Soil Mineral Nitrogen and Mobile Organic Carbon as Affected by Winter Wheat Strip Tillage and Forage Legume Intercropping
Abstract
:1. Introduction
2. Material and Methods
2.1. Site and Soil
2.2. Design and Details
2.3. Sampling, Preparation, and Analyses
2.4. Statistical Analysis
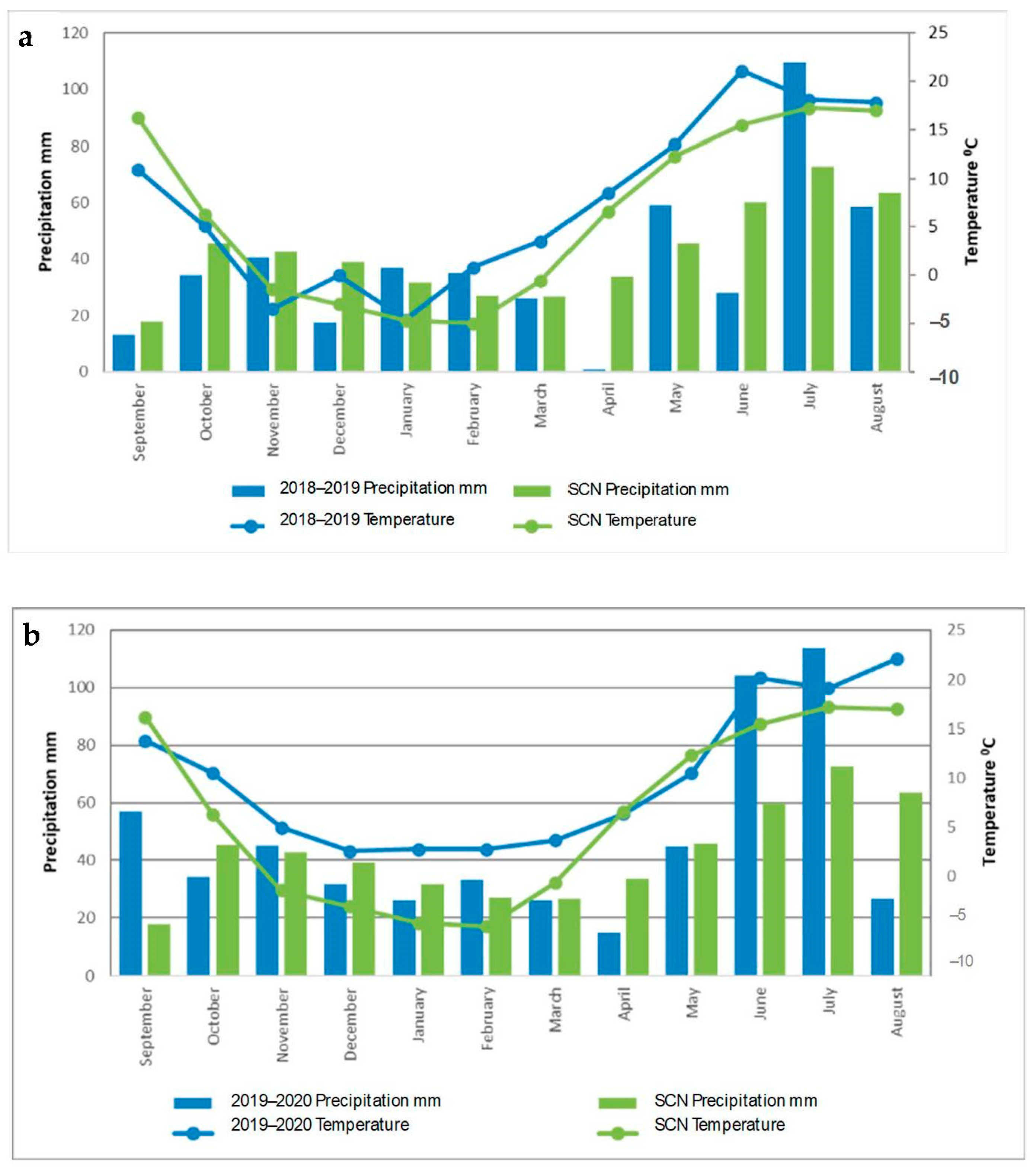
2.5. Meteorological Conditions
3. Results
3.1. Soil Mineral Nitrogen
3.2. Productivity of Winter Wheat
3.3. Forms of Soil Organic Carbon
3.4. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.; Ramanathan, A.L.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Dutta, A.; Bhattacharyya, R.; Jiménez-Ballesta, R.; Dey, A.; Saha, N.D.; Kumar, S.; Nath, C.P.; Prakash, V.; Jatav, S.S.; Patra, A. Conventional and Zero Tillage with Residue Management in Rice–Wheat System in the Indo-Gangetic Plains: Impact on Thermal Sensitivity of Soil Organic Carbon Respiration and Enzyme Activity. Int. J. Environ. Res. Public Health 2022, 20, 810. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C sequestration as a biological negative emission strategy. Front. Clim. 2019, 1, 8. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Gerke, J. Concepts and Misconceptions of Humic Substances as the Stable Part of Soil Organic Matter: A Review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Wolschick, N.H.; Barbosa, F.T.; Bertol, I.; Bagio, B.; Kaufmann, D.S. Long-term effect of soil use and management on organic carbon and aggregate stability. Rev. Bras. Cienc. Solo 2018, 42, e0170393. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, F.; Wei, D.; Zhou, B.; Cai, S.; Li, Y.; Kuang, E.; Sun, L.; Li, L.J. Impacts of Long-term Fertilization on the Molecular Structure of Humic Acid and Organic Carbon Content in Soil Aggregates in Black Soil. Sci. Rep. 2019, 9, 11908. [Google Scholar] [CrossRef]
- Mohinuzzaman, M.; Yuan, J.; Yang, X.; Senesi, N.; Li, S.L.; Ellam, R.M.; Mostofa, K.M.G.; Liu, C.Q. Insights into solubility of soil humic substances and their fluorescence characterisation in three characteristic soils. Sci. Total Environ. 2020, 720, 137395. [Google Scholar] [CrossRef]
- Scaglia, B.; Adani, F. Biodegradability of soil water soluble organic carbon extracted from seven different soils. J. Environ. Sci. 2009, 21, 641–646. [Google Scholar] [CrossRef]
- Mehra, P.; Baker, J.; Sojka, R.E.; Bolan, N.; Desbiolles, J.; Kirkham, M.B.; Ross, C.; Gupta, R. Chapter Five—A Review of Tillage Practices and Their Potential to Impact the Soil Carbon Dynamics. Adv. Agron. 2018, 150, 185–230. [Google Scholar] [CrossRef]
- Lichter, K.; Govaerts, B.; Six, J.; Sayre, K.D.; Deckers, J.; Dendooven, L. Aggregation and C and N contents of soil organic matter fractions in a permanent raised-bed planting system in the Highlands of Central Mexico. Plant Soil 2008, 305, 237–252. [Google Scholar] [CrossRef]
- Levine, U.Y.; Teal, T.K.; Robertson, G.P.; Schmidt, T.M. Agriculture’s impact on microbial diversity and associated fluxes of carbon dioxide and methane. ISME J. 2011, 5, 1683–1691. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.R.; Pu, Y.L.; Li, T.; Xu, X.X.; Jia, Y.X.; Deng, O.P.; Gong, G.S. Dynamics of soil labile organic carbon fractions and C-cycle enzyme activities under straw mulch in Chengdu Plain. Soil Tillage Res. 2016, 155, 289–297. [Google Scholar] [CrossRef]
- Ritson, J.P.; Graham, N.J.D.; Templeton, M.R.; Clark, J.M.; Gough, R.; Freeman, C. The impact of climate change on the treatability of dissolved organic matter (DOM) in upland water supplies: A UK perspective. Sci. Total Environ. 2014, 473, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H.; Wortmann, C.S. Does occasional tillage undo the ecosystem services gained with no-till? A review. Soil Tillage Res. 2020, 198, 104–534. [Google Scholar] [CrossRef]
- Pöhlitz, J.; Rücknagel, J.; Koblenz, B.; Schlüter, S.; Vogel, H.J.; Christen, O. Computed tomography and soil physical measurements of compaction behaviour under strip tillage, mulch tillage and no tillage. Soil Tillage Res. 2018, 175, 205–216. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.H.; Bao, X.G.; Zhang, F.S.; Van DerWerf, W. Intercropping enhances soil carbon and nitrogen. Glob. Change Biol. 2015, 21, 1715–1726. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.J. Global trends in wheat production, consumption and trade. In Wheat Improvement; Reynolds, M.P., Braun, H.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Gollany, H.T. Assessing the effects of crop residue retention on soil health. In Improving Soil Health; Horwath, W., Ed.; Burleigh Dodds Science Publishing: London, UK, 2022; pp. 189–218. [Google Scholar] [CrossRef]
- Gaudin, A.C.M.; Janovicek, K.; Deen, B.; Hooker, D.C. Wheat improves nitrogen use efficiency of maize and soybean-based cropping systems. Agric. Ecosyst. Environ. 2015, 210, 1–10. [Google Scholar] [CrossRef]
- Smith, R.G.; Gross, K.L.; Robertson, G.P. Effects of crop diversity on agroecosystem function: Crop yield response. Ecosystems 2008, 11, 355–366. [Google Scholar] [CrossRef]
- Domnariu, H.; Reardon, C.L.; Manning, V.A.; Gollany, H.T.; Trippe, K.M. Legume cover cropping and nitrogen fertilization influence soil prokaryotes and increase carbon content in dryland wheat systems. Agric. Ecosyst. Environ. 2024, 367, 108–959. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.J.; Li, G.D.; Zhou, D.W. Grass–legume ratio can change soil carbon and nitrogen storage in a temperate steppe grassland. Soil Tillage Res. 2016, 157, 23–31. [Google Scholar] [CrossRef]
- Huynh, H.T.; Hufnagel, J.; Wurbs, A.; Bellingrath-Kimura, S.D. Influences of soil tillage, irrigation and crop rotation on maize biomass yield in a 9-year field study in Müncheberg, Germany. Field Crops Res. 2019, 241, 107–565. [Google Scholar] [CrossRef]
- Udom, B.E.; Omovbude, S. Soil physical properties and carbon/nitrogen relationships in stable aggregates under legume and grass fallow. Acta Ecol. Sin. 2019, 39, 56–62. [Google Scholar] [CrossRef]
- Raimbault, B.A.; Vyn, T.J. Crop rotation and tillage effects on corn growth and soil structural stability. Agron. J. 1991, 83, 979–985. [Google Scholar] [CrossRef]
- Amado, T.J.; Bayer, C.; Conceicao, P.C.; Spagnollo, E.; de Campos, B.H.; da Veiga, M. Potential of carbon accumulation in no-till soils with intensive use and cover crops in southern Brazil. J. Environ. Qual. 2006, 35, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Wei, Y.X.; Li, R.C.; Chen, Z.; Wang, H.D.; Virk, A.L.; Lal, R.; Zhao, X.; Zhang, H.L. Improving soil aggregates stability and soil organic carbon sequestration by no-till and legume-based crop rotations in the North China Plain. Sci. Total Environ. 2022, 847, 157518. [Google Scholar] [CrossRef]
- Hu, L.; Huang, R.; Deng, H.; Li, K.; Peng, J.; Zhou, L.; Ou, H. Effects of different intercropping methods on soil organic carbon and aggregate stability in sugarcane field. Pol. J. Environ. Stud. 2022, 31, 3587–3596. [Google Scholar] [CrossRef]
- De Notaris, C.; Rasmussen, J.; Sørensen, P.; Olesen, J.E. Nitrogen leaching: A crop rotation perspective on the effect of N surplus, field management and use of catch crops. Agric. Ecosyst. Environ. 2018, 255, 1–11. [Google Scholar] [CrossRef]
- Carlos, F.S.; de Sousa, R.O.; Nunes, R.; de Campos Carmona, F.; Cereza, T.; Weinert, C.; Pasa, E.H.; Bayer, C.; de Oliveira Camargo, F.A. Long-term cover crops and no-tillage in Entisol increase enzyme activity and carbon stock and enable the system fertilization in southern Brazil. Geoderma Reg. 2023, 34, 100635. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The effect of digestate fertilisation on grass biogas yield and soil properties in field-biomass-biogas-field renewable energy production approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Volungevičius, J.; Amalevičiūtė, K.; Liaudanskienė, I.; Šlepetienė, A.; Šlepetys, J. Chemical properties of Pachiterric Histosol as influenced by different land use. Zemdirb.-Agric. 2015, 102, 123–132. [Google Scholar] [CrossRef]
- Ponomareva, V.V.; Plotnikova, T.A. Humus and Soil-Forming; Publishing house Nauka: Leningrad, Russia, 1980. [Google Scholar]
- Jokubauskaite, I.; Amaleviciute, K.; Lepane, V.; Slepetiene, A.; Slepetys, J.; Liaudanskiene, I.; Karcauskiene, D.; Booth, C.A. High performance liquid chromatography (HPLC)-size exclusion chromatography (SEC) for qualitative detection of humic substances and natural organic matter in mineral soils and peats in Lithuania. Int. J. Environ. Anal. Chem. 2014, 95, 508–519. [Google Scholar] [CrossRef]
- Nikitin, B.A. A method for soil humus determination. Agric. Chem. 1999, 3, 156–158. [Google Scholar]
- Gecaitė, V.; Arlauskienė, A.; Cesevičienė, J. Competition Effects and Productivity in Oat–Forage Legume Relay Intercropping Systems under Organic Farming Conditions. Agriculture 2021, 11, 99. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Faiz, M.A.; Bana, R.S.; Choudhary, A.K.; Laing, A.M.; Bansal, R.; Bhatia, A.; Bana, R.C.; Singh, Y.; Kumar, V.; Bamboriya, S.D.; et al. Zero tillage, residue retention and system-intensification with legumes for enhanced pearl millet productivity and mineral biofortification. Sustainability 2022, 14, 543. [Google Scholar] [CrossRef]
- Yogi, A.K.; Bana, R.S.; Godara, S.; Sangwan, S.; Choudhary, A.K.; Nirmal, R.C.; Bamboriya, S.; Shivay, Y.S.; Singh, T.; Yadav, A.; et al. Elucidating the interactive impact of tillage, residue retention, and system intensification on pearl millet yield stability and biofortification under rainfed agroecosystems. Agriculture 2023, 10, 1205926. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Dhaka, A.K.; Singh, B.; Kumar, A.; Choudhary, A.K.; Kumar, A.; Kamboj, N.K.; Hasanain, M.; Singh, S.; Bhupenchandra, I.; et al. Productivity, soil health, and carbon management index of soybean-wheat cropping system under double zero-tillage and natural-farming based organic nutrient management in north-Indian plains. Sci. Total Environ. 2024, 917, 170418. [Google Scholar] [CrossRef]
- Nandan, R.; Singh, V.; Singh, S.S.; Kumar, V.; Hazra, K.K.; Nath, C.P.; Poonia, S.; Malik, R.K.; Bhattacharyya, R.; McDonald, A. Impact of conservation tillage in rice–based cropping systems on soil aggregation, carbon pools and nutrients. Geoderma 2019, 340, 104–114. [Google Scholar] [CrossRef]
- Ankit Bana, R.S.; Rana, K.S.; Singh, R.; Godara, S.; Grover, M.; Yadav, A.; Choudhary, A.K.; Singh, T.; Choudhary, M.; Bansal, R.; et al. No-tillage with residue retention and foliar sulphur nutrition enhances productivity, mineral biofortification and crude protein in rainfed pearl millet under Typic Haplustepts. Elucidating the responses imposed on an eight-year long-term experiment. Plants 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Bonilla, D.; Nolot, J.M.; Raffaillac, D.; Justes, E. Cover crops mitigate nitrate leaching in cropping systems including grain legumes: Field evidence and model simulations. Agric. Ecosyst. Environ. 2016, 212, 1–12. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; Van Groenigen, K.J.; Lee, J.; Van Kessel, C. When does no-till yield more? A global meta-analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef]
- Ernst, O.R.; Kemanian, A.R.; Mazzilli, S.R.; Cadenazzi, M.; Dogliotti, S. Depressed attainable wheat yields under continuous annual no-till agriculture suggest declining soil productivity. Field Crops Res. 2016, 186, 107–116. [Google Scholar] [CrossRef]
- Virk, A.L.; Yadav, G.S.; Zhao, X.; Kan, Z.R.; Qi, J.Y.; Ahmad, N.; Lal, R.; Zhang, H.L. Soil Organic Matter and Feeding the Future: Role of Legumes in Managing Soil Organic Matter and Improving Crop Yield, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 259–270. [Google Scholar]
- Guan, S.; An, N.; Zong, N.; He, Y.; Shi, P.; Zhang, J.; He, N. Climate warming impacts on soil organic carbon fractions and aggregate stability in a Tibetan alpine meadow. Soil Biol. Biochem. 2018, 116, 224–236. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Aleksandrova, L.N.; Naidenova, O.A. Laboratory Praxis in Soil Science; Kolos: Leningrad, Russia, 1976; p. 294. [Google Scholar]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Simpson, A.J. Humic Substances in Soils: Are They Really Chemically Distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Bimüller, C.; Kreyling, O.; Kölbl, A.; von Lützow, M.; Kögel-Knabner, I. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Tillage Res. 2016, 160, 23–33. [Google Scholar] [CrossRef]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.R.; McBratney, A.B.; de Moraes Sa, J.C.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Avasthe, R.K.; Yadav, G.S.; Das, A.; Singh, V.K.; Mohapatra, K.P.; Rathore, S.S.; Chandra, P.; Kumar, A. Impact of land configuration and organic nutrient management on productivity, quality and soil properties under baby corn in eastern Himalayas. Sci. Rep. 2020, 10, 16–129. [Google Scholar] [CrossRef]
- Nath, C.P.; Hazra, K.K.; Kumar, N.; Praharaj, C.S.; Singh, S.S.; Singh, U.; Singh, N.P. Including grain legume in rice–wheat cropping system improves soil organic carbon pools over time. Ecol. Eng. 2019, 129, 144–153. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Datta, A.; Choudhury, M.; Sharma, P.C.; Kaulash, P.; Jat, H.S.; Jat, M.L.; Kar, S. Stability of humic acid carbon under conservation agriculture practices. Soil Tillage Res. 2021, 216, 105–240. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Sharma, P.C.; Yadav, A.K.; Choudhary, V.; Gathala, M.K.; Sharma, D.K.; Jat, M.L.; McDonald, A. Climate Smart Agriculture practices improve soil organic carbon pools, biological properties and crop productivity in cereal-based systems of North-West India. Catena 2019, 181, 104059. [Google Scholar] [CrossRef]
- Liaudanskiene, I.; Slepetiene, A.; Velykis, A.; Satkus, A. Distribution of organic carbon in humic and granulodensimetric fractions of soil as influenced by tillage and crop rotation. Est. J. Ecol. 2013, 62, 53–69. [Google Scholar] [CrossRef]
- Jat, H.S.; Datta, A.; Choudhary, M.; Yadav, A.K.; Choudhary, V.; Sharma, P.C.; Gathala, M.K.; Jat, M.L.; McDonald, A. Effects of tillage, crop establishment and diversification on soil organic carbon, aggregation, aggregate associated carbon and productivity in cereal systems of semi-arid Northwest India. Soil Tillage Res. 2019, 190, 128–138. [Google Scholar] [CrossRef] [PubMed]

| Rotation Sequences | |||
|---|---|---|---|
| Main Vegetation Period 2018 (Exp. I) or 2019 (Exp. II) | Autumn 2018 (Exp. I) or 2019 (Exp. II) | Main Vegetation Period 2019 (Exp. I) or 2020 (Exp. II) | Abbreviation |
| Legumes and Their Cultivation Strategy | Soil Tillage and Sowing | Crops | |
| Oats, O (Control) | Conventional deep inversion tillage at depth 23–25 cm (CTS) | Winter wheat WW (CTS) | O–WW(CTS) |
| Black medick, BM | BM–WW(CTS) | ||
| White clover, WC | WC–WW(CTS) | ||
| Egyptian clover, EC | EC–WW(CTS) | ||
| Oats, O | Strip tillage and sowing in oats or forage legumes (STS) | Winter wheat WW (STS) | O–WW(STS) |
| Oats and undersown black medick, O+BM | Black medick and winter wheat bicrop BM+WW (STS) | O+BM–BM+WW(STS) | |
| Oats and undersown white clover, O+WC | White clover and winter wheat bicrop WC+WW (STS) | O+WC–WC+WW(STS) | |
| Oats and undersown Egyptian clover, O+WC | Egyptian clover and winter wheat bicrop EC+WW (STS) | O+EC–EC+WW(STS) | |
| Effects | SMN | Grain Yield | WEOC | HSs | HAs | |||
|---|---|---|---|---|---|---|---|---|
| After WW Sowing | After WW Harvest | After WW Sowing | After WW Harvest | After WW Sowing | After WW Harvest | |||
| Year (Y) | 0.2 | 153.9 ** | 16.7 ** | 7.0 * | 57.3 ** | 23.7 ** | 4.32 ** | 24.3 ** |
| Soil tillage (ST) | 13.3 ** | 0.0 | 0.2 | 0.2 | 1.7 | 0.3 | 8.57 ** | 0.0 |
| Legumes and their cultivation strategy (LCS) | 2.6 | 14.2 ** | 1.9 | 0.2 | 5.5 ** | 7.7 ** | 1.95 | 1.5 |
| Y × ST | 16.5 ** | 0.0 | 0.0 | 0.2 | 0.0 | 2.1 | 5.67 * | 1.6 |
| Y × LCS | 1.7 | 1.3 | 0.2 | 0.6 | 9.0 ** | 7.7 ** | 4.1 * | 2.7 |
| ST × LCS | 0.8 | 11.4 ** | 1.2 | 0.3 | 0.2 | 0.1 | 0.75 | 0.7 |
| Y × ST × LCS | 0.9 | 2.2 | 3.2 * | 0.7 | 1.0 | 0.9 | 0.61 | 0.5 |
| Crops | Grain Yield kg ha−1 | Change | ||
|---|---|---|---|---|
| % | kg ha−1 | |||
| Conventional tillage and sowing of WW | ||||
| O–WW (CTS), control | 3175 ± 163.4 ab | 100 | 0 | |
| BM–WW (CTS) | 4676 ± 401.3 cd | 147 | 1501 cd | |
| WC–WW (CTS) | 5029 ± 395.2 d | 158 | 1854 d | |
| EC–WW (CTS) | 4693 ± 346.8 cd | 148 | 1519 cd | |
| Mean | 4393 ± 326.7 B | 100 | 0 | |
| Strip tillage and sowing of WW | ||||
| O–WW(STS) | 2944 ± 345.4 a | 93 | −231 a | |
| O+BM–BM+WW (STS) | 3070 ± 309.5 ab | 97 | −105 ab | |
| O+WC–WC+WW (STS) | 2827 ± 294.5 a | 89 | −348 a | |
| O+EC–EC+WW (STS) | 3497 ± 407.6 b | 110 | 322 b | |
| Mean | 3084 ± 339.3 A | 70 | 1309 | |
| Mean of years | 2019 | 2978 ± 263.1 A | 100 | 0 |
| 2020 | 4499 ± 275.2 B | 151 | 1521 | |
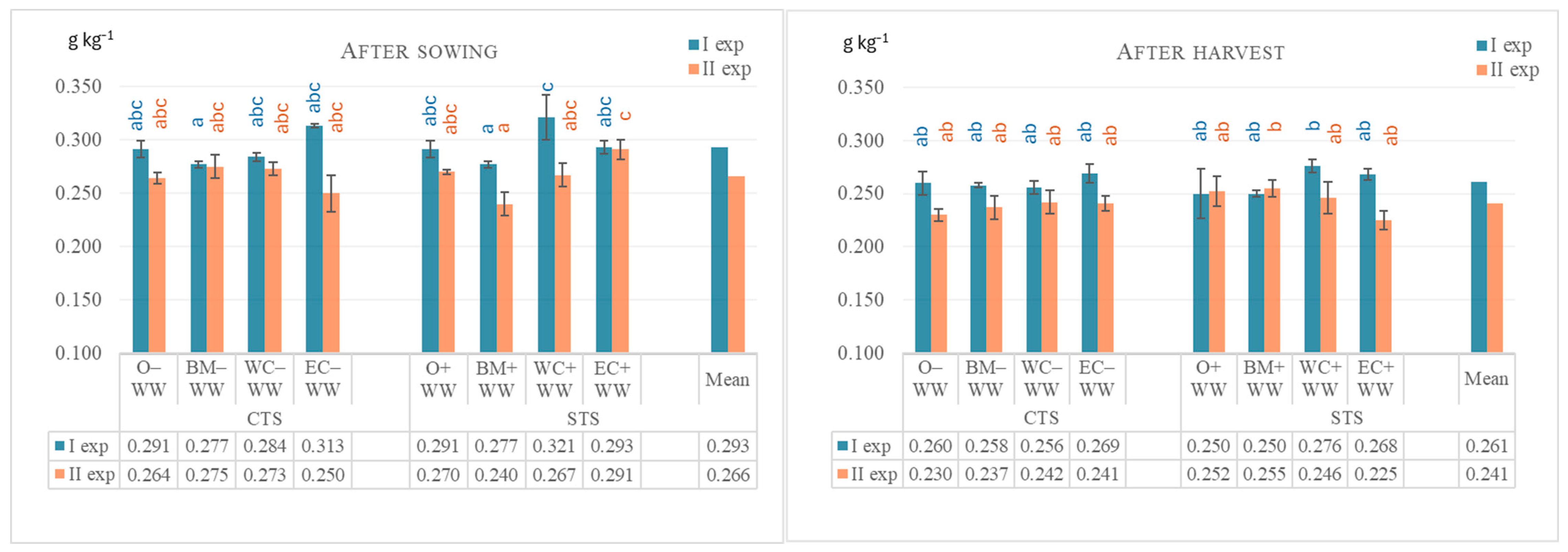
| Crops | Mobile Humic Substances % | ||||
|---|---|---|---|---|---|
| After WW Sowing | After WW Harvest | ||||
| Exp I | Exp II | Exp I | Exp II | ||
| Before experiments | 0.249 ± 0.016 | 0.276 ± 0.020 | |||
| O–WW (CTS) | 0.243 ± 0.002 a | 0.280 ± 0.009 ab | 0.288 ± 0.008 a | 0.356 ± 0.007 abc | |
| BM–WW (CTS) | 0.227 ± 0.013 a | 0.326 ± 0.003 d | 0.308 ± 0.018 ab | 0.386 ± 0.005 bc | |
| WC–WW (CTS) | 0.265 ± 0.006 abc | 0.317 ± 0.006 bcd | 0.347 ± 0.005 cde | 0.388 ± 0.005 bc | |
| EC–WW (CTS) | 0.289 ± 0.008 bc | 0.279 ± 0.009 a | 0.343 ± 0.009 cde | 0.337 ± 0.011 a | |
| O–WW (STS) | 0.243 ± 0.002 a | 0.297 ± 0.005 abcd | 0.293 ± 0.012 a | 0.341 ± 0.009 a | |
| O+BM–BM+WW (STS) | 0.237 ± 0.009 a | 0.316 ± 0.003 bcd | 0.319 ± 0.011 abc | 0.354 ± 0.007 abc | |
| O+WC–WC+WW (STS) | 0.284 ± 0.012 bc | 0.316 ± 0.008 bcd | 0.340 ± 0.008 bcde | 0.397 ± 0.009 c | |
| O+EC–EC+WW (STS) | 0.290 ± 0.015 c | 0.305 ± 0.015 abcd | 0.361 ± 0.005 e | 0.319 ± 0.017 a | |
| Mean: | |||||
| Year × Tillage/sowing | CTS | 0.256 ± 0.009 A | 0.300 ± 0.008 B | 0.322 ± 0.010 A | 0.367 ± 0.008 B |
| STS | 0.264 ± 0.010 A | 0.308 ± 0.006 B | 0.328 ± 0.009 A | 0.353 ± 0.011 B | |
| Year × Crop | O | 0.243 ±0.002 A | 0.289 ± 0.008 B | 0.291 ± 0.010 A | 0.349 ± 0.008 B |
| BM | 0.232 ± 0.011 A | 0.321 ± 0.004 B | 0.314 ± 0.014 A | 0.370 ± 0.009 B | |
| WC | 0.275 ± 0.010 B | 0.317 ± 0.007 B | 0.344 ± 0.006 B | 0.393 ± 0.007 B | |
| EC | 0.290 ± 0.011 B | 0.292 ± 0.013 B | 0.352 ± 0.008 B | 0.328 ± 0.013 B | |
| Experiment | 0.260 | 0.305 | 0.325 | 0.360 | |
| Crops | Mobile Humic Acids % | ||||
|---|---|---|---|---|---|
| After WW Sowing | After WW Harvest | ||||
| Exp I | Exp II | Exp I | Exp II | ||
| Before experiments | 0.121 ± 0.001 | 0.091 ± 0.022 | |||
| O–WW (CTS) | 0.099 ± 0.002 abc | 0.087 ± 0.011 a | 0.108 ± 0.008 a | 0.178 ± 0.009 ab | |
| BM–WW (CTS) | 0.087 ± 0.019 a | 0.125 ± 0.016 abcd | 0.111 ± 0.018 a | 0.161 ± 0.032 ab | |
| WC–WW (CTS) | 0.109± 0.007 abc | 0.112 ± 0.024 abcd | 0.131 ± 0.005 ab | 0.213 ± 0.018 b | |
| EC–WW (CTS) | 0.125 ± 0.006 bc | 0.089 ± 0.020 ab | 0.142 ± 0.005 bcd | 0.151 ± 0.011 ab | |
| O–WW (STS) | 0.099 ± 0.002 abc | 0.134 ± 0.007 cd | 0.108 ± 0.015 a | 0.174 ± 0.016 ab | |
| O+BM–BM+WW (STS) | 0.087 ± 0.011 a | 0.129 ± 0.017 bcd | 0.125 ± 0.005 ab | 0.165 ± 0.019 ab | |
| O+WC–WC+WW (STS) | 0.119 ± 0.011 bc | 0.144 ± 0.015 d | 0.141 ± 0.012 bcd | 0.165 ± 0.031 ab | |
| O+EC–EC+WW (STS) | 0.129 ± 0.013 c | 0.130 ± 0.017 bcd | 0.163 ± 0.011 d | 0.158 ± 0.016 ab | |
| Mean: | |||||
| Year × Tillage/sowing | CTS | 0.105 ± 0.006 A | 0.103 ± 0,009 A | 0.123 ± 0.006 A | 0.176 ± 0.011 B |
| STS | 0.108 ± 0.007 A | 0.134 ± 0.006 B | 0.134 ± 0.008 A | 0.165 ± 0.009 B | |
| Year × Crop | O | 0.099 ± 0.002 A | 0.111 ± 0.012 A | 0.108 ± 0.008 A | 0.176 ± 0.009 B |
| BM | 0.087 ± 0.010 A | 0.127 ±0.010 B | 0.118 ± 0.009 A | 0.163 ± 0.016 B | |
| WC | 0.114 ± 0.006 A | 0.128 ± 0.014 B | 0.136 ± 0.006 A | 0.189 ± 0.020 B | |
| EC | 0.127 ± 0.007 B | 0.110 ± 0.015 A | 0.152 ± 0.007 B | 0.155 ± 0.009 B | |
| Experiment | 0.107 | 0.119 | 0.129 | 0.171 | |
| Variable | WEOC | HSs | HAs | WEOC | HSs | HAs | SMN | Grain Yield | |
|---|---|---|---|---|---|---|---|---|---|
| CTS | After WW Sowing | After WW Harvest | |||||||
| After WW sowing | WEOC | 1.000 | |||||||
| HSs | −0.013 | 1.000 | |||||||
| HAs | 0.309 | 0.525 | 1.000 | ||||||
| After WW harvest | WEOC | 0.710 | −0.227 | 0.082 | 1.000 | ||||
| HSs | 0.024 | 0.714 | 0.274 | −0.226 | 1.000 | ||||
| HAs | −0.200 | 0.573 | −0.058 | −0.393 | 0.785 | 1.000 | |||
| SMN | 0.188 | −0.241 | −0.009 | 0.210 | 0.142 | −0.044 | 1.000 | ||
| Grain yield | −0.331 | 0.662 | 0.074 | −0.196 | 0.639 | 0.538 | −0.106 | 1.000 | |
| STS | |||||||||
| After WW sowing | WEOC | 1.000 | |||||||
| HSs | 0.001 | 1.000 | |||||||
| HAs | 0.046 | 0.900 | 1.000 | ||||||
| After WW harvest | WEOC | 0.113 | 0.012 | 0.012 | 1.000 | ||||
| HSs | −0.108 | 0.527 | 0.446 | 0.330 | 1.000 | ||||
| HAs | 0.028 | 0.555 | 0.439 | 0.290 | 0.728 | 1.000 | |||
| SMN | −0.351 | 0.213 | −0.016 | −0.312 | 0.304 | 0.361 | 1.000 | ||
| Grain yield | −0.340 | 0.364 | 0.153 | −0.435 | 0.140 | 0.393 | 0.792 | 1.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gecaite, V.; Ceseviciene, J.; Arlauskiene, A. Soil Mineral Nitrogen and Mobile Organic Carbon as Affected by Winter Wheat Strip Tillage and Forage Legume Intercropping. Agriculture 2024, 14, 1490. https://doi.org/10.3390/agriculture14091490
Gecaite V, Ceseviciene J, Arlauskiene A. Soil Mineral Nitrogen and Mobile Organic Carbon as Affected by Winter Wheat Strip Tillage and Forage Legume Intercropping. Agriculture. 2024; 14(9):1490. https://doi.org/10.3390/agriculture14091490
Chicago/Turabian StyleGecaite, Viktorija, Jurgita Ceseviciene, and Ausra Arlauskiene. 2024. "Soil Mineral Nitrogen and Mobile Organic Carbon as Affected by Winter Wheat Strip Tillage and Forage Legume Intercropping" Agriculture 14, no. 9: 1490. https://doi.org/10.3390/agriculture14091490







