Sowing Date and Seed Rate Influence on Septoria Leaf Blotch Occurrence in Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
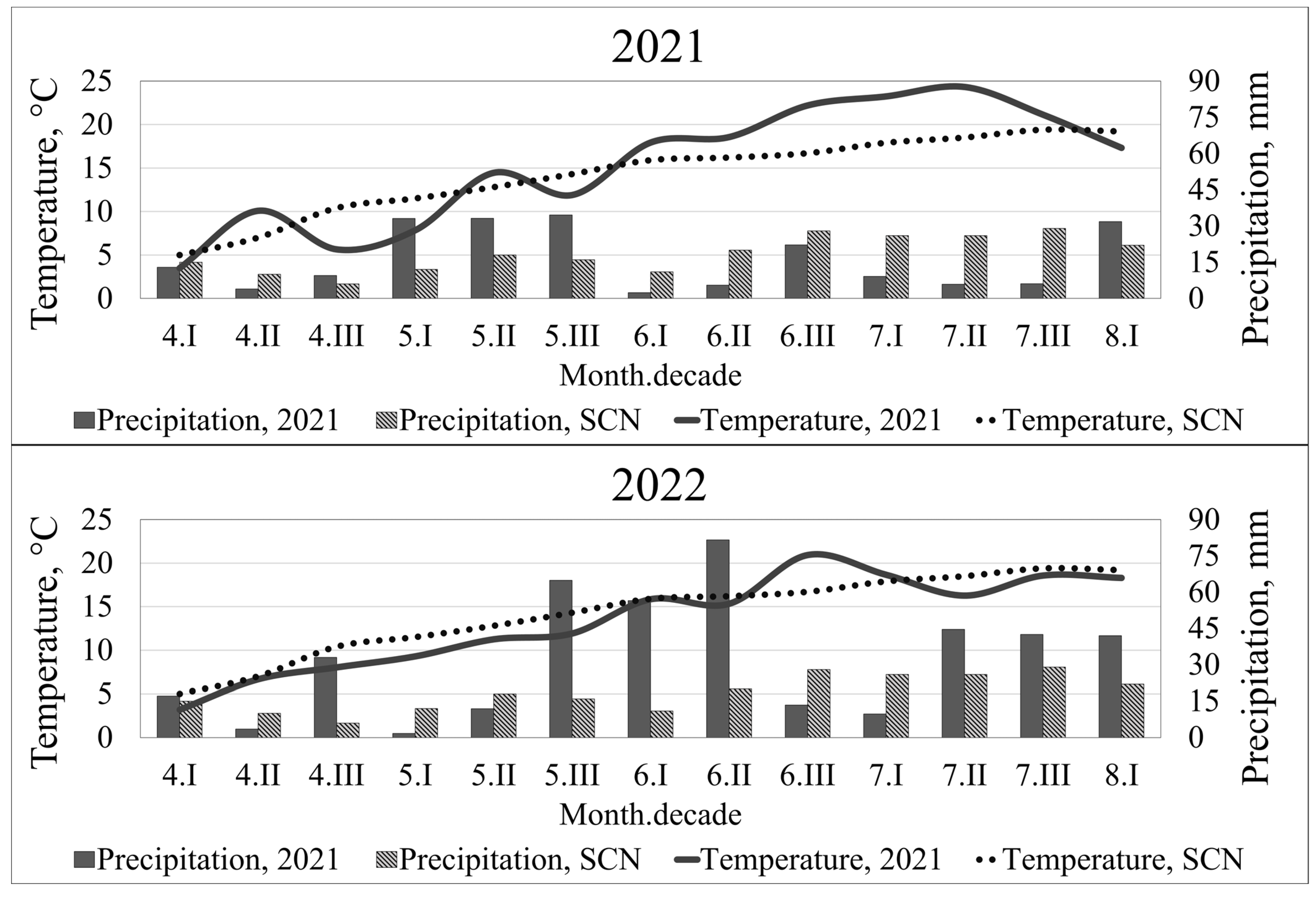
2.2. Weather Conditions
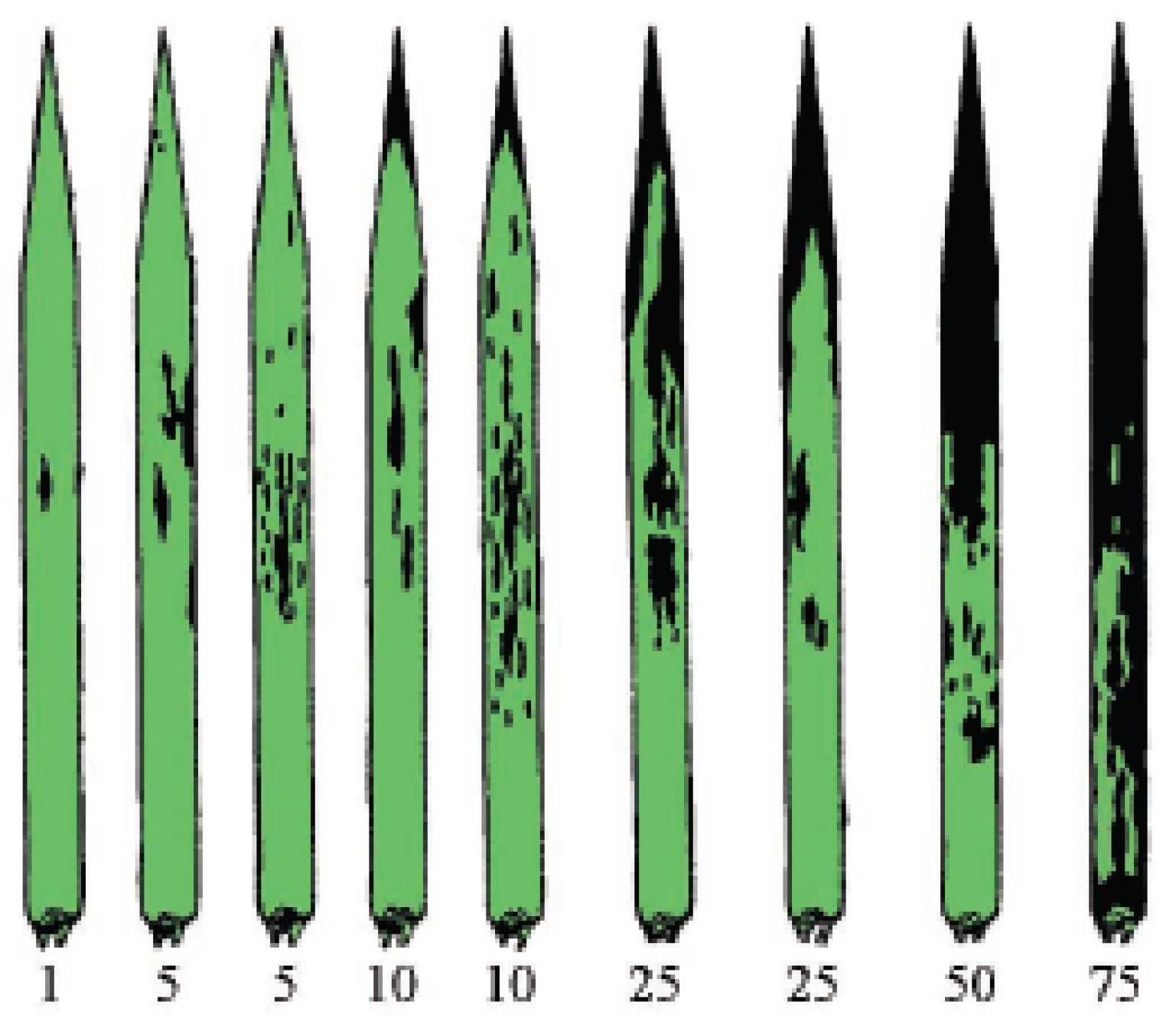
2.3. Severity of Septoria Leaf Blotch (SLB)
2.4. Statistical Analysis
3. Results
3.1. Weather Conditions during the Investigation Period
3.2. Multi-Factor Analysis of the Effect of Sowing Time and Seed Rate on the Severity of Septoria Leaf Blotch (SLB)
3.3. The Severity of Septoria Leaf Blotch on the First and Second Leaves in Winter Wheat
3.4. Effect of Sowing Time and Seed Rate on the Area under Disease Progress Curve (AUDPC) Values of Septoria Leaf Blotch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Cereal Supply and Demand Brief. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/worldfoodsituation/csdb/en (accessed on 29 April 2024).
- Crop Production—Oficialiosios Statistikos Portalas. Available online: https://osp.stat.gov.lt/lietuvos-aplinka-zemes-ukis-ir-energetika-2021/zemes-ukis/augalininkyste (accessed on 29 April 2024).
- Nepal, S.; Shrestha, S.M.; Manadhar, H.K.; Yadav, R.K. Field Response of Wheat Genotypes to Spot Blotch under Different Sowing Dates at Rampur, Chitwan, Nepal. J. Agric. For. Univ. 2020, 4, 83–90. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Delfosse, P.; Giraud, F.; Junk, J.; Hoffmann, L.; Maraite, H.; Tychon, B. Typology of the Main Fungal Diseases Affecting Winter Wheat in the Grand Duchy of Luxembourg. J. Agric. Sci. Technol. 2012, 2, 1386–1399. [Google Scholar]
- El Jarroudi, M.; Kouadio, A.L.; Mackels, C.; Tychon, B.; Delfosse, P.; Bock, C.H. A Comparison between Visual Estimates and Image Analysis Measurements to Determine Septoria Leaf Blotch Severity in Winter Wheat. Plant Pathol. 2015, 64, 355–364. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.; Gurr, S. The Impact of Septoria tritici Blotch Disease on Wheat: An EU Perspective. Fungal Genet. Biol. 2015, 79, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Berraies, S.; Gharbi, M.S.; Rezgui, S.; Yahyaoui, A. Estimating Grain Yield Losses Caused by Septoria Leaf Blotch on Durum Wheat in Tunisia. Chil. J. Agric. Res. 2014, 74, 432–437. [Google Scholar] [CrossRef]
- Tadesse, Y.; Chala, A.; Kassa, B. Yield Loss Due to Septoria Tritici Blotch (Septoria tritici) of Bread Wheat (Triticum aestivum L.) in the Central Highlands of Ethiopia. J. Biol. Agric. Healthc. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Te Beest, D.E.; Shaw, M.W.; Pietravalle, S.; Van Den Bosch, F. A Predictive Model for Early-Warning of Septoria Leaf Blotch on Winter Wheat. Eur. J. Plant Pathol. 2009, 124, 413–425. [Google Scholar] [CrossRef]
- Hardwick, N.V.; Jones, D.R.; Slough, J.E. Factors Affecting Diseases of Winter Wheat in England and Wales, 1989–1998. Plant Pathol. 2001, 50, 650–652. [Google Scholar] [CrossRef]
- Hermoso, V.; Carvalho, S.B.; Giakoumi, S.; Goldsborough, D.; Katsanevakis, S.; Leontiou, S.; Markantonatou, V.; Rumes, B.; Vogiatzakis, I.N.; Yates, K.L. The EU Biodiversity Strategy for 2030: Opportunities and Challenges on the Path towards Biodiversity Recovery. Environ. Sci. Policy 2022, 127, 263–271. [Google Scholar] [CrossRef]
- Waggoner, P.E.; Aylor, D.E. Epidemiology: A Science of Patterns. Annu. Rev. Phytopathol. 2000, 38, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Dennett, M.D. Effects of Sowing Date and the Determination of Optimum Sowing Date. In Wheat: Ecology and Physiology of Yield Determination; CRC Press: Boca Raton, FL, USA, 1999; pp. 123–140. [Google Scholar]
- Craigie, R.A.; Brown, H.E.; George, M. Grain Yield of Winter Feed Wheat in Response to Sowing Date and Sowing Rate. Agron. New Zealand 2015, 45, 1–8. [Google Scholar]
- Ponomarenko, A.; Goodwin, S.B.; Kema, G.H. Septoria tritici Blotch (STB) of Wheat. In Plant Health Instructor; American Phytopathological Society: St. Paul, MI, USA, 2011; Volume 10. [Google Scholar]
- Asseng, S.; Guarin, J.R.; Raman, M.; Monje, O.; Kiss, G.; Despommier, D.D.; Meggers, F.M.; Gauthier, P.P.G. Wheat Yield Potential in Controlled-Environment Vertical Farms. Proc. Natl. Acad. Sci. USA 2020, 117, 19131–19135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Cook, R.J.; King, J.E. Factors Affecting Development of Septoria tritici in Winter Wheat and Its Effect on Yield. Plant Pathol. 1989, 38, 246–257. [Google Scholar] [CrossRef]
- Shaw, M.W.; Royle, D.J. Factors Determining the Severity of Epidemics of Mycosphaerella graminicola (Septoria tritici) on Winter Wheat in the UK. Plant Pathol. 1993, 42, 882–899. [Google Scholar] [CrossRef]
- Shaw, M.W. Effects of Temperature, Leaf Wetness and Cultivar on the Latent Period of Mycosphaerella graminicola on Winter Wheat. Plant Pathol. 1990, 39, 255–268. [Google Scholar] [CrossRef]
- Chungu, C.; Gilbert, J.; Townley-Smith, F. Septoria tritici Blotch Development as Affected by Temperature, Duration of Leaf Wetness, Inoculum Concentration, and Host. Plant Dis. 2001, 85, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.R.; Lovell, D.J.; Royle, D.J.; Paveley, N.D. Analysing Epidemics of Septoria tritici for Improved Estimates of Disease Risk. In Septoria on Cereals: A Study of Pathosystems; CABI Publishing: Cambridge, UK, 1999; pp. 96–107. [Google Scholar]
- Hansen, J.G.; Secher, B.J.M.; Jørgensen, L.N.; Welling, B. Thresholds for Control of Septoria Spp. in Winter Wheat Based on Precipitation and Growth Stage. Plant Pathol. 1994, 43, 183–189. [Google Scholar] [CrossRef]
- Coakley, S.M.; McDaniel, L.R.; Shaner, G. Model for Predicting Severity of Septoria tritici Blotch on Winter Wheat. Phytopathology 1985, 75, 1245–1251. [Google Scholar] [CrossRef]
- Tyldesley, J.B.; Thompson, N. Forecasting Septoria Nodorum on Winter Wheat in England and Wales. Plant Pathol. 1980, 29, 9–20. [Google Scholar] [CrossRef]
- Hoogenboom, G. Contribution of Agrometeorology to the Simulation of Crop Production and Its Applications. Agric. For. Meteorol. 2000, 103, 137–157. [Google Scholar] [CrossRef]
- Kochiieru, M.; Veršulienė, A.; Feiza, V.; Feizienė, D.; Shatkovska, K.; Deveikytė, I. The Action of Environmental Factors on Carbon Dioxide Efflux per Growing Season and Non-Growing Season. Sustainability 2024, 16, 4391. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO). PP1/26(4)—Efficacy Evaluation of Fungicides. Bull. OEPP/EPPO Bull. 2012, 42, 419–425. [Google Scholar] [CrossRef]
- Simko, I.; Piepho, H.P. The Area under the Disease Progress Stairs: Calculation, Advantage, and Application. Phytopathology 2012, 102, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; p. 204. [Google Scholar]
- Dabkevičius, Z.; Brazauskienė, I. Augalų Patologija; Lietuvos žemės ūkio universitetas: Kaunas, Lithuania, 2007; p. 496. [Google Scholar]
- Akhileshwari, S.V.; Amaresh, Y.S.; Naik, M.K.; Kantharaju, V.; Shankergoud, I.; Ravi, M.V. Effect of Dates of Sowing on Powdery Mildew Severity and Yield of Sunflower. Karnataka J. Agric. Sci. 2012, 25, 129–130. [Google Scholar]
- Apeyuan, K.D.; Nwankiti, A.O.; Oluma, O.A.H.; Ekefan, E.J.; Apeyuan, K.D.; Nwankiti, A.O.; Oluma, O.A.H.; Ekefan, E.J. Effect of Different Sowing Dates on Disease Initiation and Development of Roselle (Hibiscus sabdariffa L.) Leaf Spot Disease Caused by Coniella musaiensis Var. Hibisci in Makurdi, Central Nigeria. J. Geosci. Environ. Prot. 2017, 5, 94–101. [Google Scholar] [CrossRef]
- Toropova, E.Y.; Kazakova, O.A.; Piskarev, V.V. Septoria Blotch Epidemic Process on Spring Wheat Varieties. Vavilov J. Genet. Breed. 2020, 24, 139–148. [Google Scholar] [CrossRef]
- Henze, M.; Beyer, M.; Klink, H.; Verreet, J.A. Characterizing Meteorological Scenarios Favorable for Septoria tritici Infections in Wheat and Estimation of Latent Periods. Plant Dis. 2007, 91, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Kpu, A.K.; Annih, M.G.; Ambang, A.L. Leaf Spot of Telfairia cccidentalis Incidence and Severity Influenced by Altitude and Planting Date in the West Region of Cameroon. CABI Agric. Biosci. 2022, 3, 32. [Google Scholar] [CrossRef]
- Kone, N.; Asare-Bediako, E.; Silue, S.; Kone, D.; Koita, O.; Menzel, W.; Winter, S. Influence of Planting Date on Incidence and Severity of Viral Disease on Cucurbits under Field Condition. Ann. Agric. Sci. 2017, 62, 99–104. [Google Scholar] [CrossRef]
- Ilondu, E.M. Etiology and Assessment of Leafspot Disease of Sweet Potato (Ipomoea batatas (L.) Lam) in Selected Farms in Delta State, Nigeria. Agric. Biol. J. North Am. 2013, 4, 476–484. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The Shade-Avoidance Syndrome: Multiple Signals and Ecological Consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Douma, J.C.; de Vries, J.; Poelman, E.H.; Dicke, M.; Anten, N.P.R.; Evers, J.B. Ecological Significance of Light Quality in Optimizing Plant Defence. Plant Cell Environ. 2019, 42, 1077. [Google Scholar] [CrossRef] [PubMed]
- Novoplansky, A.; Cohen, D.; Sachs, T. How Portulaca Seedlings Avoid Their Neighbours. Oecologia 1990, 82, 490–493. [Google Scholar] [CrossRef]
- Leone, M.; Keller, M.M.; Cerrudo, I.; Ballaré, C.L. To Grow or Defend? Low Red: Far-Red Ratios Reduce Jasmonate Sensitivity in Arabidopsis Seedlings by Promoting DELLA Degradation and Increasing JAZ10 Stability. N. Phytol. 2014, 204, 355–367. [Google Scholar] [CrossRef]
- Ansar, M.; Cheema, M.N.; Leitch, M.H. Effect of Agronomic Practices on the Development of Septoria Leaf Blotch and Its Subsequent Effect on Growth and Yield Components of Wheat. Pak. J. Bot. 2010, 42, 2125–2138. [Google Scholar]
- Magboul, A.M.; Geng, S.; Gilchrist, D.G.; Jackson, L.F. Environmental Influence on the Infection of Wheat by Mycosphaerella graminicola. Phytopathology 1992, 82, 1407–1413. [Google Scholar] [CrossRef]
- Juroszek, P.; Von Tiedemann, A. Potential Strategies and Future Requirements for Plant Disease Management under a Changing Climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Gladders, P.; Paveley, N.D.; Barrie, I.A.; Hardwick, N.V.; Hims, M.J.; Langton, S.; Taylor, M.C. Agronomic and Meteorological Factors Affecting the Severity of Leaf Blotch Caused by Mycosphaerella graminicola in Commercial Wheat Crops in England. Ann. Appl. Biol. 2001, 138, 301–311. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Chartrain, L.; Lasserre-Zuber, P.; Saintenac, C. Genetics of Resistance to Zymoseptoria tritici and Applications to Wheat Breeding. Fungal Genet. Biol. 2015, 79, 33–41. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Heick, T.M.; Havis, N.; Holdgate, S.; Clark, B.; Blake, J.; Glazek, M.; Korbas, M.; Danielewicz, J.; et al. Decreasing Azole Sensitivity of Z. Tritici in Europe Contributes to Reduced and Varying Field Efficacy. J. Plant Dis. Prot. 2021, 128, 287–301. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Hovmøller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Jahn, M.; Flath, K.; Goral, T.; et al. EuroWheat.org—A Support to Integrated Disease Management in Wheat. Outlooks Pest Manag. 2010, 21, 173–176. [Google Scholar] [CrossRef]
- Aryal, L.; Shrestha, S.M.; Gopal Bahadur, K.C. Effect of Date of Sowing on the Performance of Drought Tolerant Wheat Genotypes to Spot Blotch at Rampur, Chitwan, Nepal. Int. J. Appl. Sci. Biotechnol. 2013, 1, 266–271. [Google Scholar] [CrossRef]
- Gurung, S.; Sharma, R.C.; Duveiller, E.; Shrestha, S.M. Comparative Analyses of Spot Blotch and Tan Spot Epidemics on Wheat under Optimum and Late Sowing Period in South Asia. Eur. J. Plant Pathol. 2012, 134, 257–266. [Google Scholar] [CrossRef]
- Duveiller, E.; Kandel, Y.R.; Sharma, R.C.; Shrestha, S.M. Epidemiology of Foliar Blights (Spot Blotch and Tan Spot) of Wheat in the Plains Bordering the Himalayas. Phytopathology 2005, 95, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nema, K.G.; Joshi, L.M. Spot-Blotch Disease of Wheat in Relation to Host: Age, Temperature and Moisture. Indian Phytopathol. 1973, 26, 41–48. [Google Scholar]
- Regmi, A.P.; Ladha, J.K.; Pasuquin, E.; Pathak, H.; Hobbs, P.R.; Shrestha, L.L.; Gharti, D.B.; Duveiller, E. The Role of Potassium in Sustaining Yields in a Long-Term Rice-Wheat Experiment in the Indo-Gangetic Plains of Nepal. Biol. Fertil. Soils 2002, 36, 240–247. [Google Scholar] [CrossRef]

| Source | First Leaf | Second Leaf | ||||||
|---|---|---|---|---|---|---|---|---|
| DF | Mean Square | F Value | Pr > F | DF | Mean Square | F Value | Pr > F | |
| 2021 | ||||||||
| Sowing time | 2 | 60.2977 | 18.56 | <0.0001 | 2 | 50.8558 | 0.51 | 0.6054 |
| Seed rate | 2 | 0.2977 | 0.09 | 0.9127 | 2 | 45.2933 | 0.46 | 0.6390 |
| Sowing time × seed rate | 4 | 0.1661 | 0.05 | 0.9948 | 4 | 43.4516 | 0.44 | 0.7808 |
| 2022 | ||||||||
| Sowing time | 2 | 494.6319 | 28.68 | <0.0001 | 2 | 1149.5902 | 19.72 | <0.0001 |
| Seed rate | 2 | 70.9002 | 4.11 | 0.0276 | 2 | 308.5486 | 5.29 | 0.0115 |
| Sowing time × seed rate | 4 | 11.1940 | 0.65 | 0.6325 | 4 | 26.3611 | 0.45 | 0.7700 |
| Seed Rate (Seeds m−2) | Growth Stage | |||||||
|---|---|---|---|---|---|---|---|---|
| BBCH 59–65 | BBCH 75 | |||||||
| Sowing Time | ||||||||
| Early | Optimal | Late | Mean | Early | Optimal | Late | Mean | |
| 2021 | ||||||||
| 350 | 0.05 ± 0.05 aA | 0.45 ± 0.43 aA | 0.07 ± 0.09 aA | 0.19 ± 0.30 | 7.30 ± 1.58 aAB | 9.18 ± 3.9 aB | 4.32 ± 1.26 aA | 6.93 ± 3.06 |
| 400 | 0.10 ± 0.08 aA | 0.55 ± 0.34 aB | 0.12 ± 0.12 aA | 0.26 ± 0.29 | 7.05 ± 1.77 aB | 8.88 ± 0.85 aB | 4.67 ± 1.35 aA | 6.87 ± 2.18 |
| 450 | 0.03 ± 0.05 aA | 0.98 ± 0.58 aB | 0.32 ± 0.47 aAB | 0.44 ± 0.57 | 6.75 ± 0.28 aB | 8.75 ± 2.06 aB | 4.40 ± 0.81 aA | 6.63 ± 2.19 |
| Mean | 0.06 ± 0.06 * | 0.66 ± 0.48 * | 0.18 ± 0.28 * | 7.03 ± 1.27 * | 8.93 ± 2.30 * | 4.47 ± 1.06 * | ||
| 2022 | ||||||||
| 350 | 2.88 ± 0.97 aA | 2.75 ± 0.51 aA | 2.28 ± 0.57 aA | 2.64 ± 0.70 | 16.75 ± 7.04 aB | 6.00 ± 1.15 aA | 10.75 ± 2.95 aAB | 11.17 ± 6.11 |
| 400 | 1.95 ± 0.71 aA | 2.45 ± 0.78 aA | 2.55 ± 0.40 aA | 2.32 ± 0.65 | 18.15 ± 4.95 aB | 6.58± 0.75 aA | 8.00 ± 2.73 aA | 10.91 ± 6.15 |
| 450 | 2.78 ± 2.05 aA | 1.60 ± 1.01 aA | 2.65 ± 0.69 aA | 2.34 ± 1.36 | 24.13 ± 5.80 aB | 9.70 ± 1.11 bA | 11.90 ± 5.28 aA | 15.24 ± 7.81 |
| Mean | 2.54 ± 1.31 | 2.27 ± 0.88 | 2.49 ± 0.54 | 19.68 ± 6.37 * | 7.43 ± 1.93 * | 10.22 ± 3.86 * | ||
| Seed Rate (Seeds m−2) | Growth Stage | |||||||
|---|---|---|---|---|---|---|---|---|
| BBCH 59–65 | BBCH 75 | |||||||
| Sowing Time | ||||||||
| Early | Optimal | Late | Mean | Early | Optimal | Late | Mean | |
| 2021 | ||||||||
| 350 | 2.53 ± 1.14 aA | 5.65 ± 4.14 aA | 2.58 ± 1.30 aA | 3.58 ± 2.79 | 31.38 ± 4.93 aA | 38.00 ± 9.22 aA | 33.90 ± 14.67 aA | 34.43 ± 9.83 |
| 400 | 2.50 ± 0.33 aA | 4.68 ± 2.36 aA | 2.55 ± 1.28 aA | 3.24 ± 1.76 | 36.00 ± 8.25 aA | 36.88 ± 7.18 aA | 31.50 ± 13.27 aA | 34.79 ± 9.31 |
| 450 | 2.45 ± 2.17 aA | 4.75 ± 0.75 aA | 3.25 ± 1.25 aA | 3.48 ± 1.69 | 42.25 ± 6.34 aA | 36.88 ± 5.54 aA | 34.75 ± 14.26 aA | 37.96 ± 9.25 |
| Mean | 2.49 ± 1.29 * | 5.03 ± 2.56 * | 2.79 ± 1.20 * | 36.54 ± 7.60 | 37.25 ± 6.78 | 33.38 ± 12.81 | ||
| 2022 | ||||||||
| 350 | 2.73 ± 0.83 aA | 2.30 ± 0.82 aA | 6.90 ± 2.28 aB | 3.98 ± 2.54 | 40.00 ± 8.66 aB | 38.25 ± 4.27 aB | 25.38 ± 3.19 aA | 34.54 ± 8.63 * |
| 400 | 1.73 ± 0.79 aA | 1.73 ± 0.27 aA | 8.20 ± 3.94 aB | 3.89 ± 3.82 | 50.00 ± 12.19 aB | 45.38 ± 10.59 aB | 26.50 ± 8.78 aA | 40.63 ± 14.4 * |
| 450 | 2.23 ± 0.71 aA | 1.48 ± 1.01 aA | 7.03 ± 1.05 aB | 3.58 ± 2.70 | 51.63 ± 6.16 aB | 47.38 ± 3.79 aB | 34.75 ± 5.54 aA | 44.59 ± 8.87 * |
| Mean | 2.23 ± 0.82 * | 1.84 ± 0.78 * | 7.38 ± 2.51 * | 47.21 ± 10.06 * | 43.67 ± 7.49 * | 28.88 ± 7.16 * | ||
| Source | 2021 | 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| DF | Mean Square | F Value | Pr > F | DF | Mean Square | F Value | Pr > F | |
| Sowing time | 2 | 55801.9 | 10.57 | 0.0004 | 2 | 60290.5 | 22.70 | <0.0001 |
| Seed rate | 2 | 2198.6 | 0.42 | 0.6637 | 2 | 17517.7 | 6.60 | 0.0047 |
| Sowing time × seed rate | 4 | 2172.6 | 0.41 | 0.7989 | 4 | 1764.6 | 0.66 | 0.6222 |
| Years | From | To | I Sowing | II Sowing | III Sowing |
|---|---|---|---|---|---|
| 2021 | BBCH 37–41 | BBCH 59–65 | 7.5 mm (−64%) 16.4 °C (0.7 °C) | 9.9 mm (−68%) 16.3 °C (0.5 °C) | 7.8 mm (−70%) 18.3 (2.1 °C) |
| BBCH 59–65 | BBCH 75 | 24.6 mm (−50%) 20.6 °C (4.1 °C) | 22.2 mm (−43%) 22.0 °C (5.5 °C) | 22.3 mm (−54%) 22.4 °C (5.3 °C) | |
| 2022 | BBCH 37–41 | BBCH 59–65 | 128.6 mm (289%) 14.7 °C (−1.1 °C) | 72.3 mm (211%) 16.5 °C (0.4 °C) | 122.7 mm (272%) 16.1 °C (−0.1 °C) |
| BBCH 59–65 | BBCH 75 | 53.7 mm (46%) 19.9 °C (3.2 °C) | 53.9 mm (23%) 20.0 °C (3.3 °C) | 10,5 mm (−75%) 21.2 °C (4.1 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almogdad, M.; Lukošiūtė-Stasiukonienė, A.; Semaškienė, R.; Mačiulytė, V. Sowing Date and Seed Rate Influence on Septoria Leaf Blotch Occurrence in Winter Wheat. Agriculture 2024, 14, 988. https://doi.org/10.3390/agriculture14070988
Almogdad M, Lukošiūtė-Stasiukonienė A, Semaškienė R, Mačiulytė V. Sowing Date and Seed Rate Influence on Septoria Leaf Blotch Occurrence in Winter Wheat. Agriculture. 2024; 14(7):988. https://doi.org/10.3390/agriculture14070988
Chicago/Turabian StyleAlmogdad, Mohammad, Agnė Lukošiūtė-Stasiukonienė, Roma Semaškienė, and Viktorija Mačiulytė. 2024. "Sowing Date and Seed Rate Influence on Septoria Leaf Blotch Occurrence in Winter Wheat" Agriculture 14, no. 7: 988. https://doi.org/10.3390/agriculture14070988






