Intercropping Systems: An Opportunity for Environment Conservation within Nut Production
Abstract
1. Introduction
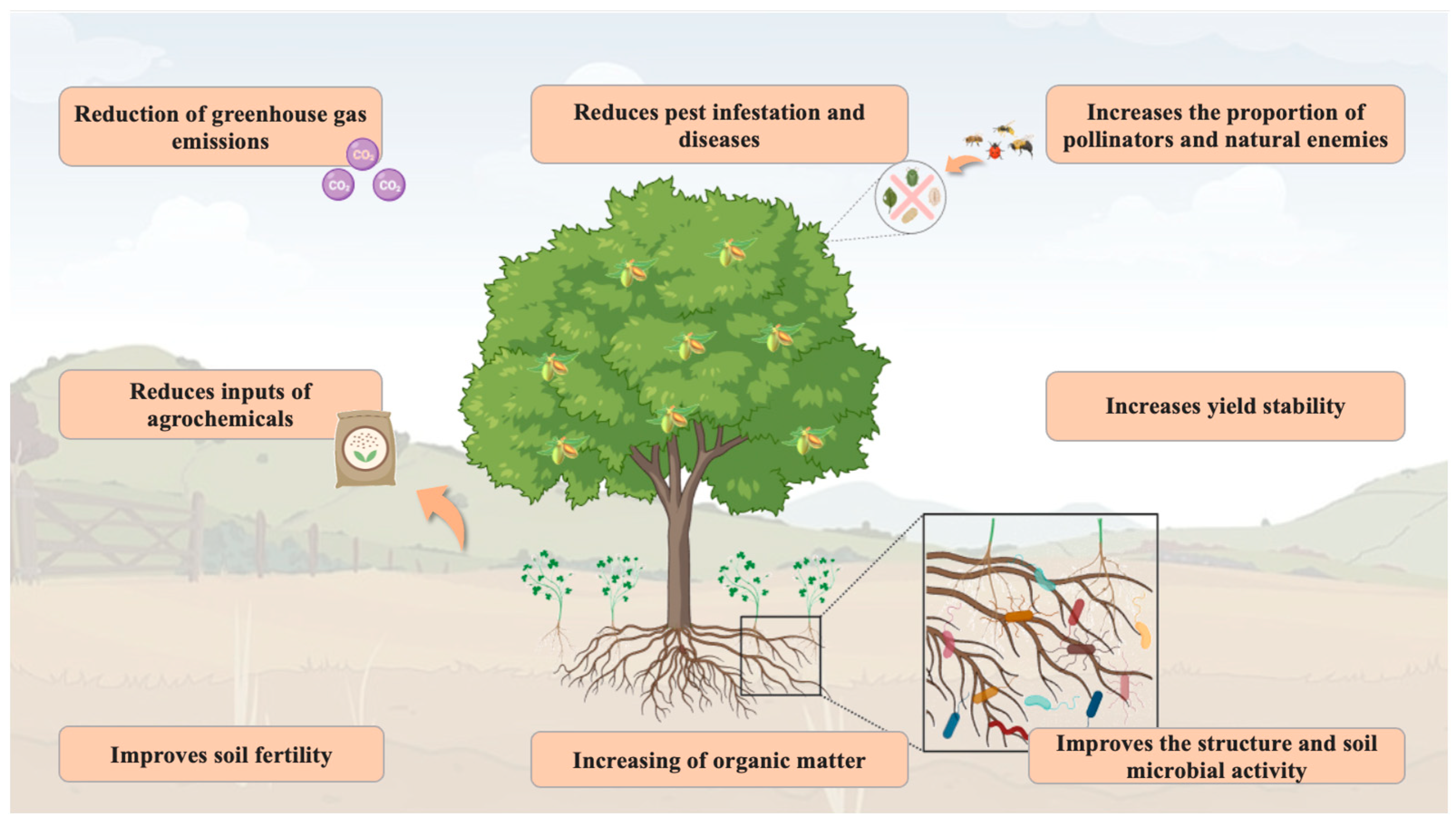
2. Features and Advantageous of Intercropping System
2.1. Improvement in the Soil Quality
2.2. Biodiversity Conservation
2.3. Yield Stability
2.4. Valorization of Bioactive Compounds
3. Types of Intercropping Systems
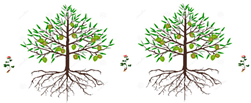
3.1. Mixed Intercropping
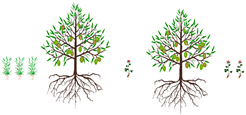
3.2. Row Intercropping
3.3. Relay Intercropping
3.4. Strip Intercropping
4. Intercropped Species
4.1. Legumes
4.2. Oilseeds
4.3. Aromatic Plants
4.4. Vegetables
5. Successful Intercropping Systems
| Nuts | Intercropped Crops | Positive Effects | Refs. |
|---|---|---|---|
| Almond (Prunus dulcis Mill.) | Legume cover (Vicia faba L., Vicia sativa L. and Vicia ervilia L.) |
| [26] |
| Almond (Prunus amygdalus B.) | Snap bean (Phaseolus vulgaris L.) |
| [115] |
| Almond (Prunus dulcis Mill.) | Caper (Capparis spinosa L.) and thyme (Thymus hyemalis L.) |
| [99] |
| Walnut (Juglans spp.) | Tea (Camellia sinensis L.) |
| [116] |
| Areca nut (Areca catechu L.) | Pandan (Pandanus amaryllifolius Roxb.) |
| [117] |
| Peanut (Arachis hypogaea L.) | Millet (Setaria itálica L.) |
| [107,118] |
| Macadamia (Macadamia integriolia Maiden & Betche) | Coffee (Coffee arabica L.) |
| [70] |
| Cashew (Anacardium occidentale L.) | Mango ginger (Curcuma amada Roxb), elephant foot yam (Amorphophallus paeoniifolius (Dennst.)), turmeric (Curcuma longa L.), east Indian arrowroot (Curcuma angustifólia Roxb), taro (Colocasia esculenta (L.)) |
| [119] |
| Peanut (Arachis hypogaea L.) | Sugarcane (Saccharum officinarum L.) |
| [120] |
| Peanut (Arachis hypogaea L.) | Maize (Zea mays L.) |
| [71] |
| Peanut (Arachis hypogaea Linn.) | Maize (Zea mays L.) |
| [77] |
6. Challenges and Limitations for the Establishment of Service Crops
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public. Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- GlobeNewswire. Global Edible Nuts Market to Reach $459.1 Billion by 2030 Report. 2023. Available online: https://www.globenewswire.com/news-release/2023/03/15/2628035/0/en/Global-Edible-Nuts-Market-to-Reach-459-1-Billion-by-2030.html (accessed on 1 May 2024).
- Wojdyło, A.; Turkiewicz, I.P.; Tkacz, K.; Nowicka, P.; Bobak, Ł. Nuts as Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their in Vitro Bioactive Properties. Food Chem. X 2022, 15, 100418. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.; Schincaglia, R.; Pimentel, G.; Mota, J. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive Procya-nidins from Dietary Sources: The Relationship between Bioactivity and Polymerization Degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Balakrishna, R.; Bjørnerud, T.; Bemanian, M.; Aune, D.; Fadnes, L.T. Consumption of Nuts and Seeds and Health Outcomes Including Cardiovascular Disease, Diabetes and Metabolic Disease, Cancer, and Mortality: An Umbrella Review. Adv. Nutr. 2022, 13, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, J.; Kennedy, G.; Attwood, S. Managing Crop Tradeoffs: A Methodology for Comparing the Water Footprint and Nutrient Density of Crops for Food System Sustainability. J. Clean. Prod. 2019, 225, 913–927. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger, J.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-Based Intercropping Systems Promote Beneficial Rhizobacterial Community and Crop Yield under Stressing Conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Dai, J.; Qiu, W.; Wang, N.; Wang, T.; Nakanishi, H.; Zuo, Y. From Leguminosae/Gramineae Intercropping Systems to See Benefits of Intercropping on Iron Nutrition. Front. Plant Sci. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.; Hossain, A.; Brestic, M.; Skalicky, M.; Ondrisik, P.; Gitari, H.; Brahmachari, K.; Shankar, T.; Bhadra, P.; Palai, J.B.; et al. Intercropping—A Low Input Agricultural Strategy for Food and Environmental Security. Agronomy 2021, 11, 343. [Google Scholar] [CrossRef]
- Kingwell-Banham, E.; Petrie, C.A.; Fuller, D.Q. Early Agriculture in South Asia. In The Cambridge World History; Cambridge University Press: Cambridge, UK, 2015; pp. 261–288. [Google Scholar]
- Carolina Lizana, X.; Sandaña, P.; Behn, A.; Ávila-Valdés, A.; Ramírez, D.A.; Soratto, R.P.; Campos, H. Potato. In Crop Physiology Case Histories for Major Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 550–587. [Google Scholar]
- Food Security & Livelihoods Cluster. Guidance on Intercropping Agriculture System, Gaziantep, Turkey. 2021, pp. 1–6. Available online: https://fscluster.org/gaziantep/document/intercropping-agriculture-system (accessed on 14 May 2024).
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and Risks of Intercropping for Crop Resilience and Pest Management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Munir, S.; Bashir, N.H.; Wang, Y.; Yang, J.; Li, C. Crop Diversity and Pest Management in Sustainable Ag-riculture. J. Integr. Agric. 2019, 18, 1945–1952. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J.; et al. Producing More Grain with Lower Environmental Costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Bardhan, S. Agroforestry for Biomass Production and Carbon Sequestration: An Overview. Agrofor. Syst. 2012, 86, 105–111. [Google Scholar] [CrossRef]
- Tully, K.; Ryals, R. Nutrient Cycling in Agroecosystems: Balancing Food and Environmental Objectives. Agroecol. Sustain. Food Syst. 2017, 41, 761–798. [Google Scholar] [CrossRef]
- Žalac, H.; Herman, G.; Ergović, L.; Jović, J.; Zebec, V.; Bubalo, A.; Ivezić, V. Ecological and Agronomic Benefits of Inter-cropping Maize in a Walnut Orchard—A Case Study. Agronomy 2022, 13, 77. [Google Scholar] [CrossRef]
- Hemel, S.A.K.; Hasan, M.K.; Wadud, M.A.; Akter, R.; Roshni, N.A.; Islam, M.T.; Yasmin, A.; Akter, K. Improvement of Farmers’ Livelihood through Choi Jhal (Piper Chaba)-Based Agroforestry System: Instance from the Northern Region of Bangladesh. Sustainability 2022, 14, 16078. [Google Scholar] [CrossRef]
- Burgess, P.J.; Rosati, A. Advances in European Agroforestry: Results from the AGFORWARD Project. Agrofor. Syst. 2018, 92, 801–810. [Google Scholar] [CrossRef]
- den Herder, M.; Moreno, G.; Mosquera-Losada, M.R.; Palma, J.H.N.; Sidiropoulou, A.; Santiago-Freijanes, J.; Crous-Duran, J.; Paulo, J.; Tomé, M.; Papanastasis, A.P.V.; et al. Current Extent and Trends of Agroforestry in the EU27. Deliv. Rep. 2016, 1, 76. [Google Scholar]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Gao, L.; Xu, H.; Bi, H.; Xi, W.; Bao, B.; Wang, X.; Bi, C.; Chang, Y. Intercropping Competition between Apple Trees and Crops in Agroforestry Systems on the Loess Plateau of China. PLoS ONE 2013, 8, e70739. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, B.C.; Zuazo, V.H.D.; Galán, J.F.H.; Lipan, L.; Soriano, M.; Hernández, F.; Sendra, E.; Carbonell-Barrachina, Á.A.; Ruiz, B.G.; García-Tejero, I.F. Soil Management Strategies in Organic Almond Orchards: Implications for Soil Rehabilitation and Nut Quality. Agronomy 2023, 13, 749. [Google Scholar] [CrossRef]
- Maitra, S.; Palai, J.B.; Manasa, P.; Kumar, D.P. Potential of Intercropping System in Sustaining Crop Productivity. Int. J. Agric. Environ. Biotechnol. 2019, 12, 39–45. [Google Scholar] [CrossRef]
- Glaze-Corcoran, S.; Hashemi, M.; Sadeghpour, A.; Jahanzad, E.; Keshavarz Afshar, R.; Liu, X.; Herbert, S.J. Understanding Intercropping to Improve Agricultural Resiliency and Environmental Sustainability. Adv. Agron. 2020, 162, 199–256. [Google Scholar]
- Khanal, U.; Stott, K.J.; Armstrong, R.; Nuttall, J.G.; Henry, F.; Christy, B.P.; Mitchell, M.; Riffkin, P.A.; Wallace, A.J.; McCaskill, M.; et al. Intercropping—Evaluating the Advantages to Broadacre Systems. Agriculture 2021, 11, 453. [Google Scholar] [CrossRef]
- Renwick, L.L.R.; Kimaro, A.A.; Hafner, J.M.; Rosenstock, T.S.; Gaudin, A.C.M. Maize-Pigeonpea Intercropping Outperforms Monocultures under Drought. Front. Sustain. Food Syst. 2020, 4, 562663. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, M.; Song, M.; Tian, J.; Song, B.; Hu, Y.; Zhang, J.; Yao, Y. Intercropping with Aromatic Plants Increased the Soil Organic Matter Content and Changed the Microbial Community in a Pear Orchard. Front. Microbiol. 2021, 12, 616932. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Zhang, X.; Zhao, K.; Chen, H.Y.H. Intercropping Improves Soil Nutrient Availability, Soil Enzyme Activity and Tea Quantity and Quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Roohi, M.; Saleem Arif, M.; Guillaume, T.; Yasmeen, T.; Riaz, M.; Shakoor, A.; Hassan Farooq, T.; Muhammad Shahzad, S.; Bragazza, L. Role of Fertilization Regime on Soil Carbon Sequestration and Crop Yield in a Maize-Cowpea Intercropping System on Low Fertility Soils. Geoderma 2022, 428, 116152. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Short-Term Dynamics of Soil Organic Matter Fractions and Microbial Activity in Smallholder Potato-Legume Intercropping Systems. Appl. Soil Ecol. 2019, 142, 123–135. [Google Scholar] [CrossRef]
- Sharma, R.C.; Banik, P. Baby Corn-Legumes Intercropping Systems: I. Yields, Resource Utilization Efficiency, and Soil Health. Agroecol. Sustain. Food Syst. 2015, 39, 41–61. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual Intercrops: An Alternative Pathway for Sus-tainable Agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Ehrmann, J.; Ritz, K. Plant: Soil Interactions in Temperate Multi-Cropping Production Systems. Plant Soil 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Sönmez, O.; Turan, V.; Kaya, C. The Effects of Sulfur, Cattle, and Poultry Manure Addition on Soil Phosphorus. Turk. J. Agric. For. 2016, 40, 536–541. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M. Optimizing Soil Nitrogen Balance in a Potato Cropping System through Legume Intercropping. Nutr. Cycl. Agroecosyst 2020, 117, 43–59. [Google Scholar] [CrossRef]
- Clermont-Dauphin, C.; Dissataporn, C.; Suvannang, N.; Pongwichian, P.; Maeght, J.; Hammecker, C.; Jourdan, C. Intercrops Improve the Drought Resistance of Young Rubber Trees. Agron. Sustain. Dev. 2018, 38, 56. [Google Scholar] [CrossRef]
- Schmutz, A.; Schöb, C. Crops Grown in Mixtures Show Niche Partitioning in Spatial Water Uptake. J. Ecol. 2023, 111, 1151–1165. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil Organic Carbon Sequestration in Agroforestry Systems: A Review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Nair, P.K.R. Carbon Sequestration Studies in Agroforestry Systems: A Reality-Check. Agrofor. Syst. 2012, 86, 243–253. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Cai, H.; You, M.; Lin, C. Effects of Intercropping Systems on Community Composition and Diversity of Predatory Arthropods in Vegetable Fields. Acta Ecol. Sin. 2010, 30, 190–195. [Google Scholar] [CrossRef]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Beneficial Effect of the Rhizosphere Microbial Community for Plant Growth and Health. Biotechnol. Agron. Soc. Environ. 2011, 15, 327–337. [Google Scholar]
- Fan, L.; Tarin, M.W.K.; Zhang, Y.; Han, Y.; Rong, J.; Cai, X.; Chen, L.; Shi, C.; Zheng, Y. Patterns of Soil Microorganisms and Enzymatic Activities of Various Forest Types in Coastal Sandy Land. Glob. Ecol. Conserv. 2021, 28, e01625. [Google Scholar] [CrossRef]
- Veres, Z.; Kotroczó, Z.; Fekete, I.; Tóth, J.A.; Lajtha, K.; Townsend, K.; Tóthmérész, B. Soil Extracellular Enzyme Activities Are Sensitive Indicators of Detrital Inputs and Carbon Availability. Appl. Soil. Ecol. 2015, 92, 18–23. [Google Scholar] [CrossRef]
- Vera-Reyes, I.; Vázquez-Núñez, E.; Castellano, L.E.; Bautista, D.I.A.; Valenzuela Soto, J.H.; Valle-García, J.D. Biointeractions of Plants–Microbes–Engineered Nanomaterials. In Physicochemical Interactions of Engineered Nanoparticles and Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–231. [Google Scholar]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S.; et al. Designing Intercrops for High Yield, Yield Stability and Efficient Use of Resources: Are There Principles? Adv. Agron. 2020, 160, 1–50. [Google Scholar]
- Pergner, I.; Lippert, C. On the Effects That Motivate Pesticide Use in Perspective of Designing a Cropping System without Pesticides but with Mineral Fertilizer—A Review. Agron. Sustain. Dev. 2023, 43, 24. [Google Scholar] [CrossRef]
- Madembo, C.; Mhlanga, B.; Thierfelder, C. Productivity or Stability? Exploring Maize-Legume Intercropping Strategies for Smallholder Conservation Agriculture Farmers in Zimbabwe. Agric. Syst. 2020, 185, 102921. [Google Scholar] [CrossRef]
- Chimonyo, V.G.P.; Snapp, S.S.; Chikowo, R. Grain Legumes Increase Yield Stability in Maize Based Cropping Systems. Crop Sci. 2019, 59, 1222–1235. [Google Scholar] [CrossRef]
- Luo, S.; Yu, L.; Liu, Y.; Zhang, Y.; Yang, W.; Li, Z.; Wang, J. Effects of Reduced Nitrogen Input on Productivity and N2O Emissions in a Sugarcane/Soybean Intercropping System. Eur. J. Agron. 2016, 81, 78–85. [Google Scholar] [CrossRef]
- Raseduzzaman, M.; Jensen, E.S. Does Intercropping Enhance Yield Stability in Arable Crop Production? A Meta-Analysis. Eur. J. Agron. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen Fertilization Decreases the Yield of Bioactive Compounds in Carlina acaulis L. grown in the field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Rezaei-Chiyaneh, E.; Mahdavikia, H.; Rahimi, A.; Gheshlaghi, M.; Battaglia, M.L.; Harrison, M.T. Effect of Intercropping and Bio-Fertilizer Application on the Nutrient Uptake and Productivity of Mung Bean and Marjoram. Land 2022, 11, 1825. [Google Scholar] [CrossRef]
- Seeno, E.; Naumann, H.; Ates, S.S. Production and Chemical Composition of Pasture Forbs with High Bioactive Compounds in a Low Input Production System in the Pacific Northwest. Anim. Feed Sci. Technol. 2022, 289, 115324. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Con-centration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Wu, T.; Zou, R.; Pu, D.; Lan, Z.; Zhao, B. Non-Targeted and Targeted Metabolomics Profiling of Tea Plants (Camellia Sinensis) in Response to Its Intercropping with Chinese Chestnut. BMC Plant Biol. 2021, 21, 55. [Google Scholar] [CrossRef]
- Sergieieva, K. Consorciação de Culturas (Interplantação): O Que é? Available online: https://eos.com/pt/blog/consorciacao-de-culturas/ (accessed on 28 May 2024).
- Ali, S.; Baloch, A.M. Overview of Sustainable Plant Growth and Differentiation and the Role of Hormones in Controlling Growth and Development of Plants under Various Stresses. Recent. Pat. Food Nutr. Agric. 2020, 11, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, C.I.; Tuulos, A.; Jokela, V.; Mäkelä, P.S.A. Sustainable Mixed Cropping Systems for the Boreal-Nemoral Region. Front. Sustain. Food Syst. 2020, 4, 103. [Google Scholar] [CrossRef]
- Pan, P.; Qin, Y. Genotypic Diversity of Soybean in Mixed Cropping Can Affect the Populations of Insect Pests and Their Natural Enemies. Int. J. Pest. Manag. 2014, 60, 287–292. [Google Scholar] [CrossRef]
- Gałęzewski, L.; Jaskulska, I.; Jaskulski, D.; Wilczewski, E.; Kościński, M. Strip Intercrop of Barley, Wheat, Triticale, Oat, Pea and Yellow Lupine—A Meta-Analysis. Sustainability 2022, 14, 15651. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Gałęzewski, L. Peas and Barley Grown in the Strip-Till One Pass Technology as Row Intercropping Components in Sustainable Crop Production. Agriculture 2022, 12, 229. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Tang, W.; Huang, Y.; Coulter, J.A.; Nan, Z. Common Vetch Cultivars Improve Yield of Oat Row Inter-cropping on the Qinghai-Tibetan Plateau by Optimizing Photosynthetic Performance. Eur. J. Agron. 2020, 117, 126088. [Google Scholar] [CrossRef]
- Jakhar, P.; Dass, A.; Sudhishri, S.; Naik, B.S.; Panda, R.K. Multitier Cropping Systems for Resource Conservation and Higher Productivity in Eastern Ghat Highland Zone of Odisha; Indian Council of Agricultural Research: New Delhi, India, 2012; Volume 33, pp. 240–243. [Google Scholar]
- Wang, T.; Zhu, B.; Xia, L. Effects of Contour Hedgerow Intercropping on Nutrient Losses from the Sloping Farmland in the Three Gorges Area, China. J. Mt. Sci. 2012, 9, 105–114. [Google Scholar] [CrossRef]
- Perdoná, M.J.; Soratto, R.P. Higher Yield and Economic Benefits Are Achieved in the Macadamia Crop by Irrigation and Intercropping with Coffee. Sci. Hortic. 2015, 185, 59–67. [Google Scholar] [CrossRef]
- Lu, J.; Dong, Q.; Lan, G.; He, Z.; Zhou, D.; Zhang, H.; Wang, X.; Liu, X.; Jiang, C.; Zhang, Z.; et al. Row Ratio Increasing Improved Light Distribution, Photosynthetic Characteristics, and Yield of Peanut in the Maize and Peanut Strip Intercropping System. Front. Plant Sci. 2023, 14, 1135580. [Google Scholar] [CrossRef] [PubMed]
- Amossé, C.; Jeuffroy, M.-H.; David, C. Relay Intercropping of Legume Cover Crops in Organic Winter Wheat: Effects on Performance and Resource Availability. Field Crops Res. 2013, 145, 78–87. [Google Scholar] [CrossRef]
- Raza, M.A.; Feng, L.Y.; van der Werf, W.; Iqbal, N.; Khan, I.; Khan, A.; Din, A.M.U.; Naeem, M.; Meraj, T.A.; Hassan, M.J.; et al. Optimum Strip Width Increases Dry Matter, Nutrient Accumulation, and Seed Yield of Intercrops under the Relay Inter-cropping System. Food Energy Secur. 2020, 9, e199. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Liao, D.; Raza, M.A.; Wang, B.; Zhang, J.; Chen, J.; Feng, L.; Wu, X.; Liu, C.; et al. Uptake and Utilization of Nitrogen, Phosphorus and Potassium as Related to Yield Advantage in Maize-Soybean Intercropping under Different Row Configurations. Sci. Rep. 2020, 10, 9504. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Du, Q.; Liu, X.; Zhou, L.; Hussain, S.; Lei, L.; Song, C.; Wang, X.; Liu, W.; Yang, F.; et al. Effects of Reduced Nitrogen Inputs on Crop Yield and Nitrogen Use Efficiency in a Long-Term Maize-Soybean Relay Strip Intercropping System. PLoS ONE 2017, 12, e0184503. [Google Scholar] [CrossRef]
- van Oort, P.A.J.; Gou, F.; Stomph, T.J.; van der Werf, W. Effects of Strip Width on Yields in Relay-Strip Intercropping: A Simulation Study. Eur. J. Agron. 2020, 112, 125936. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Z.; Zhang, L.; Yang, N.; Feng, L.; Bai, W.; Zhang, D.; Wang, Q.; Evers, J.B.; Liu, Y.; et al. Border-Row Proportion Determines Strength of Interspecific Interactions and Crop Yields in Maize/Peanut Strip Intercropping. Field Crops Res. 2020, 253, 107819. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving Intercropping: A Synthesis of Research in Agronomy, Plant Physiology and Ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Nord, A.; Bekunda, M.; McCormack, C.; Snapp, S. Barriers to Sustainable Intensification: Overlooked Disconnects between Agricultural Extension and Farmer Practice in Maize-Legume Cropping Systems in Tanzania. Int. J. Agric. Sustain. 2022, 20, 576–594. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.-F.; Celette, F. Intercropping with Legume for Agroecological Cropping Systems: Complementarity and Facilitation Processes and the Importance of Soil Microorganisms. A Review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A. Leguminous Crops. In Food Crop Production by Smallholder Farmers in Southern Africa Challenges and Opportunities for Improvement; Academic Press: Cambridge, MA, USA, 2018; pp. 173–203. [Google Scholar]
- Chamkhi, I.; El Omari, N.; Balahbib, A.; El Menyiy, N.; Benali, T.; Ghoulam, C. Is the Rhizosphere a Source of Applicable Multi-Beneficial Microorganisms for Plant Enhancement? Saudi J. Biol. Sci. 2022, 29, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Layek, J.; Das, A.; Mitran, T.; Nath, C.; Meena, R.S.; Yadav, G.S.; Shivakumar, B.G.; Kumar, S.; Lal, R. Cereal+Legume In-tercropping: An Option for Improving Productivity and Sustaining Soil Health. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 347–386. [Google Scholar]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; Zhang, L.; van der Werf, W. Meta-Analysis of Relative Crop Yields in Cereal/Legume Mixtures Suggests Options for Management. Field Crops Res. 2016, 198, 269–279. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Sys-tems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Shah, A.N.; Iqbal, J.; Ullah, A.; Yang, G.; Yousaf, M.; Fahad, S.; Tanveer, M.; Hassan, W.; Tung, S.A.; Wang, L.; et al. Alle-lopathic Potential of Oil Seed Crops in Production of Crops: A Review. Environ. Sci. Pollut. Res. 2016, 23, 14854–14867. [Google Scholar] [CrossRef]
- Dowling, A.; Roberts, P.; Doolette, A.; Zhou, Y.; Denton, M.D. Oilseed-Legume Intercropping Is Productive and Profitable in Low Input Scenarios. Agric. Syst. 2023, 204, 103551. [Google Scholar] [CrossRef]
- Xia, H.; Wang, L.; Jiao, N.; Mei, P.; Wang, Z.; Lan, Y.; Chen, L.; Ding, H.; Yin, Y.; Kong, W.; et al. Luxury Absorption of Phosphorus Exists in Maize When Intercropping with Legumes or Oilseed Rape—Covering Different Locations and Years. Agronomy 2019, 9, 314. [Google Scholar] [CrossRef]
- Dayoub, E.; Piva, G.; Shirtliffe, S.J.; Fustec, J.; Corre-Hellou, G.; Naudin, C. Species Choice Influences Weed Suppression, N Sharing and Crop Productivity in Oilseed Rape–Legume Intercrops. Agronomy 2022, 12, 2187. [Google Scholar] [CrossRef]
- Chen, S.; Yang, D.; Wei, Y.; He, L.; Li, Z.; Yang, S. Changes in Soil Phosphorus Availability and Microbial Community Structures in Rhizospheres of Oilseed Rapes Induced by Intercropping with White Lupins. Microorganisms 2023, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Segura, V.; Grass, I.; Breustedt, G.; Rohlfs, M.; Tscharntke, T. Strip Intercropping of Wheat and Oilseed Rape En-hances Biodiversity and Biological Pest Control in a Conventionally Managed Farm Scenario. J. Appl. Ecol. 2022, 59, 1513–1523. [Google Scholar] [CrossRef]
- Marc (Vlaic), R.A.; Mureșan, V.; Mureșan, A.E.; Mureșan, C.C.; Tanislav, A.E.; Pușcaș, A.; Marţiș (Petruţ), G.S.; Ungur, R.A. Spicy and Aromatic Plants for Meat and Meat Analogues Applications. Plants 2022, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Christaki, E.; Giannenas, I.; Bonos, E.; Florou-Paneri, P. Innovative Uses of Aromatic Plants as Natural Supplements in Nu-trition. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–34. [Google Scholar]
- EIP-AGRI Focus Group. Plant-Based Medicinal and Cosmetic Product; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Rydlová, J.; Jelínková, M.; Dušek, K.; Dušková, E.; Vosátka, M.; Püschel, D. Arbuscular Mycorrhiza Differentially Affects Synthesis of Essential Oils in Coriander and Dill. Mycorrhiza 2016, 26, 123–131. [Google Scholar] [CrossRef]
- Rao, E.V.S.P. Economic and Ecological Aspects of Aromatic-Plant-Based Cropping Systems. CABI Rev. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Marotti, I.; Whittaker, A.; Bağdat, R.B.; Akin, P.A.; Ergün, N.; Dinelli, G. Intercropping Perennial Fruit Trees and Annual Field Crops with Aromatic and Medicinal Plants (MAPs) in the Mediterranean Basin. Sustainability 2023, 15, 12054. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Shahrokh, V.; Martínez-Martínez, S.; Acosta, J.A.; Almagro, M.; Martínez-Mena, M.; Boix-Fayos, C.; Díaz-Pereira, E.; Zornoza, R. Perennial Alley Cropping Contributes to Decrease Soil CO2 and N2O Emissions and Increase Soil Carbon Sequestration in a Mediterranean Almond Orchard. Sci. Total Environ. 2022, 845, 157225. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mena, M.; Boix-Fayos, C.; Carrillo-López, E.; Díaz-Pereira, E.; Zornoza, R.; Sánchez-Navarro, V.; Acosta, J.A.; Martínez-Martínez, S.; Almagro, M. Short-Term Impact of Crop Diversification on Soil Carbon Fluxes and Balance in Rainfed and Irrigated Woody Cropping Systems under Semiarid Mediterranean Conditions. Plant Soil 2021, 467, 499–514. [Google Scholar] [CrossRef]
- Almagro, M.; Díaz-Pereira, E.; Boix-Fayos, C.; Zornoza, R.; Sánchez-Navarro, V.; Re, P.; Fernández, C.; Martínez-Mena, M. The Combination of Crop Diversification and No Tillage Enhances Key Soil Quality Parameters Related to Soil Functioning without Compromising Crop Yields in a Low-Input Rainfed Almond Orchard under Semiarid Mediterranean Conditions. Agric. Ecosyst. Env. 2023, 345, 108320. [Google Scholar] [CrossRef]
- Shanmugam, S.; Hefner, M.; Pelck, J.S.; Labouriau, R.; Kristensen, H.L. Complementary Resource Use in Intercropped Faba Bean and Cabbage by Increased Root Growth and Nitrogen Use in Organic Production. Soil Use Manag. 2022, 38, 729–740. [Google Scholar] [CrossRef]
- Gitari, H.I.; Nyawade, S.O.; Kamau, S.; Karanja, N.N.; Gachene, C.K.K.; Raza, M.A.; Maitra, S.; Schulte-Geldermann, E. Revisiting Intercropping Indices with Respect to Potato-Legume Intercropping Systems. Field Crops Res. 2020, 258, 107957. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Zuo, S.; Ali, M.; Wang, Z. Soil Salinity Control and Cauliflower Quality Promotion by Intercropping with Five Turfgrass Species. J. Clean. Prod. 2020, 266, 121991. [Google Scholar] [CrossRef]
- Santos, R.H.S.; Gliessman, S.R.; Cecon, P.R. Crop Interactions in Broccoli Intercropping. Biol. Agric. Hortic. 2002, 20, 51–75. [Google Scholar] [CrossRef]
- Unlu, H.; Süleyman, T.C.; Üniversitesi, D.; Dasgan, H.Y.; Solmaz, I.; Sari, N. Effects of Intercropping on Plant Nutrient Uptake in Various Vegetables Species. Asian J. Chem. 2008, 20, 4781. [Google Scholar]
- Zhu, L.; He, J.; Tian, Y.; Li, X.; Li, Y.; Wang, F.; Qin, K.; Wang, J. Intercropping Wolfberry with Gramineae Plants Improves Productivity and Soil Quality. Sci. Hortic. 2022, 292, 110632. [Google Scholar] [CrossRef]
- Zou, X.-X.; Shi, P.-X.; Zhang, C.-J.; Si, T.; Wang, Y.-F.; Zhang, X.-J.; Yu, X.-N.; Wang, H.-X.; Wang, M.-L. Rotational Strip Intercropping of Maize and Peanuts Has Multiple Benefits for Agricultural Production in the Northern Agropastoral Ecotone Region of China. Eur. J. Agron. 2021, 129, 126304. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, J.; Liu, Y.; You, Z.; Zhang, J.; Guo, F.; Gao, H.; Li, L.; Wan, S. Maize/Peanut Intercropping Reduces Carbon Footprint Size and Improves Net Ecosystem Economic Benefits in the Huang-Huai-Hai Region: A Four-Year Study. Agronomy 2023, 13, 1343. [Google Scholar] [CrossRef]
- Chi, B.; Zhang, Y.; Zhang, D.; Zhang, X.; Dai, J.; Dong, H. Wide-Strip Intercropping of Cotton and Peanut Combined with Strip Rotation Increases Crop Productivity and Economic Returns. Field Crops Res. 2019, 243, 107617. [Google Scholar] [CrossRef]
- Biswas, S.; Chandra, B.; Viswavidyalaya, K.; Khan, O.; Bose, S. The Role of Agroforestry-Based Farming in Sustainable Agriculture; AkiNik Publications: New Delhi, India, 2023; pp. 99–115. [Google Scholar]
- Waldén, P.; Eronen, M.; Kaseva, J.; Negash, M.; Kahiluoto, H. Determinants of the Economy in Multistrata Agroforestry in Ethiopia. Land. Use Policy 2024, 141, 107162. [Google Scholar] [CrossRef]
- Abbasi Surki, A.; Nazari, M.; Fallah, S.; Iranipour, R.; Mousavi, A. The Competitive Effect of Almond Trees on Light and Nutrients Absorption, Crop Growth Rate, and the Yield in Almond–Cereal Agroforestry Systems in Semi-Arid Regions. Agrofor. Syst. 2020, 94, 1111–1122. [Google Scholar] [CrossRef]
- Abbasi Surki, A.; Nazari, M.; Fallah, S.; Iranipour, R. Improvement of the Soil Properties, Nutrients, and Carbon Stocks in Different Cereal–Legume Agroforestry Systems. Int. J. Environ. Sci. Technol. 2021, 18, 123–130. [Google Scholar] [CrossRef]
- Abourayya, M.S.; Kaseem, N.E.; Mahmoud, T.S.M.; Marzouk, N.M.; Rakha, A.M. Intercropping Young Almond Trees with Snap Bean under Nubaria Region Conditions. Bull. Natl. Res. Cent. 2022, 46, 77. [Google Scholar] [CrossRef]
- Bai, Y.-C.; Li, B.-X.; Xu, C.-Y.; Raza, M.; Wang, Q.; Wang, Q.-Z.; Fu, Y.-N.; Hu, J.-Y.; Imoulan, A.; Hussain, M.; et al. Inter-cropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, A.; Qin, X.; Yu, H.; Ji, X.; He, S.; Zong, Y.; Wang, J.; Tang, J. Effects of Intercropping Pandanus Amaryl-lifolius on Soil Properties and Microbial Community Composition in Areca Catechu Plantations. Forests 2022, 13, 1814. [Google Scholar] [CrossRef]
- Liu, Z.; Nan, Z.; Lin, S.; Yu, H.; Xie, L.; Meng, W.; Zhang, Z.; Wan, S. Millet/Peanut Intercropping at a Moderate N Rate Increases Crop Productivity and N Use Efficiency, as Well as Economic Benefits, under Rain-Fed Conditions. J. Integr. Agric. 2023, 22, 738–751. [Google Scholar] [CrossRef]
- Ramteke, V.; Thakur, P.; Kerketta, A.; Paramesh, V.; Nirala, Y.S.; Netam, R.S.; Adiga, J.D. Assessment of Cashew-Based Intercropping System in Chhattisgarh, India. Natl. Acad. Sci. Lett. 2022, 45, 485–489. [Google Scholar] [CrossRef]
- Tang, X.; He, Y.; Zhang, Z.; Wu, H.; He, L.; Jiang, J.; Meng, W.; Huang, Z.; Xiong, F.; Liu, J.; et al. Beneficial Shift of Rhizo-sphere Soil Nutrients and Metabolites under a Sugarcane/Peanut Intercropping System. Front. Plant Sci. 2022, 13, 1018727. [Google Scholar] [CrossRef]
- Perdoná, M.J.; Soratto, R.P. Irrigation and Intercropping with Macadamia Increase Initial Arabica Coffee Yield and Profita-bility. Agron. J. 2015, 107, 615–626. [Google Scholar] [CrossRef]
- Ferreira, F.; Mesquita, A.; Mota, M.d.S.; Fuck Júnior, S.; Miranda, F. Grau de Infestação da Traça-da-Castanha em Clones de Cajueiro-anão Consorciados com Fruteiras. Educação Ambiental—uso, Manejo e Gestão dos Recursos Naturais; Embrapa: Ituiutaba, Brazil, 2022; pp. 143–149.
- Wang, C.; Liang, Q.; Liu, J.; Zhou, R.; Lang, X.; Xu, S.; Li, X.; Gong, A.; Mu, Y.; Fang, H.; et al. Impact of Intercropping Grass on the Soil Rhizosphere Microbial Community and Soil Ecosystem Function in a Walnut Orchard. Front. Microbiol. 2023, 14, 1137590. [Google Scholar] [CrossRef]
- Gao, P.; Zheng, X.; Wang, L.; Liu, B.; Zhang, S. Changes in the Soil Bacterial Community in a Chronosequence of Temperate Walnut-Based Intercropping Systems. Forests 2019, 10, 299. [Google Scholar] [CrossRef]
- Boukid, F. Peanut Protein—An Underutilised By-product with Great Potential: A Review. Int. J. Food Sci. Technol. 2022, 57, 5585–5591. [Google Scholar] [CrossRef]
- Brannan, T.; Bickler, C.; Hansson, H.; Karley, A.; Weih, M.; Manevska-Tasevska, G. Overcoming Barriers to Crop Diversifi-cation Uptake in Europe: A Mini Review. Front. Sustain. Food Syst. 2023, 7, 1107700. [Google Scholar] [CrossRef]
- Himanen, S.; Mäkinen, H.; Rimhanen, K.; Savikko, R. Engaging Farmers in Climate Change Adaptation Planning: Assessing Intercropping as a Means to Support Farm Adaptive Capacity. Agriculture 2016, 6, 34. [Google Scholar] [CrossRef]
- Burgess, A.J.; Correa Cano, M.E.; Parkes, B. The Deployment of Intercropping and Agroforestry as Adaptation to Climate Change. Crop Environ. 2022, 1, 145–160. [Google Scholar] [CrossRef]
- Jose, S.; Holzmueller, E. Black Walnut Allelopathy: Implications for Intercropping. In Allelopathy in Sustainable Agriculture and Forestry; Springer: New York, NY, USA, 2008; pp. 303–319. [Google Scholar]
- Žalac, H.; Zebec, V.; Ivezić, V.; Herman, G. Land and Water Productivity in Intercropped Systems of Walnut—Buckwheat and Walnut–Barley: A Case Study. Sustainability 2022, 14, 6096. [Google Scholar] [CrossRef]
- Blessing, D.J.; Gu, Y.; Cao, M.; Cui, Y.; Wang, X.; Asante-Badu, B. Overview of the Advantages and Limitations of Maize-Soybean Intercropping in Sustainable Agriculture and Future Prospects: A Review. Chil. J. Agric. Res. 2022, 82, 177–188. [Google Scholar] [CrossRef]
- Ai, P.; Ma, Y.; Hai, Y. Jujube Is at a Competitiveness Disadvantage to Cotton in Intercropped System. Agron. J. 2021, 113, 3475–3488. [Google Scholar] [CrossRef]
- Goleva, I.; Zebitz, C.P.W. Suitability of Different Pollen as Alternative Food for the Predatory Mite Amblyseius Swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 259–283. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D.L. Control of Crop Diseases through Integrated Crop Management to Deliver Climate-smart Farming Systems for Low- and High-input Crop Production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Hou, P.; Liu, G.; Liu, W.; Wang, Y.; Zhao, R.; Ming, B.; Xie, R.; Wang, K.; et al. Improving Maize Grain Yield by Matching Maize Growth and Solar Radiation. Sci. Rep. 2019, 9, 3635. [Google Scholar] [CrossRef] [PubMed]
- Chimonyo, V.G.P.; Modi, A.T.; Mabhaudhi, T. Perspective on Crop Modelling in the Management of Intercropping Systems. Arch. Agron. Soil Sci. 2015, 61, 1511–1529. [Google Scholar] [CrossRef]
- Li, L. Intercropping Enhances Agroecosystem Services and Functioning: Current Knowledge and Perspectives. Zhongguo Shengtai Nongye Xuebao Chin. J. Eco-Agric. 2016, 24, 403–415. [Google Scholar]
- Weih, M.; Adam, E.; Vico, G.; Rubiales, D. Application of Crop Growth Models to Assist Breeding for Intercropping: Op-portunities and Challenges. Front. Plant Sci. 2022, 13, 720486. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-P.; Yang, H.; Xing, Y.; Zhang, W.-P.; Lambers, H.; Li, L. Belowground Processes and Sustainability in Agroecosystems with Intercropping. Plant Soil 2022, 476, 263–288. [Google Scholar] [CrossRef]
- Snapp, S.S.; DeDecker, J.; Davis, A.S. Farmer Participatory Research Advances Sustainable Agriculture: Lessons from Michigan and Malawi. Agron. J. 2019, 111, 2681–2691. [Google Scholar] [CrossRef]
- Çakmakçı, S.; Çakmakçı, R. Quality and Nutritional Parameters of Food in Agri-Food Production Systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef]
| Schematic | Uses | Advantages | Disadvantages |
|---|---|---|---|
 Mixed intercropping |
|
|
|
 Row intercropping |
|
|
|
 Relay intercropping |
|
|
|
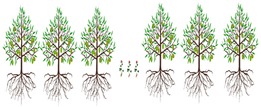 Strip intercropping |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, B.; Gonçalves, A.; Pinto, L.; Prieto, M.A.; Carocho, M.; Caleja, C.; Barros, L. Intercropping Systems: An Opportunity for Environment Conservation within Nut Production. Agriculture 2024, 14, 1149. https://doi.org/10.3390/agriculture14071149
Moreira B, Gonçalves A, Pinto L, Prieto MA, Carocho M, Caleja C, Barros L. Intercropping Systems: An Opportunity for Environment Conservation within Nut Production. Agriculture. 2024; 14(7):1149. https://doi.org/10.3390/agriculture14071149
Chicago/Turabian StyleMoreira, Bruna, Alexandre Gonçalves, Luís Pinto, Miguel A. Prieto, Márcio Carocho, Cristina Caleja, and Lillian Barros. 2024. "Intercropping Systems: An Opportunity for Environment Conservation within Nut Production" Agriculture 14, no. 7: 1149. https://doi.org/10.3390/agriculture14071149
APA StyleMoreira, B., Gonçalves, A., Pinto, L., Prieto, M. A., Carocho, M., Caleja, C., & Barros, L. (2024). Intercropping Systems: An Opportunity for Environment Conservation within Nut Production. Agriculture, 14(7), 1149. https://doi.org/10.3390/agriculture14071149












