Scholar] [CrossRef]
- Gan, Z.; Zhou, Q.a.; Zheng, C.; Wang, J. Challenges and Applications of Volatile Organic Compounds Monitoring Technology in Plant Disease Diagnosis. Biosens. Bioelectron. 2023, 237, 115540. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, B.; Wu, Y.; Lu, Q.; Zhu, H. Identifying Pine Wood Nematode Disease Using UAV Images and Deep Learning Algorithms. Remote Sens. 2021, 13, 162. [Google Scholar] [CrossRef]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H.M. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2014, 35, 1–25. [Google Scholar] [CrossRef]
- Ge, Y.; Thomasson, J.; Sui, R. Remote Sensing of Soil Properties in Precision Agriculture: A Review. Front. Earth Sci. 2011, 5, 229–238. [Google Scholar] [CrossRef]
- Shi, Y.; Thomasson, J.A.; Murray, S.C.; Pugh, N.A.; Rooney, W.L.; Shafian, S.; Rajan, N.; Rouze, G.; Morgan, C.L.; Neely, H.L.; et al. Unmanned Aerial Vehicles for High-Throughput Phenotyping and Agronomic Research. PLoS ONE 2016, 11, e0159781. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.; Bdolach, E.; Montekyo, Y.; Rachmilevitch, S.; Townsend, P.; Karnieli, A. Assessment of Maize Yield and Phenology by Drone-Mounted Superspectral Camera. Precis. Agric. 2020, 21, 51–76. [Google Scholar] [CrossRef]
- Bauriegel, E.; Herppich, W.B. Hyperspectral and Chlorophyll Fluorescence Imaging for Early Detection of Plant Diseases, with Special Reference to Fusarium Spec. Infections on Wheat. Agriculture 2014, 4, 32–57. [Google Scholar] [CrossRef]
- Kuska, M.; Wahabzada, M.; Leucker, M.; Dehne, H.W.; Kersting, K.; Oerke, E.C.; Steiner, U.; Mahlein, A.K. Hyperspectral Phenotyping on the Microscopic Scale: Towards Automated Characterization of Plant-Pathogen Interactions. Plant Methods 2015, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, X.; Zhang, J.; Lan, Y.; Xu, C.; Liang, D. Detection of Rice Sheath Blight Using an Unmanned Aerial System with High-Resolution Color and Multispectral Imaging. PLoS ONE 2018, 13, e0187470. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, L.; Shu, M.; Gu, X.; Yang, G.; Zhou, L. Monitoring Maize Lodging Grades via Unmanned Aerial Vehicle Multispectral Image. Plant Phenomics 2019, 2019, 5704154. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Carter, A.H.; Johnson, D.A.; Cummings, T.F. UAS Imaging-Based Decision Tools for Arid Winter Wheat and Irrigated Potato Production Management. Int. J. Remote Sens. 2016, 37, 125–137. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat Yellow Rust Detection Using UAV-Based Hyperspectral Technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Shahi, T.; Xu, C.-Y.; Neupane, A.; Guo, W. Recent Advances in Crop Disease Detection Using UAV and Deep Learning Techniques. Remote Sens. 2023, 15, 2450. [Google Scholar] [CrossRef]
- Franceschini, M.H.D.; Bartholomeus, H.M.; Apeldoorn, D.v.; Suomalainen, J.M.; Kooistra, L. Assessing Changes in Potato Canopy Caused by Late Blight in Organic Production Systems through UAV-Based Pushbroom Imaging Spectrometer. ISPRS-Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2017, 42, 109–112. [Google Scholar] [CrossRef]
- Yamamoto, S.; Nomoto, S.; Hashimoto, N.; Maki, M.; Hongo, C.; Tatsuhiko, S.; Homma, K. Monitoring Spatial and Time-Series Variations in Red Crown Rot Damage of Soybean in Farmer Fields Based on UAV Remote Sensing. Plant Prod. Sci. 2023, 26, 36–47. [Google Scholar] [CrossRef]
- Abdulridha, J.N.; Ampatzidis, Y.; Kakarla, S.C.; Roberts, P.D. Detection of Target Spot and Bacterial Spot Diseases in Tomato Using UAV-Based and Benchtop-Based Hyperspectral Imaging Techniques. Precis. Agric. 2019, 21, 955–978. [Google Scholar] [CrossRef]
- Tallapragada, P.; Ross, S.D.; Schmale, D.G. Lagrangian Coherent Structures Are Associated with Fluctuations in Airborne Microbial Populations. Chaos 2011, 21, 033122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, M.; Tao, M.; Zhou, W.; Lu, X.; Xiong, Y.; Li, F.; Wang, Q. The Role of Satellite Remote Sensing in Mitigating and Adapting to Global Climate Change. Sci. Total Environ. 2023, 904, 166820. [Google Scholar] [CrossRef]
- Wang, J.; Long, X.; Chern, M.; Chen, X. Understanding the Molecular Mechanisms of Trade-Offs between Plant Growth and Immunity. Sci. China Life Sci. 2021, 64, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Plett, J.M.; Plett, K.L.; Bithell, S.L.; Mitchell, C.; Moore, K.; Powell, J.R.; Anderson, I.C. Improved Phytophthora Resistance in Commercial Chickpea (Cicer arietinum) Varieties Negatively Impacts Symbiotic Gene Signalling and Symbiotic Potential in Some Varieties. Plant Cell Environ. 2016, 39, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, B.; Yu, J.; Dou, D. Pathogen-Informed Breeding for Crop Disease Resistance. J. Integr. Plant Biol. 2021, 63, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Jiang, H.; Liu, B.; Wei, R.; Zhang, Y.; Zhang, Q.; Liang, Y.; Zhao, Y. Chitin Degradation and the Temporary Response of Bacterial Chitinolytic Communities to Chitin Amendment in Soil under Different Fertilization Regimes. Sci. Total Environ. 2020, 705, 136003. [Google Scholar] [CrossRef]
- Shen, Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.; Ruan, Y.; Li, R.; Li, R.; Shen, Q. Suppression of Banana Panama Disease Induced by Soil Microbiome Reconstruction through an Integrated Agricultural Strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Troy, T.; Kipgen, C.; Pal, I. The Impact of Climate Extremes and Irrigation on US Crop Yields. Environ. Res. Lett. 2015, 10, 054013. [Google Scholar] [CrossRef]
- Wheeler, T.A.; Bordovsky, J.P.; Keeling, J.W. The Effectiveness of Crop Rotation on Management of Verticillium Wilt over Time. Crop Prot. 2019, 121, 157–162. [Google Scholar] [CrossRef]
- Kpu, K.A.; Annih, M.G.; Ambang, A.L.; Agwah, E.D. Influence of Tillage Systems and Sowing Dates on the Incidence of Leaf Spot Disease in Telfairia occidentalis Caused by Phoma sorghina in Cameroon. Sci. Rep. 2022, 12, 19790. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Sadhu, A.; Chinchmalatpure, A.; Prasad, I.; Kumar, S.; Camus, D. Fertigation-Modern Technique of Fertilizer Application. Indian Farmer 2018, 5, 1062–1071. [Google Scholar]
- Breda, C.C.; Soares, M.B.; Tavanti, R.F.R.; Viana, D.G.; Freddi, O.d.S.; Piedade, A.R.; Mahl, D.; Traballi, R.C.; Guerrini, I.A. Successive Sewage Sludge Fertilization: Recycling for Sustainable Agriculture. Waste Manag. 2020, 109, 38–50. [Google Scholar] [CrossRef]
- Saccon, P. Water for Agriculture, Irrigation Management. Appl. Soil Ecol. 2018, 123, 793–796. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jørgensen, H.J.L.; Latz, M.A.C.; Manzotti, A.; Ntana, F.; Rojas, E.C.; Jensen, B. Searching for Novel Fungal Biological Control Agents for Plant Disease Control Among Endophytes. In Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H.M. The Benefits of Pesticides to Mankind and the Environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Kawasaki, K.; Lichtenberg, E. Quality Versus Quantity Effects of Pesticides: Joint Estimation of Quality Grade and Crop Yield. In Proceedings of the 2015 AAEA & WAEA Joint Annual Meeting, San Francisco, CA, USA, 26–28 July 2015. [Google Scholar]
- Carvalho, F. Agriculture, Pesticides, Food Security and Food Safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Zhu, L. Organophosphorus Pesticides in Greenhouse and Open-Field Soils across China: Distribution Characteristic, Polluted Pathway and Health Risk. Sci. Total Environ. 2021, 765, 142757. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Influence of Fungicide Application and Vine Age on Trichoderma Diversity as Source of Biological Control Agents. Agronomy 2021, 11, 446. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, T.; Li, Y.; Liang, H.; Zhao, Y.; Chen, H.; Li, L.; Liang, H. Enantioselective Bioaccumulation and Toxicity of the Novel Chiral Antifungal Agrochemical Penthiopyrad in Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 228, 113010. [Google Scholar] [CrossRef] [PubMed]
- Borel, B. When the Pesticides Run Out. Nature 2017, 543, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The Global Status of Insect Resistance to Neonicotinoid Insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Parker, J.E.; Warrilow, A.; Kelly, D.E.; Kelly, S.L. Azole Fungicides—Understanding Resistance Mechanisms in Agricultural Fungal Pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Z.; Kah, M.; Lin, D.; Filser, J. Nanopesticides: A Comprehensive Assessment of Environmental Risk Is Needed before Widespread Agricultural Application. Environ. Sci. Technol. 2019, 53, 7923–7924. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Z.; Yang, T.; Pei, H.; Zhang, F. A Dual Pesticide–Fertilizer Silicon-Base Nanocomposite to Synergistically Control Fungal Disease and Provide Nutrition. Environ. Sci. Nano 2023, 10, 3462–3475. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When Is It Biological Control? A Framework of Definitions, Mechanisms, and Classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Veras, F.F.; Silveira, R.D.; Welke, J.E. Bacillus spp. As a Strategy to Control Fungi and Mycotoxins in Food. Curr. Opin. Food Sci. 2023, 52, 101068. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Long, T.; Ma, J.; Wu, N.; Mo, W.; Zhou, Z.; Jin, J.; Zhou, H.; Ding, H. Effects of Bacillus velezensis GUAL210 Control on Edible Rose Black Spot Disease and Soil Fungal Community Structure. Front. Microbiol. 2023, 14, 1199024. [Google Scholar] [CrossRef] [PubMed]
- Nuangmek, W.; Aiduang, W.; Kumla, J.; Lumyong, S.; Suwannarach, N. Evaluation of a Newly Identified Endophytic Fungus, Trichoderma phayaoense for Plant Growth Promotion and Biological Control of Gummy Stem Blight and Wilt of Muskmelon. Front. Microbiol. 2021, 12, 634772. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, P.; Suganya, K.; Parwin Banu, K.S.; Ramalakshmi, A.; Nalina, L. Effects of Root Exudates of Vetiver on Physicochemical Properties and Fractionation of Heavy Metals in Tannery Effluent Contaminated Soil. Ecol. Environ. Conserv. 2023, 29, 164–169. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Ryan, M.H.; Power, S.A. CO2 Concentration and Water Availability Alter the Organic Acid Composition of Root Exudates in Native Australian Species. Plant Soil 2023, 485, 507–524. [Google Scholar] [CrossRef]
- Wu, J.; Yu, S. Effect of Root Exudates of Eucalyptus urophylla and Acacia mearnsii on Soil Microbes under Simulated Warming Climate Conditions. BMC Microbiol. 2019, 19, 224. [Google Scholar] [CrossRef]
- Climate Warming Threatens Soil Microbial Diversity. Nat. Microbiol. 2022, 7, 935–936. [CrossRef] [PubMed]
- Li, X.; Dong, J.; Chu, W.; Chen, Y.; Duan, Z. The Relationship between Root Exudation Properties and Root Morphological Traits of Cucumber Grown under Different Nitrogen Supplies and Atmospheric CO2 Concentrations. Plant Soil 2018, 425, 415–432. [Google Scholar] [CrossRef]
- Vicente, E.J.; Dean, D.R. Keeping the Nitrogen-Fixation Dream Alive. Proc. Natl. Acad. Sci. USA 2017, 114, 3009–3011. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Nazari, M.; Antar, M.S.M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.A.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Zytynska, S.E.; Eicher, M.; Rothballer, M.; Weisser, W.W. Microbial-Mediated Plant Growth Promotion and Pest Suppression Varies Under Climate Change. Front. Plant Sci. 2020, 11, 573578. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen Fertilizer Rate Affects Root Exudation, the Rhizosphere Microbiome and Nitrogen-Use-Efficiency of Maize. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi u Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific Plant Interaction via Root Exudates Structures the Disease Suppressiveness of Rhizosphere Microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Li, S.; Yang, Q. The Combination of Biochar and Bacillus subtilis Biological Agent Reduced the Relative Abundance of Pathogenic Bacteria in the Rhizosphere Soil of Panax notoginseng. Microorganisms 2024, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Z.; Liu, C.; Zhang, Z.; Liu, X. Biochar Combined with Bacillus subtilis SL-44 as an Eco-Friendly Strategy to Improve Soil Fertility, Reduce Fusarium Wilt, and Promote Radish Growth. Ecotoxicol. Environ. Saf. 2023, 251, 114509. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Reynolds, M.; Xu, Y. Climate Change Challenges Plant Breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef] [PubMed]
- Samada, L.H.; Tambunan, U.S.F. Biopesticides as Promising Alternatives to Chemical Pesticides: A Review of Their Current and Future Status. J. Biol. Sci. 2020, 20, 66–76. [Google Scholar] [CrossRef]
- Chandrika, K.S.V.P.; Prasad, R.D.; Godbole, V. Development of Chitosan-PEG Blended Films using Trichoderma: Enhancement of Antimicrobial Activity and Seed Quality. Int. J. Biol. Macromol. 2019, 126, 282–290. [Google Scholar] [CrossRef] [PubMed]

| Type of Climate Change | Crop | Disease | Pathogenic Fungus | Hazard | Reference |
|---|---|---|---|---|---|
| Desertification | Faba bean | Root rot | Fusarium equiseti, Fusarium graminearum, Fusarium brachygibbosum | Growth rate of pathogenic fungi increased by 30–44% | [21] |
| Chickpea | Root rot | Macrophomina phaseolina | Chickpea production down 66.99% | [27] | |
| Rice | Rice blast | Magnaporthe oryzae | Plants under drought for longer have higher fungal abundance and poorer germination | [28] | |
| Flooding and high humidity | Cucumber | Damping-off disease | Pythium aphanidermatum | Significant increase in morbidity from 40% to 93% | [35] |
| Sugar cane | False floral smut | Epicoccum andropogonis, Claviceps purpurea | Heavy precipitation during the bloom period resulted in a higher incidence than in previous years | [40] | |
| Cashew | Leaf blight | Cryptosporiopsis spp. | 2% increase in incidence per mm increase in rainfall | [41] | |
| Quinoa | Downy mildew | Peronospora variabilis | Decreased chlorophyll in quinoa after disease onset | [43] | |
| High temperatures | Grapevine trunk | Grapevine trunk disease | Lasiodiplodia theobromae | Pathogenic fungi grow at 4 °C–40 °C and prefer higher temperatures | [46] |
| Celery | Root rot | Fusarium oxysporum | Fusarium acanthamoeba levels and disease severity increased with increasing temperature | [48] | |
| Soybean | Root rot | Fusarium solani | Longer root lesion lengths and higher disease incidence of Fusarium at 30 °C | [49] |
| Transmission Route | Crop | Pathogenic Fungus | Mechanisms of Influence | Reference |
|---|---|---|---|---|
| Soil-borne disease | Tomato | Fusarium solani, F. oxysporum or Ilyonectria destructans | Crops are more susceptible to extreme summer temperatures and drought | [54,57,58,59] |
| Maize | Fusarium graminearum | High temperatures trigger oxidative stress in grains that damages enzymes and tissues, making fungi more susceptible to infestation | [61] | |
| Air-borne disease | Cucurbit | Pseudoperonospora cubensis | Relative humidity > 90% and temperature 15 °C–20 °C are optimal conditions for cucurbit downy mildew development | [78,79] |
| Potato | Alternaria solani | Streptomyces levels exceed 50 spores/m3 at average temperature > 18 °C and leaf humidity > 80% | [84,85] | |
| Seed-borne disease | Wheat | Tilletia caries | Stored seeds are affected by temperature and humidity | [91] |
| Peanut | Aspergillus flavus | Increase in the proportion of Aspergillus flavus colonizing peanuts and aflatoxin concentration with increasing temperature | [92] | |
| Rice | Fusarium fujikuroi | At >25 °C, mycelium grows most vigorously | [93] |
| Method Category | Diagnostic Method | Principle | Advantages and Disadvantages | Reference | |
|---|---|---|---|---|---|
| Traditional detection methods | Visual inspection method | Isolation of pathogens and interpretation of visual symptoms of disease through microscopy | Highly subjective and error-prone | [96] | |
| Culture | Morphological characterization of pathogens by medium culture and microscopic observation | Cheap, simple, but accuracy and reliability depend on experience and skill, time-consuming | [97] | ||
| Modern testing methods | Direct detection methods | Immunological methods | Based on antigen–antibody binding | Short detection time, low sensitivity and accuracy | [98] |
| Polymerase chain reaction (PCR) | Detection of target nucleic acids specific to the target pathogen | Fast detection, lack of standardization, additional sample preparation, difficult on-site operation, high detection cost | [99,100] | ||
| Indirect detection methods | spectroscopy | Measurement of the intensity of reflected light wavelengths irradiated by a specific light source to assess the health of the crop | Inability to detect disease prior to onset, and inability to detect multiple diseases occurring at the same time | [101,102] | |
| Biosensor detection | Provides selective quantitative or semiquantitative analytical information using biometric elements | Quantitative information on crop diseases is available, but commercially available equipment is limited | [103,104] | ||
| GC–MS | Assessment of changes in volatiles due to fungal infection of crops | No crop damage, continuous monitoring over long periods of time, environmental factors interfere with sensor readings, pre-collection of samples required | [105,106,107] | ||
| Imaging technology | Indirect diagnosis of diseases by detecting changes in color, texture, or temperature of crop leaves | Efficient and inexpensive, suitable for climate change conditions | [108,109] | ||
| Preventive and Curative Measures | Principles of Prevention and Treatment | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Breeding for disease resistance | Targeted selection or alteration of certain genotypes to produce new varieties of crops that are resistant to disease | One of the most effective and economical measures | High-quality resistance results in the targeted selection of pathogens | [126,127,128] |
| Agronomic measures | Fertilization, irrigation, and so on to create environmental conditions suitable for the growth and development of vegetables and the survival and reproduction of beneficial organisms, but not conducive to the occurrence of pathogenic fungi | Improve the physical and chemical properties of the soil to provide a good growing environment for crops | High upfront costs, and labor-intensive and time-consuming process | [129,130,131,134,135,136] |
| Chemical control | Chemical pesticides kill pathogenic fungi | By far the most commonly used control method | Pathogen resistance, environmental risks | [137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152] |
| Biological control | Use of organisms such as crops, insects, and microorganisms to limit diseases caused by pathogens | A green, healthy, and promising approach | Colonization by free microorganisms is difficult, and the preventive effect is unstable in extreme climate conditions such as drought and high temperature | [153,154,155,156,157] |
| Ecological regulation | Provide a favorable environment for healthy crop growth and maintain crop–biology–soil dynamic balance | Maintaining a dynamic crop–biology–soil balance | the complexity of its application and long time to obtain results. | [158,159,160,161,162,163,164,165,166,167,168,169,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, X.; Qu, Z.; Zhang, C.; Wang, F.; Gao, T.; Yao, Y.; Liang, J. Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change. Agriculture 2024, 14, 1108. https://doi.org/10.3390/agriculture14071108
Zhou J, Zhang X, Qu Z, Zhang C, Wang F, Gao T, Yao Y, Liang J. Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change. Agriculture. 2024; 14(7):1108. https://doi.org/10.3390/agriculture14071108
Chicago/Turabian StyleZhou, Jien, Xueyan Zhang, Zheng Qu, Chenchen Zhang, Feng Wang, Tongguo Gao, Yanpo Yao, and Junfeng Liang. 2024. "Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change" Agriculture 14, no. 7: 1108. https://doi.org/10.3390/agriculture14071108
APA StyleZhou, J., Zhang, X., Qu, Z., Zhang, C., Wang, F., Gao, T., Yao, Y., & Liang, J. (2024). Progress in Research on Prevention and Control of Crop Fungal Diseases in the Context of Climate Change. Agriculture, 14(7), 1108. https://doi.org/10.3390/agriculture14071108






