Obtaining Phenolic-Enriched Liquid Fractions and Compostable Pomace for Agriculture from Alperujo Using Standard Two-Phase Olive Oil Mill Equipment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin and Characterization of Raw Material
2.2. Chemicals and Analytical Determinations
2.3. Industrial Pretreatments
2.4. Phenolics Purification Trial
2.5. Experimental Design and Statistical Analysis
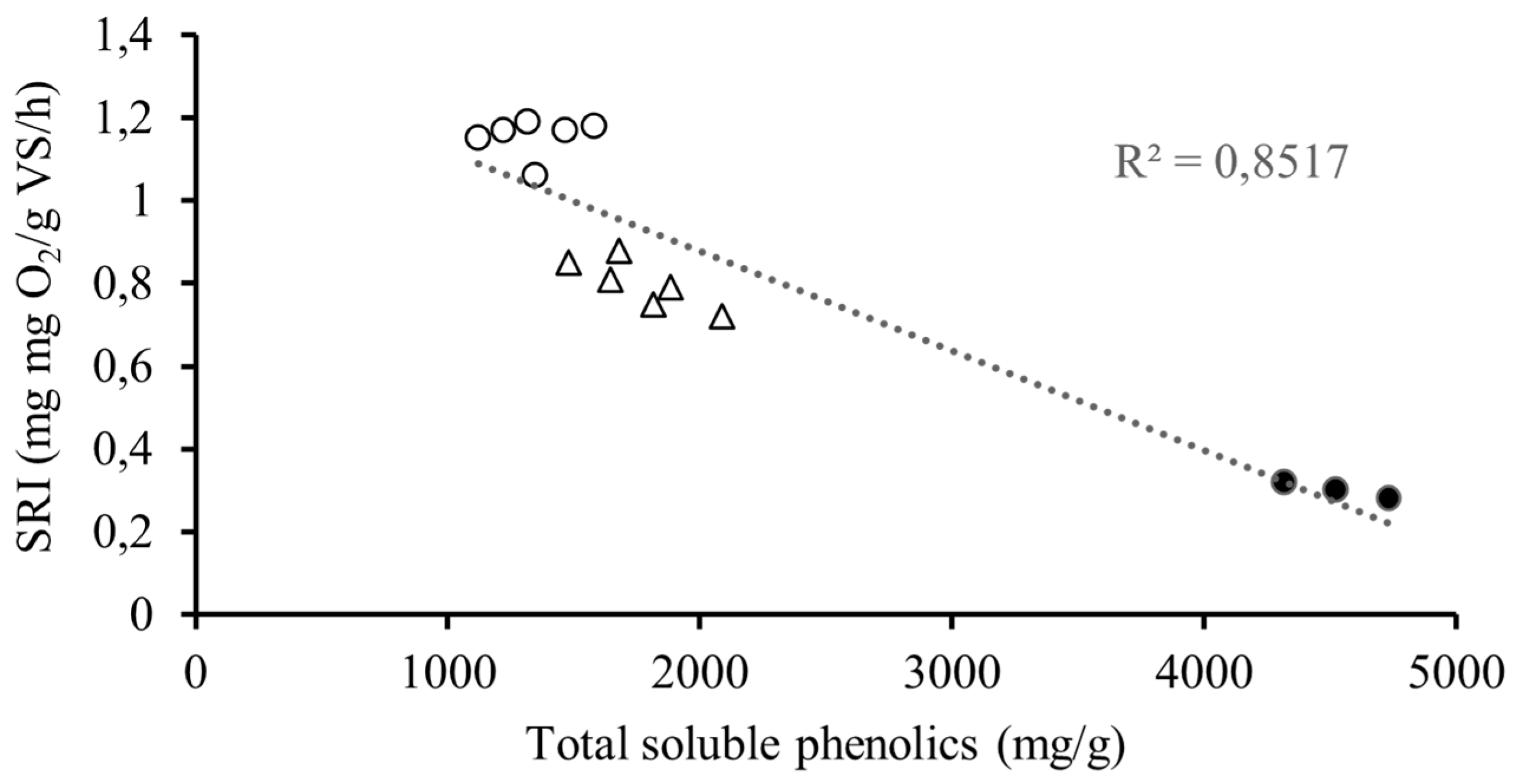
3. Results and Discussion
3.1. Raw Material Characterization
3.2. Applied Pretreatments and Analysis of Obtained Fractions
3.3. Phenolics Purification Trial
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canitrot, L.; Méndez, Y. Informe de Cadena de Valor Olivícola. Secr. Polít. Econ. Minist. Hacienda Pres. Nación. 2018, 3, 23. Available online: https://www.argentina.gob.ar/sites/default/files/sspmicro_cadenas_de_valor_olivicola.pdf (accessed on 1 March 2018).
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical Characterisation of “Alperujo”, a Solid by-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Monetta, P.; Bueno, L.; Cornejo, V.; González-Aubone, F.; Babelis, G. Short-Term Dynamics of Soil Chemical Parameters after Application of Alperujo in High-Density Drip-Irrigated Olive Groves in Argentina. Int. J. Environ. Stud. 2012, 69, 578–588. [Google Scholar] [CrossRef]
- Monetta, P.; Sanchez-Montilla, R.; Bosch-Rubia, G.; Rizzo, P.F.F.; Crespo, D.; Gouiric, S.; Vallejo Herrera, M. Co-Composting Alperujo with Other Agro-Industrial Residues as Sustainable Practice for Its Recycling. Acta Hortic. 2014, 1057, 709–716. [Google Scholar] [CrossRef]
- Borja, R.; Raposo, F.; Rincón, B. Treatment Technologies of Liquid and Solid Wastes from Two-Phase Olive Oil Mills. Grasas Aceites 2006, 57, 32–46. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Ait-Baddi, G.; Cegarra, J. The Production of Commercial Organic Amendments and Fertilisers by Composting of Two-Phase Olive Mill Waste (“alperujo”). J. Clean. Prod. 2012, 26, 48–55. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive Mill Waste Composting: A Review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Phenolic Extract Obtained from Steam-Treated Olive Oil Waste: Characterization and Antioxidant Activity. LWT Food Sci. Technol. 2013, 54, 114–124. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Millán-Linares, M.D.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-Inflammatory and Antioxidant Activity of Hydroxytyrosol and 3,4-Dihydroxyphenyglycol Purified from Table Olive Effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Prior, Á.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Synergistic Effect of 3,4-Dihydroxyphenylglycol with Hydroxytyrosol and α-Tocopherol on the Rancimat Oxidative Stability of Vegetable Oils. Innov. Food Sci. Emerg. Technol. 2019, 51, 100–106. [Google Scholar] [CrossRef]
- Lama-muñoz, A.; Gómez-carretero, A.; Rubio-senent, F.; Bermúdez-oria, A.; Maya, I.; Fernández-bolaños, J.G.; Vioque, B.; Fernández-bolaños, J. Inhibitory Effect of Olive Phenolic Compounds Isolated from Olive Oil By-Product on Melanosis of Shrimps. Antioxidants 2021, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Cardazzo, B.; Novelli, E.; Carraro, L.; Fontana, F.; Currò, S.; Laghetto, M.; Trocino, A.; Xiccato, G.; Taticchi, A.; et al. Employment of Phenolic Compounds from Olive Vegetation Water in Broiler Chickens: Effects on Gut Microbiota and on the Shelf Life of Breast Fillets. Molecules 2021, 26, 4307. [Google Scholar] [CrossRef] [PubMed]
- Bartella, L.; Mazzotti, F.; Talarico, I.R.; Santoro, I.; Di Donna, L. Hydroxytyrosol-fortified Foods Obtained by Supercritical Fluid Extraction of Olive Oil. Antioxidants 2021, 10, 1619. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of Edible Pectin-Fish Gelatin Films Containing the Olive Antioxidants Hydroxytyrosol and 3,4-Dihydroxyphenylglycol on Beef Meat during Refrigerated Storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Nieto, G.; Pateiro, M.; Lorenzo, J.M. Phenolic Compounds Obtained from Olea Europaea By-products and Their Use to Improve the Quality and Shelf Life of Meat and Meat Products—A Review. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Heredia, A.; Guillén, R.; Jimínez, A. Production in Large Quantities of Highly Purified Hydroxytyrosol from Liquid-Solid Waste of Two-Phase Olive Oil Processing or “Alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Rodriguez, M.; Cornejo, V.; Rodriguez-Gutierrez, G.; Monetta, R. Optimization of Low Thermal Treatments to Increase Hydrophilic Phenols in the Alperujo Liquid Fraction. Grasas Aceites 2023, 74, e491. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Rubio-Senent, F.; Villanueva-Lazo, Á.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Application of Thermo-Malaxation Followed by Three-Phase Centrifugation to Enable the Biorefinery of Alperujo, the Main By-Product of Olive Oil. Foods 2023, 12, 4023. [Google Scholar] [CrossRef]
- Gimenez, M.; Rodríguez, M.; Montoro, L.; Sardella, F.; Rodríguez-Gutierrez, G.; Monetta, P.; Deiana, C. Two Phase Olive Mill Waste Valorization. Hydrochar Production and Phenols Extraction by Hydrothermal Carbonization. Biomass Bioenergy 2020, 143, 105875. [Google Scholar] [CrossRef]
- Niknam, S.M.; Kashaninejad, M.; Escudero, I.; Sanz, M.T.; Beltrán, S.; Benito, J.M. Valorization of Olive Mill Solid Residue through Ultrasound-Assisted Extraction and Phenolics Recovery by Adsorption Process. J. Clean. Prod. 2021, 316, 128340. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Prior, Á.F.; Rodríguez-Gutiérrez, G.; Fernández, Á.; Rodríguez-Gutiérrez, G. The Use of Industrial Thermal Techniques to Improve the Bioactive Compounds Extraction and the Olive Oil Solid Waste Utilization. Innov. Food Sci. Emerg. Technol. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Fernández-Prior, M.Á.; Charfi, A.; Bermúdez-Oria, A.; Rodríguez-Juan, E.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Deep Eutectic Solvents Improve the Biorefinery of Alperujo by Extraction of Bioactive Molecules in Combination with Industrial Thermal Treatments. Food Bioprod. Process. 2020, 121, 131–142. [Google Scholar] [CrossRef]
- Gil, K.A.; Tuberoso, C.I.G. Crucial Challenges in the Development of Green Extraction Technologies to Obtain Antioxidant Bioactive Compounds from Agro-Industrial by-Products. Chem. Biochem. Eng. Q. 2021, 35, 105–138. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Uceda, M.; Frías, L. Épocas de Recolección. Evolución del Contenido Graso del Fruto y de la Composición y Calidad del Aceite. In Proceedings of the II Seminario Oleícola International, Córdoba, Spain, 6–17 October 1975; pp. 25–46. [Google Scholar]
- Martínez, L.E.; Rizzo, P.F.; Bres, P.A.; Riera, N.I.; Beily, M.E.; Young, B.J. Compendio de Métodos Analíticos Para La Caracterización de Residuos, Compost y Efluentes de Origen Agropecuario y Agroindustrial Para La Caracterización de Origen Agropecuario y Agroindustrial, 1st ed.; INTA Ediciones: Buenos Aires, Argentina, 2021; 159p, Available online: http://hdl.handle.net/20.500.12123/10587 (accessed on 20 October 2021).
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Iannotti, D.A.; Pang, T.; Toth, B.L.; Elwell, D.L.; Keener, H.M.; Hoitink, H.A.J. A Quantitative Respirometric Method for Monitoring Compost Stability. Compost Sci. Util. 1993, 1, 52–65. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, E.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and Biovalorisation of Olive-Mill Wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.; Mailer, R.; Prenzler, P.D.; Robards, K. Impact of Cultivar, Harvesting Time, and Seasonal Variation on the Content of Biophenols in Olive Mill Waste. J. Agric. Food Chem. 2008, 56, 8851–8858. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; Yousfi, K.; García, P.; García, A.; Garrido, A. Effect of Cultivar and Processing Method on the Contents of Polyphenols in Table Olives. J. Agric. Food Chem. 2004, 52, 479–484. [Google Scholar] [CrossRef]
- Ceci, L.N.; Mattar, S.B.; Carelli, A.A. Chemical Quality and Oxidative Stability of Extra Virgin Olive Oils from San Juan Province (Argentina). Food Res. Int. 2017, 100, 764–770. [Google Scholar] [CrossRef]
- Abaza, L.; Taamalli, A.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutierrérez, A.; Zarrouk, M.; Youssef, N. Ben Changes in Phenolic Composition in Olive Tree Parts According to Development Stage. Food Res. Int. 2017, 100, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Barrena Gómez, R.; Vázquez Lima, F.; Sánchez Ferrer, A. The Use of Respiration Indices in the Composting Process: A Review. Waste Manag. Res. 2006, 24, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cormenzana, A.; Juárez-Jiménez, B.; Garcia-Pareja, M.P. Antimicrobial Activity of Olive Mill Waste-Waters (Alpechin) and Biotransformed Olive Oil Mill Wastewater. Int. Biodeterior. Biodegrad. 1996, 38, 283–290. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Fernández-Bolaños, J.; García-Borrego, A.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G. Influence of PH on the Antioxidant Phenols Solubilised from Hydrothermally Treated Olive Oil By-Product (Alperujo). Food Chem. 2017, 219, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Effect of Steam Treatment of Alperujo on the Composition, Enzymatic Saccharification, and in Vitro Digestibility of Alperujo. J. Agric. Food Chem. 2007, 55, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Yuan, J.; Zhang, C. Adsorption Characteristics of Adsorbent Resins and Antioxidant Capacity for Enrichment of Phenolics from Two-Phase Olive Waste. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 38–46. [Google Scholar] [CrossRef]
- Çelik, G.; Saygın, Ö.; Akmehmet Balcıoğlu, I. Multistage Recovery Process of Phenolic Antioxidants with a Focus on Hydroxytyrosol from Olive Mill Wastewater Concentrates. Sep. Purif. Technol. 2021, 259, 117757. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry Dietary Fiber Functionalized with Phenolic Antioxidants from Olives. Interactions between Polysaccharides and Phenolic Compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef]
| Analyzed Parameter | n | Mean Value ± SD |
|---|---|---|
| pH | 3 | 5.5 ± 0.05 |
| Water content (%) | 3 | 69.0 ± 0.4 |
| Organic matter (%) | 3 | 28.5 ± 2.0 |
| Ash content (%) | 3 | 2.5 ± 0.3 |
| Total soluble phenolics (mg/kg) | 3 | 3121.0 ± 142.2 |
| HT (mg/kg) | 3 | 127.2 ± 54.4 |
| Ty (mg/kg) | 3 | 44.7 ± 9.3 |
| DHPG (mg/kg) | 3 | 5.4 ± 1.2 |
| SRI (mg O2/g VS/h) | 3 | 0.31 ± 0.02 |
| Treatment | Water Content (%) | Organic Matter (%) | Ash (%) | pH | Total Phenolics (mg/kg) | SRI (mg O2/g VS/h) |
|---|---|---|---|---|---|---|
| 70 °C, 45 min | 64.4 ± 1.1 c | 34.2 ± 0.6 b | 1.4 ± 0.3 b | 5.50 ± 0.15 b | 885 ± 63 a | 1.17 ± 0.12 b |
| 70 °C, 90 min | 60.7 ± 0.5 b | 37.6 ± 0.4 b | 1.7 ± 0.4 b | 5.35 ± 0.14 b | 889 ± 71 a | 1.17 ± 0.20 b |
| 70 °C, 45 min, 0.25% H2SO4 w/w | 62.0 ± 0.7 b | 36.4 ± 0.2 b | 1.6 ± 0.2 b | 4.75 ± 0.18 a | 1021 ± 104 b | 0.81 ± 0.09 a |
| 70 °C, 90 min, 0.25% H2SO4 w/w | 56.7 ± 1.1 a | 42.3 ± 0.1 a | 1.0 ± 0.2 a | 4.80 ± 0.10 a | 1073 ± 117 b | 0.79 ± 0.30 a |
| Treatment | Liquid Fraction | |||||
|---|---|---|---|---|---|---|
| Percent of Raw (% w/w) | Insoluble Solids (mg/L) | Phenolics (mg/L) | ||||
| Total | HT | Ty | DHPG | |||
| 70 °C, 45 min | 36 ± 3 a | 18.0 ± 0.7 b | 4230 ± 47 b | 475 ± 30 a | 137 ± 6 c | 43 ± 2 c |
| 70 °C, 90 min | 39 ± 2 a | 23.8 ± 0.5 c | 4334 ± 112 b | 615 ± 19 b | 127 ± 10 c | 37 ± 1 c |
| 70 °C, 45 min, 0.25% H2SO4 w/w | 38 ± 1 a | 11.7 ± 0.2 a | 3472 ± 24 a | 849 ± 33 c | 61 ± 8 b | 27 ± 2 b |
| 70 °C, 90 min, 0.25% H2SO4 w/w | 43 ± 3 b | 13.0 ± 0.4 a | 3551 ± 136 a | 519 ± 18 a | 47 ± 8 a | 16 ± 3 a |
| Treatment | Phenolics in Liquid Fractions (g) | |||
|---|---|---|---|---|
| Total | HT | Ty | DHPG | |
| 70 °C, 45 min | 304 ± 15.3 b | 34.8 ± 3.5 a | 9.5 ± 0.4 c | 3.0 ± 0.3 c |
| 70 °C, 90 min | 338 ± 8.7 c | 47.5 ± 0.6 c | 9.5 ± 0.6 c | 3.0 ± 0.1 c |
| 70 °C, 45 min, 0.25% H2SO4 w/w | 264 ± 6.7 a | 66.1 ± 1.3 d | 4.2 ± 0.5 a | 2.2 ± 0.1 b |
| 70 °C, 90 min, 0.25% H2SO4 w/w | 307 ± 4.2 b | 43.7 ± 0.5 b | 4.0 ± 0.5 b | 1.6 ± 0.2 a |
| Treatment-Derived Liquid Fraction | Liquid Fraction (mL) | Purification System | Total Solids (g) | Total Sugars (mg/g) | Ash Content (mg/g) |
|---|---|---|---|---|---|
| 70 °C, 90 min | 250 | Non-ionic resin | 3.05 ± 0.12 b | 122.8 ± 11.6 c | 1.5 ± 0.1 b |
| 250 | Ionic resin | 0.91 ± 0.07 a | 40.2 ± 3.8 b | 1.5 ± 0.3 b | |
| 250 | Ethyl acetate | 0.83 ± 0.05 a | 29.8 ± 1.4 a | 0.3 ± 0.1 a | |
| 70 °C, 45 min,0.25% H2SO4 w/w | 250 | Non-ionic resin | 2.49 ± 0.17 b | 121.3 ± 13.1 c | 2.5 ± 0.3 c |
| 250 | Ionic resin | 0.55 ±0.09 a | 23.3 ± 4.4 a | 1.7 ± 0.3 b | |
| 250 | Ethyl acetate | 0.78 ±0.15 a | 14.0 ± 1.9 a | 0.6 ± 0.2 a |
| Treatment-Derived Liquid Fraction | Purification System | Phenolics in Ethanol-Reconstituted Fractions (mg/L) | ||||
|---|---|---|---|---|---|---|
| Total | HT | Ty | DHPG | HP/Total | ||
| 70 °C, 90 min | Non-ionic resin | 45,780 ± 938 d | 13,558 ± 677 e | 1696 ± 84 d | 662 ± 33 e | 0.35 |
| Ionic resin | 19,047 ± 276 b | 4763 ± 238 b | 856 ± 42 b | 90 ± 5 b | 0.30 | |
| Ethyl acetate | 16,828 ± 712 b | 6968 ± 348 d | 1020 ± 51 c | 234 ± 22 c | 0.49 | |
| 70 °C, 45 min, 0.25% H2SO4 w/w | Non-ionic resin | 38,844 ± 546 c | 7287 ± 364 d | 1100 ± 55 c | 394 ± 22 d | 0.22 |
| Ionic resin | 9773 ± 307 a | 1714 ± 86 a | 446 ± 22 a | 41 ± 2 a | 0.23 | |
| Ethyl acetate | 14,232 ± 644 b | 5941 ± 297 c | 1001 ± 53 c | 208 ± 9 c | 0.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Márquez, M.; Rodríguez Gutiérrez, G.; Giménez, M.; Rizzo, P.F.; Bueno, L.; Deiana, C.; Monetta, P. Obtaining Phenolic-Enriched Liquid Fractions and Compostable Pomace for Agriculture from Alperujo Using Standard Two-Phase Olive Oil Mill Equipment. Agriculture 2024, 14, 1427. https://doi.org/10.3390/agriculture14081427
Rodríguez Márquez M, Rodríguez Gutiérrez G, Giménez M, Rizzo PF, Bueno L, Deiana C, Monetta P. Obtaining Phenolic-Enriched Liquid Fractions and Compostable Pomace for Agriculture from Alperujo Using Standard Two-Phase Olive Oil Mill Equipment. Agriculture. 2024; 14(8):1427. https://doi.org/10.3390/agriculture14081427
Chicago/Turabian StyleRodríguez Márquez, Manuel, Guillermo Rodríguez Gutiérrez, Marianela Giménez, Pedro Federico Rizzo, Luis Bueno, Cristina Deiana, and Pablo Monetta. 2024. "Obtaining Phenolic-Enriched Liquid Fractions and Compostable Pomace for Agriculture from Alperujo Using Standard Two-Phase Olive Oil Mill Equipment" Agriculture 14, no. 8: 1427. https://doi.org/10.3390/agriculture14081427






