Relationships of Circulating and Preovulatory Follicular Fluid Hydrogen Peroxide Levels with Body Condition Score and Metabolome Profiles of Lactating Beef Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Serum and Preovulatory Follicular Fluid Collection
2.3. Body Condition, Hormone, and Metabolome Data
2.4. ROS Assay
2.5. Statistical Analysis
3. Results
3.1. Relationship between Serum and Preovulatory Follicular Fluid H2O2 Levels and Cow BCS
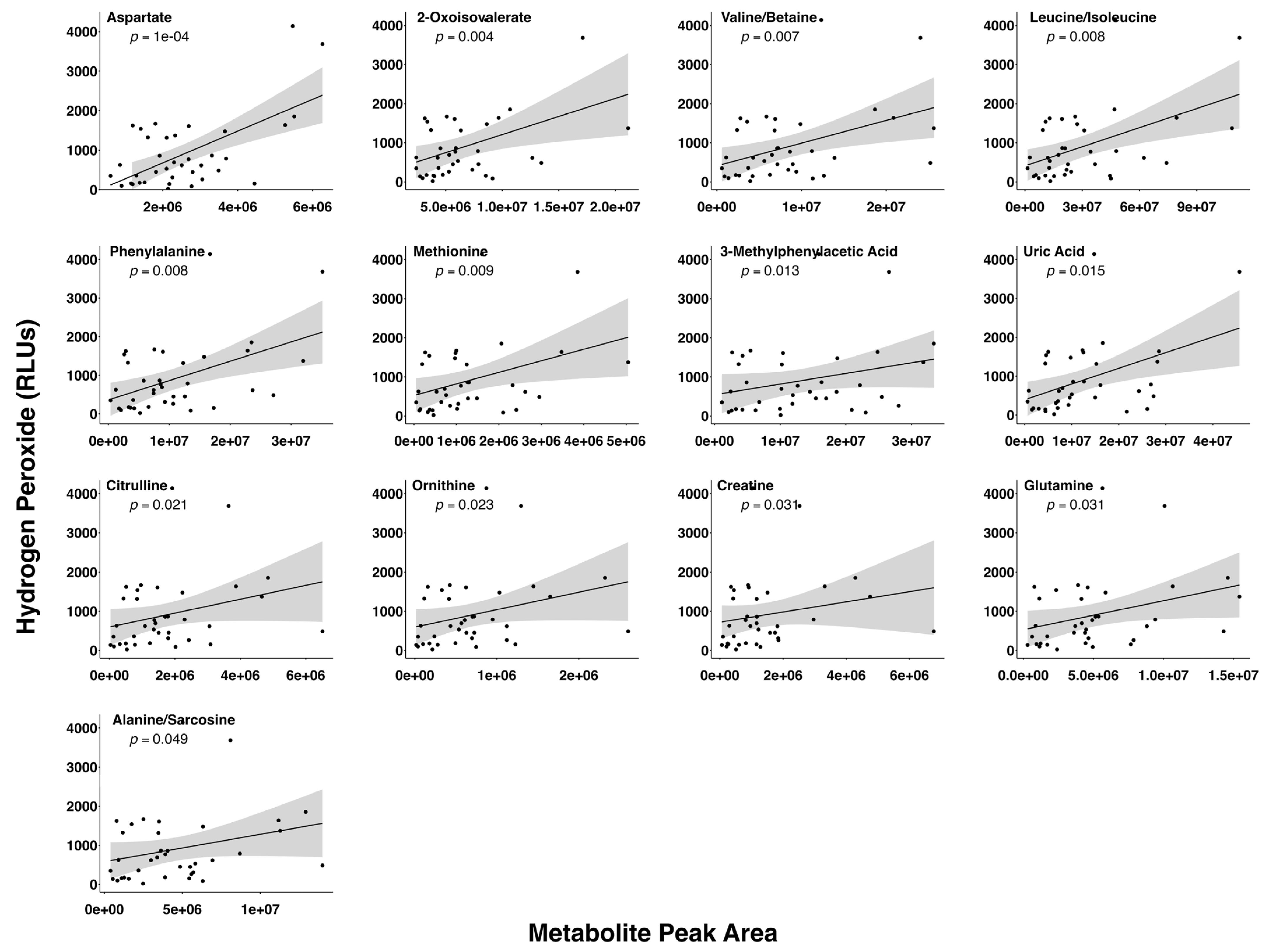
3.2. Relationship between Serum H2O2 and Metabolite Levels
3.3. Relationship between Preovulatory Follicular Fluid H2O2 and Metabolite Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, M.M.; Spitzer, J.C.; Warner, M.B. Effect of varying levels of postpartum nutrition and body condition at calving on subsequent reproductive performance in beef cattle. J. Anim. Sci. 1986, 62, 300–306. [Google Scholar] [CrossRef]
- Moraes, J.C.F.; Jaume, C.M.; Souza, C.J.H. Body condition score to predict the postpartum fertility of crossbred beef cows. Pesq. Agropec. 2007, 42, 741–746. [Google Scholar] [CrossRef]
- Gu, L.; Liu, H.; Gu, C.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Herring, A.D. Beef Cattle. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–20. [Google Scholar] [CrossRef]
- Selk, G.E.; Wettemann, R.P.; Lusby, K.S.; Oltjen, J.W.; Mobley, S.L.; Rasby, R.J.; Garmendia, J.C. Relationship among weight change, body condition and reproductive performance of range beef cows. J. Anim. Sci. 1988, 66, 3153–3159. [Google Scholar] [CrossRef]
- DeRouen, S.M.; Franke, D.E.; Morrison, D.G.; Wyatt, W.E.; Coombs, D.F.; White, T.W.; Humes, P.E.; Greene, B.B. Prepartum body condition and weight influences on reproductive performance of first-calf beef cows. J. Anim. Sci. 1994, 72, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.M. Effects of body condition, reproductive status and breed on follicular population and oocyte quality in cows. Theriogenology 1995, 43, 1405–1418. [Google Scholar] [CrossRef]
- Snijders, S.E.M.; Dillon, P.; O’Callaghan, D.; Boland, M.P. Effect of genetic merit, milk yield, body condition and lactation number on in vitro oocyte development in dairy cows. Theriogenology 2000, 53, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Dorice, A.K.; Ferdinand, N.; Justin, K.; Augustave, K.; Linda, K.K. Effects of breed, age, body condition score, and nutritional status on follicular population, oocyte yield, and quality in three cameroonian Zebus cattle Bos indicus. Adv. Agric. 2019, 2019, 2979740. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Ting, H.; Shabnam, F.; Linbao, J.; Tianyi, L.; Xi, M. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Celi, P. Oxidative stress in ruminants. In Studies on Veterinary Medicine. Oxidative Stress in Applied Basic Research and Clinical Practice; Mandelker, L., Vajdovich, P., Eds.; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reative oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2016, 16, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.B.; Parchment, R.E.; Lewellyn, A.L. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation 1991, 46, 181–186. [Google Scholar] [CrossRef]
- Das, S.; Chattopadhyay, R.; Ghosh, S.; Ghosh, S.; Goswami, S.K.; Chakravarty, B.N.; Chaudhury, K. Reactive oxygen species level in follicular fluid-embryo quality marker in IVF? Hum. Reprod. 2006, 21, 2403–2407. [Google Scholar] [CrossRef]
- Tarín, J.J.; Vendrell, F.J.; Ten, J.; Blanes, R.; van Blerkom, J.; Cano, A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol. Hum. Reprod. 1996, 2, 895–901. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.J.; Read, C.C.; Edwards, J.L.; Schrick, F.N.; Rhinehard, J.D.; Payton, R.R.; Campagna, S.R.; Klabnik, J.L.; Clark, H.M.; Myer, P.R.; et al. Preovulatory follicular fluid and serum metabolome profiles in lactating beef cows with thin, moderate, and obese body condition. J. Anim. Sci. 2022, 100, skac152. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Moorey, S.E.; Monning, J.M.; Smith, M.F.; Ortega, M.S.; Green, J.A.; Pohler, K.G.; Bridges, G.A.; Behura, S.K.; Geary, T.W. Differential transcript profiles in cumulus-oocyte complexes originating from pre-ovulatory follicles of varied physiological maturity in beef cows. Genes 2021, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Read, C.C.; Edwards, L.; Schrick, N.; Rhinehart, J.D.; Payton, R.R.; Campagna, S.R.; Castro, H.F.; Klabnik, J.L.; Horn, E.J.; Moorey, S.E. Correlation between pre-ovulatory follicle diameter and follicular fluid metabolome profiles in lactating beef cows. Metabolites 2021, 11, 623. [Google Scholar] [CrossRef]
- Whitman, R.W. Weight Change, Body Condition and Beef-Cow Reproduction. Colorado State University: Fort Collins, CO, USA, 1975. [Google Scholar]
- Kirby, C.J.; Smith, M.F.; Keisler, D.H.; Lucy, M.C. Follicular function in lactating dairy cows treated with sustained-release bovine somatotropin. J. Dairy Sci. 1997, 80, 273–285. [Google Scholar] [CrossRef]
- García-Guerra, A.; Canavessi, A.M.O.; Monteiro, P.L.J., Jr.; Mezera, M.A.; Sartori, R.; Kirkpatrick, B.W.; Wiltbank, M.C. Trio, a novel bovine high fecundity allele: III. acquisition of dominance and ovulatory capacity at a smaller follicle size. Biol. Reprod. 2018, 98, 350–365. [Google Scholar] [CrossRef]
- Pohler, K.G.; Pereira, M.H.C.; Lopes, F.R.; Lawrence, J.C.; Keisler, D.H.; Vasconcelos, J.L.M.; Green, J.A. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy Sci. 2016, 99, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.C.; Butler, W.R. Metabolic signals of the beef cow in negative energy balance. In Proceedings of the 4th Grazing Livestock Nutrition Conference, Ithaca, NY, USA, 9–10 July 2010. [Google Scholar]
- Hohenboken, W.D.; Dudley, A.; Moody, D.E. A comparison among equations to characterize lactation curves in beef cows. Anim. Sci. 1992, 55, 23–28. [Google Scholar] [CrossRef]
- Albertini, T.Z.; Medeiros, S.R.; Torres Junior, R.A.A.; Zocchi, S.S.; Oltjen, J.W.; Strathe, A.B.; Lanna, D.P.D. A methodological approach to estimate the lactation curve and net energy and protein requirements of beef cows using nonlinear mixed–effects modeling. J. Anim. Sci. 2012, 90, 3867–3878. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Adamaki, M.; Ioannou, P.; Pappa, A.; Panayiotidis, M.I.; Spandidos, D.A.; Christodoulou, I.; Kyriakopoulos, A.M.; Zoumpourlis, V. Protective role of taurine against oxidative stress (Review). Mol. Mel. Rep. 2021, 24, 605. [Google Scholar] [CrossRef]
- Cozzi, R.; Ricordy, R.; Bartolini, F.; Ramadori, L.; Perticone, P.; De Salvia, R. Taurine and el-lagic acid: Two differently-acting natural antioxidants. Environ. Mol. Mutagen. 1995, 26, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox. Biol. 2020, 28, 101365. [Google Scholar] [CrossRef] [PubMed]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta. Bioenerg. 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Stepien, K.M.; Heaton, R.; Rankin, S.; Murphy, A.; Bentley, J.; Sexton, D.; Hargreaves, I.P. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J. Clin. Med. 2017, 6, 71. [Google Scholar] [CrossRef]
- Shalgi, R.; Kraicer, P.; Rimon, A.; Pinto, M.; Soferman, N. Proteins of human follicular fluid: The blood-follicle barrier. Fertil. Steril. 1973, 24, 429–434. [Google Scholar] [CrossRef]
- Shin, S.; Gombedza, F.C.; Bandyopadhyay, B.C. l-ornithine activates Ca2+ signaling to exert its protective function on human proximal tubular cells. Cell Signal. 2020, 67, 109484. [Google Scholar] [CrossRef]
- Stegman, L.D.; Zheng, H.; Neal, E.R.; Ben-Yoseph, O.; Pollegioni, L.; Pilone, M.S.; Ross, B.D. Induction of cytotoxic oxidative stress by D-alanine in brain tumor cells expressing Rhodotorula gracilis D-amino acid oxidase: A cancer gene therapy strategy. Hum. Gene Ther. 1998, 9, 185–193. [Google Scholar] [CrossRef]
- Dastmalchi, F.; Karachi, A.; Yang, C.; Azari, H.; Sayour, E.J.; Dechkovskaia, A.; Vlasak, A.L.; Saia, M.E.; Lovaton, R.E.; Mitchell, D.A.; et al. Sarcosine promotes trafficking of dendritic cells and improves efficacy of anti-tumor dendritic cell vaccines via CXC chemokine family signaling. J. Immunother. Cancer 2019, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Hessock, E.A.; Edwards, J.L.; Schrick, F.N.; Payton, R.R.; Campagna, S.R.; Pollock, A.B.; Clark, H.M.; Stokes, A.E.; Klabnik, J.L.; Hill, K.S.; et al. Metabolite abundance in bovine preovulatory follicular fluid is influenced by follicle developmental progression post estrous onset in cattle. Front. Cell Dev. Biol. 2023, 11, 1156060. [Google Scholar] [CrossRef] [PubMed]
- Heras, S.S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.A.; Smith, M.F.; MacNeil, M.D.; Jinks, E.M.; Abreu, F.M.; Alexander, L.J.; Geary, T.W. Pregnancy establishment and maintenance in cattle. J. Anim. Sci. 2013, 91, 722–733. [Google Scholar] [CrossRef]
- D’Aniello, G.; Grieco, N.; Di Filippo, M.A.; Cappiello, F.; Topo, E.; D’Aniello, E.; Ronsini, S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007, 22, 3178–3183. [Google Scholar] [CrossRef]
- Marzyieh, S.; Rasoul, K.; Mohammad, H.A.A.; Nima, S.; Masoud, B.J. The relationship between bovine blastocyst formation in vitro and follicular fluid amino acids. Theriogenology 2023, 206, 197–204. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine supplementation, physical exercise and oxidative stress markers: A review of the mechanisms and effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Wu, P.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Protective role of phenylalanine on the ROS-induced oxidative damage, apoptosis and tight junction damage via Nrf2, TOR and NF-κB signalling molecules in the gill of fish. Fish Shellfish. Immun. 2017, 60, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef]
- Stefani, G.P.; Nunes, R.B.; Dornelles, A.Z. Effects of creatine supplementation associated with resistance training on oxidative stress in different tissues of rats. J. Int. Soc. Sports Nutr. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron. Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Amores-Sanchez, M.I.; Medina, M.A. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Freitas, C.; Neto, A.C.; Matos, L.; Silva, E.; Ribeiro, Â.; Silva-Carvalho, J.L.; Almeida, H. Follicular fluid redox involvement for ovarian follicle growth. J. Ovarian Res. 2017, 10, 44. [Google Scholar] [CrossRef]
- Sutovsky, P.; Schatten, G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol. Reprod. 1997, 56, 1503–1512. [Google Scholar] [CrossRef]
- Brad, A.; Bormann, C.; Swain, J.; Durkin, R.; Johnson, A.; Clifford, A.; Krisher, R.L. Glutathione and adenosine triphosphate content of in vivo and in vitro matured porcine oocytes. Mol. Reprod. Dev. 2003, 64, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Zuelke, K.A.; Jeffay, S.C.; Zucker, R.M.; Perreault, S.D. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol. Reprod. Dev. 2003, 64, 106–112. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wu, Q.; Li, J.; Sun, S.; Sun, S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020, 53, 12891. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, V.; Ansari, S.; Kumar, A.; Thakur, A.; Malik, H.; Kumar, S.; Malakar, D. Folate supplementation during oocyte maturation positively impacts the folate-methionine metabolism in pre-implantation embryos. Theriogenology 2022, 182, 63–70. [Google Scholar] [CrossRef]
- Ikeda, S.; Namekawa, T.; Sugimoto, M.; Kume, S. Expression of methylation pathway enzymes in bovine oocytes and preimplantation embryos. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 129–136. [Google Scholar] [CrossRef] [PubMed]

| Interaction BCS * H2O2 | Thin H2O2 | Moderate H2O2 | Obese H2O2 | |
|---|---|---|---|---|
| 1 Metabolite Name | 2 p | 3 p (estimate) | 3 p (estimate) | 3 p (estimate) |
| 2-Aminoadipate | 0.0035 | 0.0661 (112.3) | 0.0693 (−47.4) | 0.2252 (27.9) |
| 2-Dehydro-D-Gluconate | 0.0115 | 0.1527 (77) | 0.0148 (−83) | 0.2466 (69) |
| Glyoxylate | 0.0310 | 0.0809 (358) | 0.1909 (−190) | 0.2092 (162) |
| Alanine/Sarcosine | 0.0255 | 0.1572 (174) | 0.0242 (−255) | 0.6234 (−61) |
| Creatinine | 0.0513 | 0.1432 (155) | 0.043 (−135) | 0.6370 (−49) |
| Proline | 0.0455 | 0.4954 (53) | 0.0468 (−116) | 0.2622 (60) |
| 2-Oxoisovalerate | 0.0265 | 0.3769 (51) | 0.0719 (−105) | 0.1628 (105) |
| Succinate/Methylmalonate | 0.0008 | 0.0001 (653) | 0.1082 (−201) | 0.2775 (146) |
| Taurine | 0.0009 | 0.0095 (424) | 0.1436 (−135) | 0.0764 (240) |
| Methyl Succinic Acid | 0.0003 | 0.0016 (230) | 0.1572 (−60) | 0.6935 (−23) |
| Ornithine | 0.0319 | 0.0677 (84) | 0.0703 (−50) | 0.8930 (−4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, K.S.; Edwards, J.L.; Payton, R.R.; Schrick, F.N.; Campagna, S.R.; Hessock, E.A.; Moorey, S.E. Relationships of Circulating and Preovulatory Follicular Fluid Hydrogen Peroxide Levels with Body Condition Score and Metabolome Profiles of Lactating Beef Cows. Agriculture 2024, 14, 1406. https://doi.org/10.3390/agriculture14081406
Hill KS, Edwards JL, Payton RR, Schrick FN, Campagna SR, Hessock EA, Moorey SE. Relationships of Circulating and Preovulatory Follicular Fluid Hydrogen Peroxide Levels with Body Condition Score and Metabolome Profiles of Lactating Beef Cows. Agriculture. 2024; 14(8):1406. https://doi.org/10.3390/agriculture14081406
Chicago/Turabian StyleHill, Kennedy S., J. Lannett Edwards, Rebecca R. Payton, F. Neal Schrick, Shawn R. Campagna, Emma A. Hessock, and Sarah E. Moorey. 2024. "Relationships of Circulating and Preovulatory Follicular Fluid Hydrogen Peroxide Levels with Body Condition Score and Metabolome Profiles of Lactating Beef Cows" Agriculture 14, no. 8: 1406. https://doi.org/10.3390/agriculture14081406
APA StyleHill, K. S., Edwards, J. L., Payton, R. R., Schrick, F. N., Campagna, S. R., Hessock, E. A., & Moorey, S. E. (2024). Relationships of Circulating and Preovulatory Follicular Fluid Hydrogen Peroxide Levels with Body Condition Score and Metabolome Profiles of Lactating Beef Cows. Agriculture, 14(8), 1406. https://doi.org/10.3390/agriculture14081406








