Abstract
Antimony (Sb) is a toxic trace element for plants and animals. With the development of industrial applications and mining, Sb pollution is becoming more serious. Phytoremediation is regarded as an eco-friendly technique to reduce the threat of Sb to the environment and human health, and tall fescue that is highly adaptable to heavy metal stress can be a candidate species for Sb-contaminated soil phytoremediation. However, the mechanism of the Sb stress response in tall fescue is not clear. Therefore, transcriptomic analysis was used in this study to reveal the molecular mechanisms of Sb stress response regulation in tall fescue. The results suggested that the roots and leaves of tall fescue responded to Sb stress in different ways. In roots, the lignin and flavonoids might reduce the toxicity of Sb by anti-oxidation and Sb chelation. At the same time, the DEGs in leaves were mainly enriched in the pathways of glutathione metabolism, β-alanine metabolism, and glycine, serine, and threonine metabolism. Additionally, genes related to the pathways, such as 4CL, GST, AGXT2, and ALDH7A1, especially cytochrome P450 family genes (e.g., CYP73A, CYP75A, and CYP98A), might play key roles in the regulation of the Sb stress response in tall fescue. These findings provided a theoretical reference for the efficient use of tall fescue to control Sb-contaminated soil in the future.
1. Introduction
Sb is a metalloid element with potential toxicity and carcinogenicity for living organisms [1]. The mining and smelting of Sb ore, as well as the application of Sb and its compounds, will cause serious Sb pollution in the environment. China is the world’s largest Sb reservoir and the most important producer of Sb, and the exploitation quantity of Sb is about 50% of the world’s production [2]. Sb tailings leakage and Sb mining will induce soil pollution, which restricts soil utilization. Meanwhile, Sb in the soil is easily transferred into the food chain through the absorption of plants, which threatens the health of humans. Substances containing Sb have toxic effects on the human respiratory system, causing diseases such as Sb pneumoconiosis and chronic bronchitis and causing damage to the heart, gastrointestinal tract, and skin. In addition, Sb and its compounds may also be carcinogenic and genotoxic [3]. Therefore, the management of soil pollution caused by Sb should be careful. Sb in the environment can be subdivided into organic and inorganic forms and has four valence states (+3, −3, 0, 5). Among these states, trivalent Sb is the most noxious, followed by pentavalent Sb, and finally, organic Sb [4]. The management methods for Sb in Sb-contaminated areas include solidification/stabilization, cement kiln, photocatalytic degradation, and bioremediation [5,6,7]. In these different ways, bioremediation technology is environmentally friendly and sustainable. Plants are usually less susceptible to Sb toxicity than microorganisms and animals [8], the cost of phytoremediation is low, and there is little secondary pollution. Therefore, in situ green remediation technology is considered to be among the most appropriate methods to deal with mine waste pollution [9].
Studies have shown that Amorpha fruticosa L. [10], Catalpa bungei C. A. Mey. [11], and Broussonetia papyrifera (L.) L’Hér. ex Vent. [12] have strong enrichment capacity for Sb. However, there are limited studies on herbaceous plants, especially the grass family. The family Poaceae has the advantages of a fast growth rate, high density, and strong stress resistance, so it may be tolerant to heavy metal (HM) stress [13]. Festuca arundinacea is a perennial cold-season turf grass with less stringent requirements on soil and climate [14], and it also has a long green period [15]. Owing to the deep roots of tall fescue, it has a wide range of adaptability to the environment [16]. Compared to other cold-season grasses, it is tolerant to heat, drought, shade, and disease stresses [17,18], which makes it possible for tall fescue to have robust tolerance and rejection of HMs. Numerous studies have proved that tall fescue is tolerant to various HM stresses. Through the evaluation of cadmium tolerance among Festuca arundinacea Schreb., Lolium perenne L., Poa pratensis L., and Agrostis stolonifera L., it was shown that tall fescue had the strongest tolerance [19]. Moreover, Festuca arundinacea is also more tolerant of lead stress than Cynodon dactylon (L.) Persoon and Lolium perenne L. [20]. In addition, tall fescue is invoked as a remediation plant to control chromium pollution [21]. Reports indicate that tall fescue exhibits a notable capacity for the translocation of heavy metals, including lead (Pb), cadmium (Cd), copper (Cu), zinc (Zn), nickel (Ni), and chromium (Cr), in the vicinity of Daqingshan Mountain in Inner Mongolia, China [22]. In the context of Beijing, China, tall fescue demonstrates a significant translocation factor for chromium (Cr), nickel (Ni), copper (Cu), and zinc (Zn) [23]. Likewise, this species has shown efficacious remediation capability for lead (Pb), zinc (Zn), chromium (Cr), and cadmium (Cd) in the rehabilitation of abandoned gold mine tailings in Beijing [24]. Therefore, it is speculated that tall fescue should be forbearing to Sb stress and that it is feasible to apply for Sb-polluted soil remediation.
However, the mechanisms of Sb stress response in tall fescue remain unclear, which hinders the application of tall fescue in Sb-contaminated phytoremediation. By analyzing the transcriptome data, unknown or rare genes can be discovered, and potential major genes controlling specific traits can be found [25]. With this technology, pathways, such as the plant hormone signal pathway, the hydrogen peroxide signaling pathway, and the ethylene pathway, and genes, such as MKK9 and PYR/PYL, were identified to be involved in the cadmium stress response of Erigeron acris L. and Tagetes erecta L. [26,27]. In transcriptome analysis of reed (Phragmites australis) under copper stress, it was found that ribosome and chaperon-related genes were significantly up-regulated, while photosynthesis-related genes were significantly down-regulated, indicating that photosynthesis of reed under copper stress was inhibited, and reed mainly responded to copper toxicity through chaperon-related methods [28]. So, the main technology used in this study is transcriptome sequencing to analyze the gene expression in tall fescue under the Sb stress conditions. The purpose of this study is to identify the differentially expressed genes in the root and leaves of tall fescue under Sb stress and analyze the molecular mechanism differences in the roots and leaves in response to Sb stress, then provide an important theoretical basis for the application of tall fescue to remediate the Sb-contaminated soil.
2. Materials and Methods
2.1. Plant Growth and Treatment
Two-month seedlings of tall fescue ‘K31’ were used as the plant material in this study. The seedlings were grown in plastic pots (12 cm diameter × 12 cm height) filled with a soil mixture (nutritive soil–sandy soil = 1:1, v/v). The growth conditions were 25/20 °C (day/night) with a photoperiod of 14/10 h (day/night). The plants were watered with 50 mL, 200 μg/L Sb potassium tartrate solution every other day, and the same amount of distilled water was adjusted as a control group. Three replicates were established in each group. After 72 h of treatment, 0.5 g of leaves and roots were sampled, respectively (three replicates for each treatment and tissue). The Illumina NovaSeq 6000 (Illumina, San Diego, CA, USA) platform was used for transcriptome sequencing. In order to preserve the genetic stability of the biological materials, the samples were preserved at ultra-low temperatures (−80 °C). For physiological analysis, 0.3 g of leaves were sampled after Sb treatment for 18 d.
2.2. Measurement of Physiological Index
2.2.1. Determination of Chlorophyll Content
A total of 0.1 g of leaves were submerged in 10 mL DMSO (dimethyl sulfoxide) and treated in the dark for 72 h. After that, the light absorption values of each sample at 645 nm and 663 nm were measured by a spectrophotometer, and the chlorophyll content was calculated according to the following formula:
where A645 and A663 are the absorbances of 645 and 663 nm, respectively; Ca is chlorophyll a content (mg/L); Cb is chlorophyll b content (mg/L); and Ct is the total chlorophyll content (mg/L) [29].
Ca = 12.72A663 − 2.59A645
Cb = 22.88A645 − 4.67A663
Ct = Ca + Cb
2.2.2. Determination of Peroxidase (POD) and Catalase (CAT) Activities
For the measurement of antioxidative enzyme activities, 0.2 g of the leaf tissues were ground in liquid nitrogen, and then 4.0 mL of pre-cooled phosphate buffer (pH 7.8) was added and homogenized. The homogenates were transferred into 5 mL tubes. After centrifugation at 12,000 rpm at 4 °C for 15 min, the supernatant was stored at 4 °C for enzymatic assays as the crude enzyme preparation.
The reaction mixture for the POD activity assay contained 3 mL of potassium phosphate buffer (0.1 M, pH 6.0) mixed with 28 μL of guaiacol, 30%, 19 μL of 30% H2O2, and 20 μL of enzyme extract [30]. The reaction mixture for the CAT activity assay contained 2 mL of potassium phosphate buffer (0.1 M, pH 7.0), 0.5 mL of 0.1 M H2O2, and 100 μL enzyme extract. Since the addition of H2O2, the absorbance changes were monitored for 180 s at 470 nm and 240 nm for calculating the POD and CAT activities, respectively [31].
2.2.3. Measurement of Electrical Leakage (EL)
Cutting 0.1 g of fresh leaves and placing them in a 50 mL centrifuge tube containing 25 mL deionized water. Then, the centrifuge tubes were oscillated for 24 h in a shaker (Honour, Tianjin, China). The initial conductivity (Ci) was measured with a conductivity meter (Qiwei, Hangzhou, China). The centrifuge tube was then placed at 121 °C to autoclave the leaf tissue for 20 min to completely release the electrolyte. The electrical conductivity (Cmax) was measured again after the solution was cooled to 25 °C. EL is calculated by the following formula: EL (%) = Ci/Cmax × 100%.
2.2.4. Measurement of Plant Height, Root Length, and Biomass
Finally, the plant height and root length of tall fescue were observed, recorded, and accurately measured using vernier calipers. Simultaneously, the biomass of the plants was further measured, both above and below ground. The effects of antimony stress on plant height, root length, and biomass of tall fescue were analyzed by comparing the data of different treatment groups and control groups.
2.3. RNA Extraction and Preparation of cDNA Libraries
The polysaccharide polyphenol total RNA extraction kit (QIAGEN, Düsseldorf, Germany) was used to extract and purify the total RNA of roots and leaves from all samples following the manufacturer’s manual. The total RNA was quantified and assessed with an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA).
Initially, oligo(dT) magnetic beads were used for enriching mRNA with a poly-A tail. Subsequently, divalent cations were used for the fragmentation of the mRNA in the NEB Fragmentation Buffer. Random oligonucleotides were then used for the synthesis of the first cDNA strand under the M-MuLV Reverse Transcriptase (RNase H) system, followed by the use of RNase H and DNA Polymerase I to synthesize the second strand and yield blunt ends. The 3′ fragment ends then underwent adenylation, followed by ligation to NEBNext hairpin loop adaptors. Subsequently, the AMPure XP beads were used to select 250–300 bp cDNA fragments, after which the purified cDNA molecules were amplified by PCR. The PCR products were purified again using AMPure XP beads to establish a library. The qualified libraries assessed on the Agilent 2100 bioanalyzer were sequenced on the Illumina NovaSeq 6000 (Illumina, San Diego, CA, USA) [32].
2.4. Raw Sequence Procession, Assembly and Functional Annotation
Raw short reads of Illumina RNA-seq were filtered by removing reads containing adaptors, reads containing N (N indicates that the base information cannot be determined), and low-quality reads. Simultaneously, GC content levels, Q20, and Q30 of the clean data were evaluated. Clean-read assembly was accomplished using Trinity. Unigenes were obtained by Corset hierarchical clustering of all assembled transcripts from total samples.
The functions of the unigenes were annotated using a series of databases, including NCBI non-redundant protein sequences (NR), the KEGG Ortholog database (KO), Gene Ontology (GO), NCBI non-redundant nucleotide sequences (Nt), the Protein Family (Pfam), Clusters of Orthologous Groups of Proteins (KOG/COG), and Swiss-Prot.
2.5. Gene Expression Level Quantification and Differential Expression Analysis
The transcriptome obtained by Trinity is used as a reference transcriptome (Ref). All clean reads of Illumina RNA-seq were each mapped to the reference transcriptome using RSEM software (version 1.2.15). Gene expression levels were calculated using the fragments per kilobase of transcript sequence per million base pairs sequenced (FPKM) method. The intra-group and inter-group correlation coefficients were calculated based on the FPKM values of all genes in the sample, sample differences between groups, and sample duplication within groups visualized as heat maps. Differentially expressed transcripts were identified using EdgeR at a |log2FC(FoldChange)| > 1 and p adj < 0.05, log2FC > 0 for up-regulated transcripts, and Log2 FC < 0 for down-regulated transcripts.
2.6. Function Assessment of DEGs
The GO enrichment analysis was implemented to assess the functions of DEGs using clusterProfiler (Version 3.0.3). GO enrichment was significant at p < 0.05. The KEGG pathways were applied to enrich the DEGs with different biochemical metabolic pathways and signal transduction pathways using clusterProfiler (Version 3.0.3). All KEGG pathways that had a p < 0.05 were regarded as being significantly enriched.
2.7. Statistical Analysis
The physiological data were analyzed by one-way ANOVA using SPSS 13.0 for Windows (p < 0.05 was significant).
3. Results
3.1. Effects of Sb on the Physiology of Tall Fescue
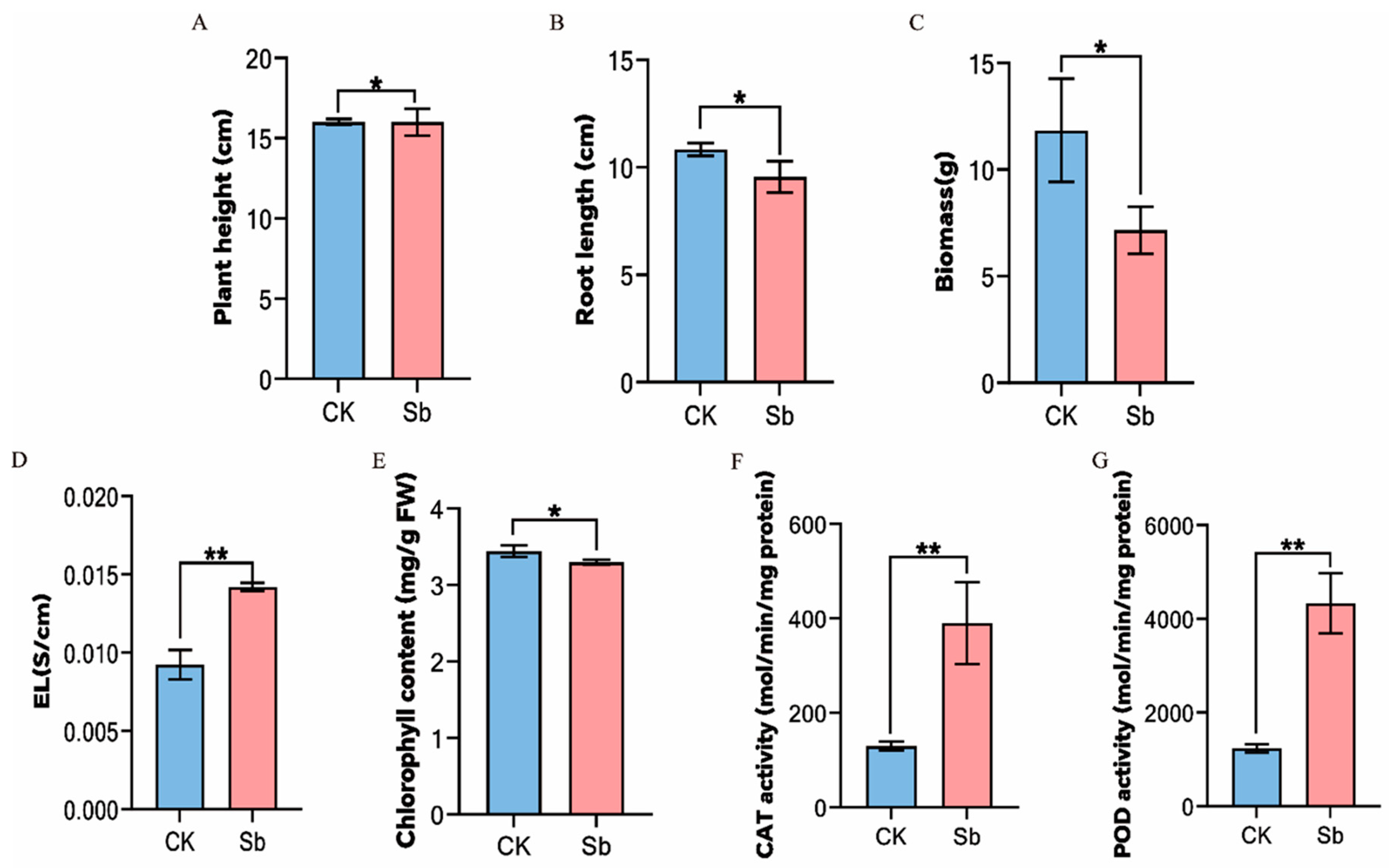
To investigate the physiological changes in tall fescue after Sb treatment, the plant height, root length, biomass, antioxidant enzyme activities, chlorophyll content, and EL were measured. Compared with control, Sb significantly increased the EL of tall fescue by 1.54-fold and the chlorophyll content by 1.04-fold (Figure 1D,E). The results indicated that osmotic stress may occur in tall fescue under Sb treatment, and the accumulation of heavy metal ions may disturb the ion balance in the plant. In addition, it also affected the photosynthesis of tall fescue. The CAT enzyme activity significantly increased by 3-fold following Sb treatment, with POD activity increasing by about 3.51-fold (Figure 1F,G). The increased activity of CAT and POD reflected that plant cells reduce oxidative damage by mobilizing the antioxidant enzyme system to clear excess ROS produced by Sb stress. Most importantly, the height of tall fescue plants under Sb stress did not change significantly, but the root length and biomass decreased significantly by 1.13 times and 1.66 times, respectively (Figure 1A–C). The results showed that Sb had different effects on the roots and leaves of tall fescue, which might be related to the response mode of the roots and leaves to Sb.
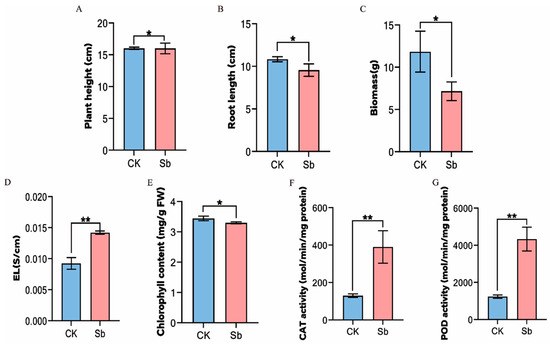
Figure 1.
Physiological changes in tall fescue under Sb stress. (A) Plant height. (B) Root length. (C) Biomass. (D) EL (electrical leakage). (E) Chlorophyll content. (F) Catalase (CAT) activity. (G) Peroxidase (POD) activity. CK = control. Sb = treatment with 200 mg/L potassium antimony tartrate for 18 days. * represents p < 0.05, ** represents p < 0.01.
3.2. Sequence Analysis and Assembly
To understand the in-depth underlying molecular mechanisms of Sb stress tolerance in tall fescue, RNA-sequencing of the Sb-treated leaves and roots was performed. In total, each RNA-seq sample generated more than 20,219,000 clean reads. The average GC content ranged from 53.01% to 55.18%. Q20 values range from 95.87 to 97.4 (an error percent of 3%), and Q30 values range from 89.81 to 92.96 (Table 1). The high-quality sequencing results obtained were appropriate for further analysis.

Table 1.
Sequencing quality statistics table.
Furthermore, to ensure the reliability and completeness of the transcriptome assembly, N50 statistics and BUSCO analysis were performed. The assembly achieved an N50 value of 1340 bp (Table S4), reflecting the presence of longer contigs and, thus, a high-quality assembly. The BUSCO analysis revealed that 76.4% of the BUSCOs were completely detected, with 13.8% being single-copy and 62.6% being duplicated. This indicates that our transcriptome assembly exhibits a high level of integrity, and the assembly quality is excellent (Table S5).
3.3. Identification of Differentially Expressed Genes (DEGs)
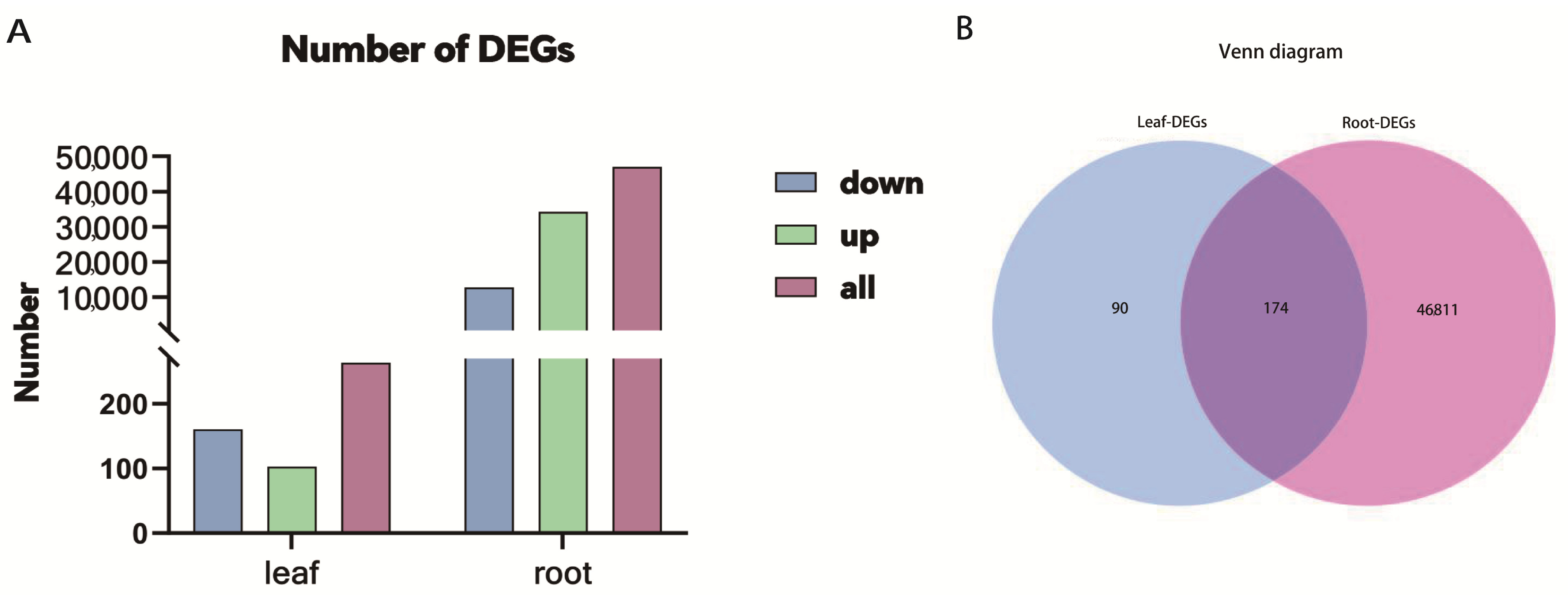
The filtered clean reads from Illumina RNA-seq were mapped to the reference transcriptome. In total, 59.61–66.18% of clean reads were mapped to the reference sequence (Table 2). The p < 0.05 or |log2FoldChange| ≥ 1 was used to determine the DEGs. In total, 46,985 DEGs were identified in roots. Among these, 34,246 DEGs were up-regulated, and 12,739 DEGs were down-regulated. However, only 264 DEGs were identified in the leaves after Sb treatment. Among them, 103 were up-regulated, and 161 were down-regulated (Figure 2A). The number of DEGs in roots was much higher than that in leaves, indicating that roots should play crucial roles in the Sb stress response in tall fescue. Furthermore, the Venn diagram analysis displayed that 174 DEGs were co-expressed in roots and leaves, 46,811 DEGs were expressed specifically in roots, and 90 were expressed specifically in leaves (Figure 2B).

Table 2.
Clean reads mapping rate.
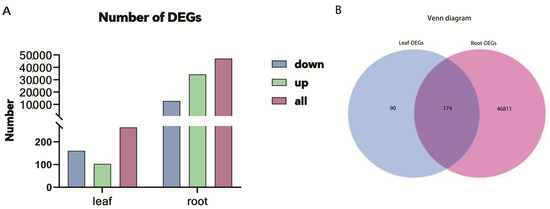
Figure 2.
Basic conditions of DEGs in the roots and leaves of tall fescue. (A) The number of differentially expressed genes (DEGs) up-regulated, down-regulated, and total in the roots and leaves of tall fescue. (B) Venn plot of the DEGs of tissue comparisons.
3.4. Functional Annotation and Classification
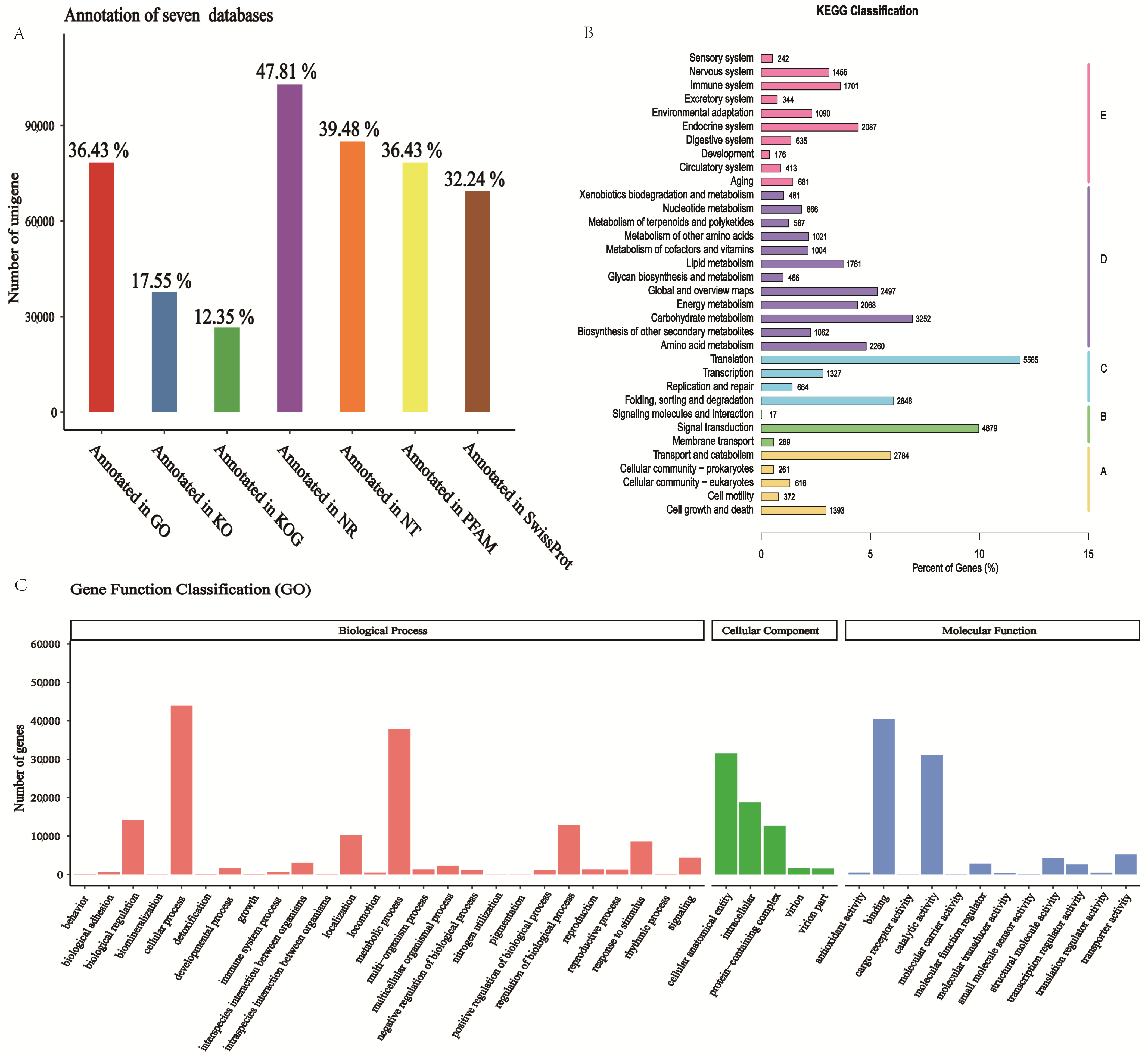
As shown in Table S6, in total, 215,107 unigenes were annotated; there were 102,863 unigenes annotated in the Nr database, 78,372 unigenes annotated in the GO database, and 37,752 unigenes annotated in the KEGG database. A total of 133,956 (62.27%) were annotated in at least one database. The success rate of functional annotation in the seven databases is presented in Figure 3A.
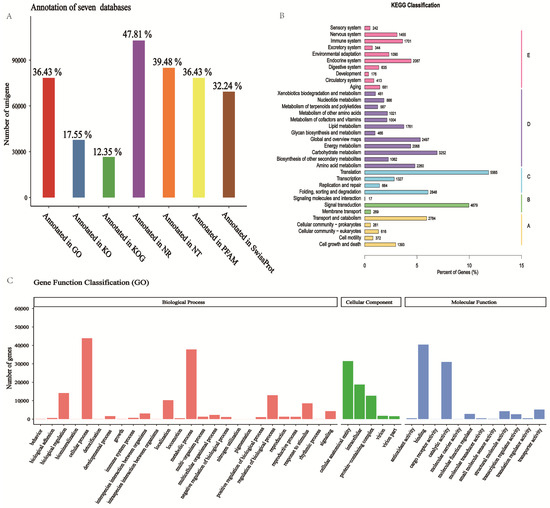
Figure 3.
Functional annotation of unigenes. (A) The annotation rate of unigenes in the seven databases. (B) Classification situation of unigenes that are annotated to the KEGG database. A: Cellular Processes; B: Environmental Information Processing; C: Genetic Information Processing; D: Metabolism; E: Organismal Systems. These are Level 1 categories in KEGG Pathway Hierarchy. (C) Classification situation of unigenes that are annexed to the GO database.
For GO analysis, the most representative terms under BP (biological process) were ‘cellular process’, ‘metabolic process’, and ‘biological regulation’. Under cellular component (CC), the most functional terms were ‘cellular anatomical entity’ and ‘intracellular’. For molecular function (MF), ‘binding’ and ‘catalytic activity’ were the most significant terms in the MF (molecular function) (Figure 3C).
To further profile the pathways of the unigenes, a KEGG analysis was conducted. The result showed that a total of 46,944 unigenes were classified into five metabolic pathways (Hierarchy 1). Among these pathways, ‘translation’ was the group with the greatest number of genes (5565, 11.85%), followed by ‘signal transduction’ (4679, 9.97%) and ‘transport and catabolism’ (2784, 5.93%). Ribosome and protein processing in endoplasmic reticulum pathways belong to genetic information processing, and carbon metabolism belongs to metabolism (Figure 3B, Table S1).
3.5. Functions and Processes Influenced by the Sb Treatment
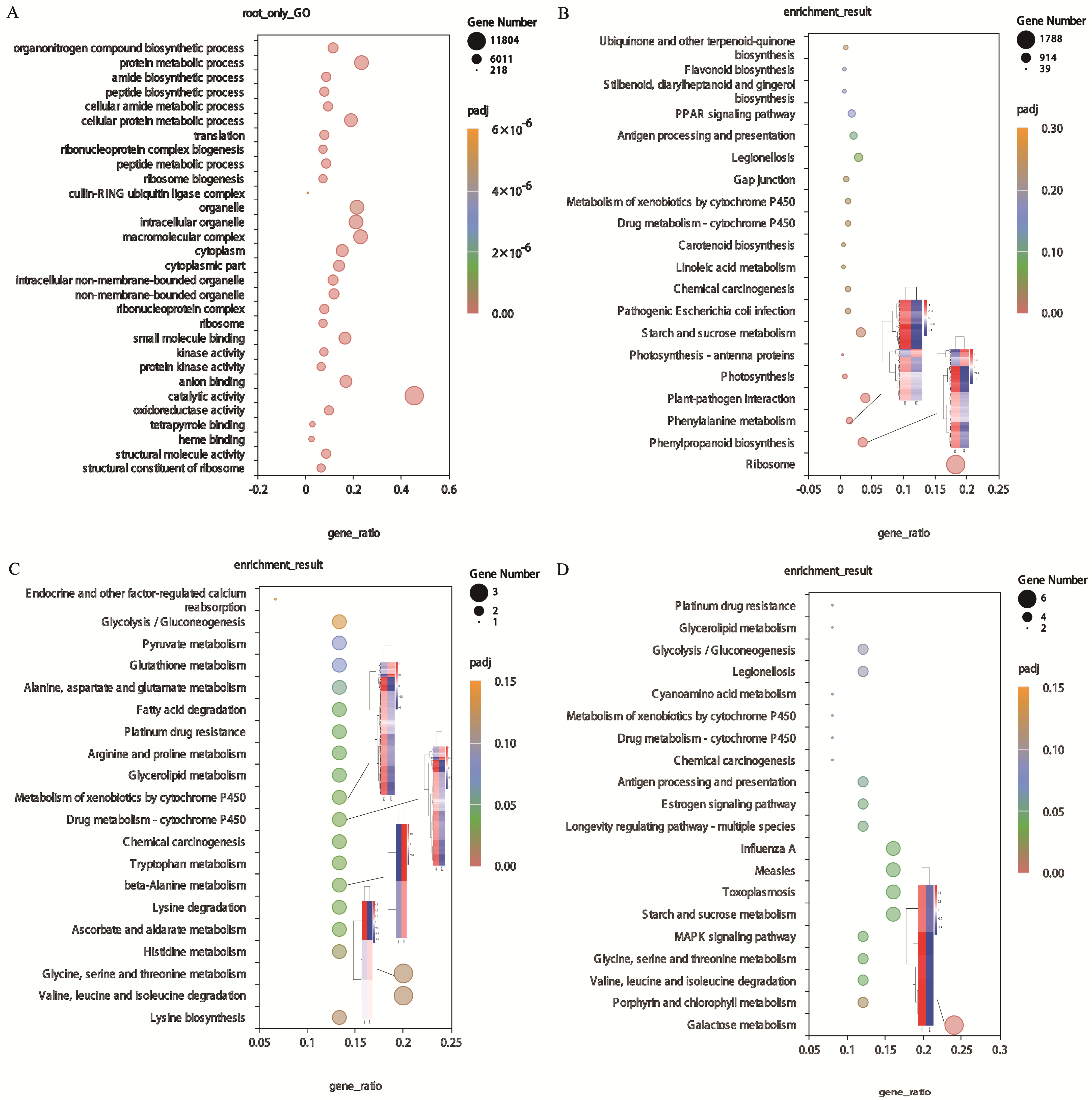
To better understand the modulated expression of the biological functions of DEGs in the roots and leaves of tall fescue under Sb stress, GO enrichment analysis was carried out. The DEGs significantly annotated in the top 30 terms in the BP, MF, and CC categories are presented in Figure 4A. The six terms involved in anion binding (GO:0043168,4301), small molecule binding (GO:0036094,227), oxidoreductase activity (GO:0016491,2455), kinase activity (GO:0016301,1905), heme binding (GO:0020037,637) and protein kinase activity (GO:0004672,1607) were assigned to the MF category. The macromolecular complex (GO:0032991,5898), organelle (GO:0043226,5504), and intracellular organelle (GO:0043229,5449) were the three most important terms in the CC category. For the BP category, the two largest GO terms were linked to the protein metabolic process (GO:0019538,6083) and the cellular protein metabolic process (GO:0044267,4878) (Table S2).
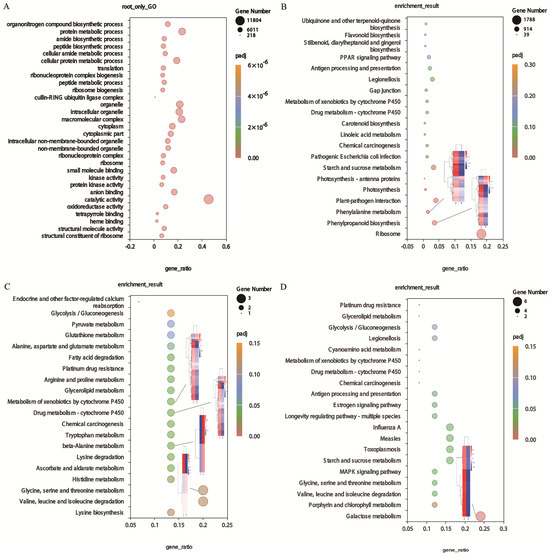
Figure 4.
Functional analysis of DEGs. (A) GO enrichment pathway of DEGs in the root. (B) KEGG enrichment pathway of DEGs and the heat map of important DEGs in roots. (C) KEGG enrichment pathway of DEGs and the heat map of important DEGs in leaves. (D) KEGG enrichment pathway of DEGs and the heat map of important DEGs in roots and leaves.
In the root of tall fescue, a total of 29,454 DEGs were assigned to 354 KEGG pathways. Table 3 shows the top significant pathways involving DEGs. Among those pathways, the ‘ribosome’ (ko03010,1788) was the most significant pathway, followed by ‘phenylpropanoid biosynthesis’ (ko00940,349) and ‘starch and sucrose metabolism’ (ko00940,318). Significantly, the ‘PPAR signaling pathway’ (ko03320,179) and ‘flavonoid biosynthesis’ (ko00941,58) were also associated with the root of the tall fescue response to Sb stress (Figure 4B). In leaves, 80 DEGs were allocated to 52 pathways. The DEGs significantly enriched in the uppermost 20 significant pathways are shown in Figure 4C. The highly enriched pathways of DEGs were valine, leucine, and isoleucine degradation (ko00280,3) and glycine, serine, and threonine metabolism (ko00260,3). In addition, the ascorbate and aldarate metabolism (ko00053,2), drug metabolism—cytochrome P450 (ko00982,2), glycerolipid metabolism (ko00561,2), and glutathione metabolism (ko00480,2) are also the main KEGG pathways of the DEGs (Table 4). KEGG analysis was also performed on DEGs in both root and leaf, and the results showed that galactose metabolism (ko00052,6), starch and sucrose metabolism (ko00500,4), valine, leucine, and isoleucine degradation (ko00280,3), and MAPK signaling pathway (ko04010,3) were significantly enriched. The results suggested that these pathways play a major role in the response of roots and leaves to Sb stress. Furthermore, it should be emphasized that the co-expression DEGs were significantly enriched in drug metabolism—cytochrome P450 (ko00982,2)—and metabolism of xenobiotics by cytochrome P450 (ko00980,2). These two pathways were also noted in the KEGG enrichment pathway of DEGs specifically expressed in leaves (Figure 4D, Table 5).

Table 3.
Statistical table of KEGG enrichment analysis in root.

Table 4.
Statistical table of KEGG enrichment analysis in leaf.

Table 5.
Statistical table of KEGG enrichment analysis in root and leaf.
3.6. DEGs Involved in Root and Leave of Tall Fescue Response to Sb Stress
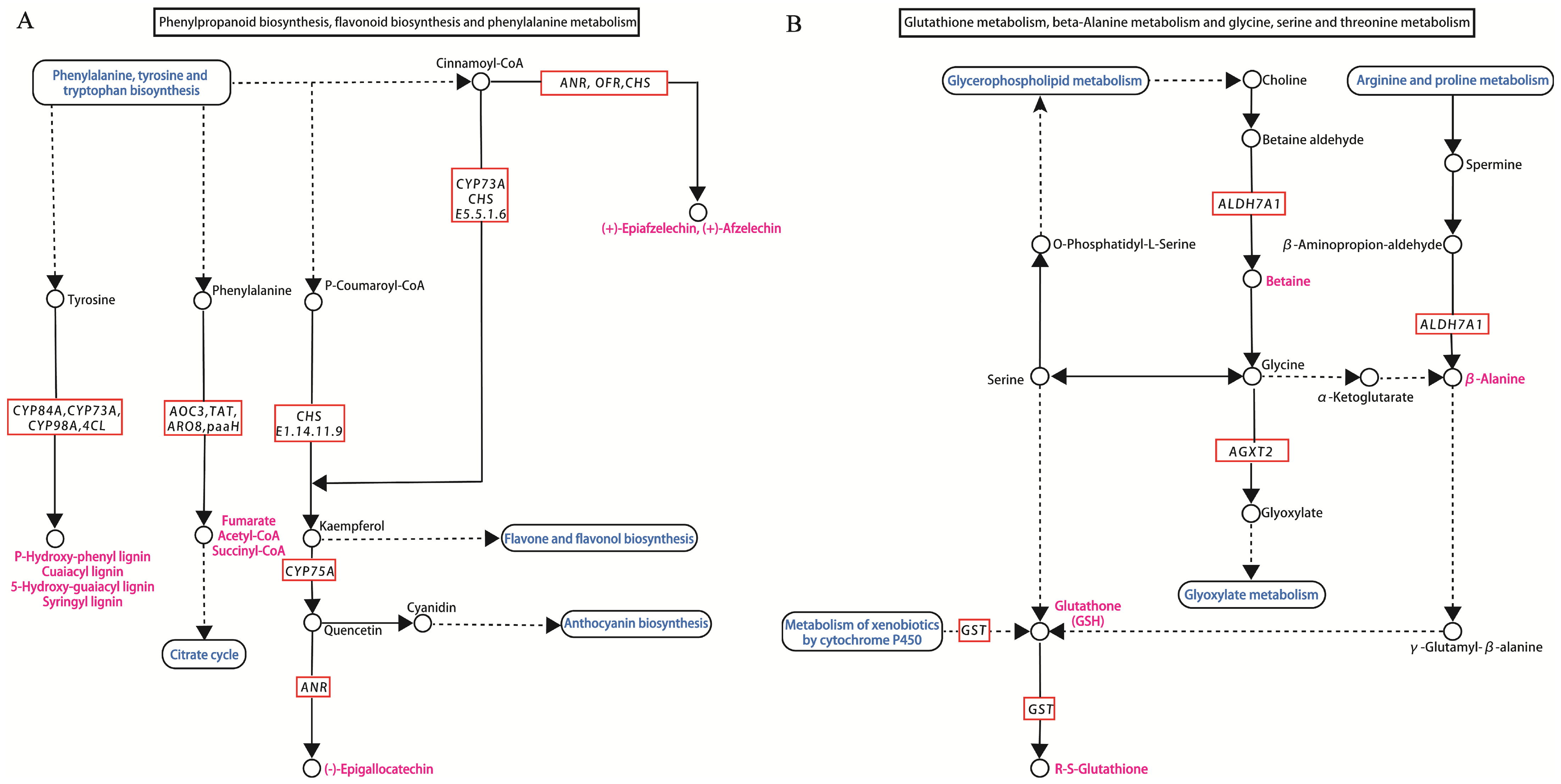
KEGG enrichment analysis revealed that the expression of all DEGs involved in phenylpropanoid biosynthesis, phenylalanine metabolism, and flavonoid biosynthesis (Figure 5A) was up-regulated in the root. The genes include genes encoding key enzymes in phenylpropanoid biosynthesis (4CL, PAL), in the phenylalanine metabolism pathway (CYP73A, PAL, 4CL), and in flavonoid biosynthesis (HCT, CYP73A) (Figure 4B). The enzymes encoded by these genes are predominantly involved in the synthesis of lignin.
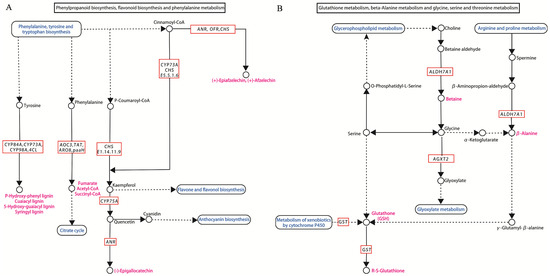
Figure 5.
Pathways and genes associated with response to Sb stress in tall fescue roots and leaves. (A) The pathway of phenylpropanoid biosynthesis, flavonoid biosynthesis, and phenylalanine metabolism in the roots of tall fescue. (B) The pathway of glutathione metabolism, beta-alanine metabolism, glycine, serine, and threonine metabolism in the leaves of tall fescue.
In leaf, Figure 5B illustrates that GST, which was related to glutathione metabolism, and ALDH7A1, which was associated with β-alanine metabolism, were up-regulated under Sb stress (Figure 4C). In addition, AGXT2 and ALDH7A1, which were the DEGs associated with both the valine, leucine, and isoleucine degradation pathways and the glycine, ser1ine, and threonine metabolism pathways, were activated under Sb stress (Figure 5B). In leaves, ALDH7A1 regulates 14 metabolic pathways (Table S3). The results suggested that this gene may play an important role in leaf responses to Sb stress.
DEGs associated with both metabolism of xenobiotics by cytochrome P450 (2 DEGs all up-regulated) and drug metabolism—cytochrome P450 (2 DEGs all up-regulated)—all mainly encoding glutathione S-transferase (GST) were expressed in both root and leaf (Figure 5B). Meanwhile, most of the genes encoding hexokinase (HK), invertase (INV), sucrose isomerase (sacA), and heat shock protein A1 (HSPA1_8) were induced by Sb treatment in roots and leaves (Table S3). In this study, the GEGs belonging to the cytochrome P450 genes include CYP73A, CYP75A, CYP84A, CYP98A, CYP1A1, CYP2J, and CYP97C1. These genes may be the key genes in response to Sb stress in tall fescue (Table S3).
4. Discussion
Sb is a hazardous element closely related to human activities [33]. Under Sb stress, leaf growth and biomass of plants are inhibited, and lignification occurs in the roots. Sb exposure also activated the antioxidant defense response in the plant. It causes lipid peroxidation and interferes with chlorophyll synthesis, thus affecting photosynthesis [34]. The root can reduce the damage of HMs to plants by enhancing antioxidant capacity, chelating metal ions by transporters, and modifying cell wall composition [35,36]. In addition to chelating metal ions by phytochelatin (PC), leaves can also sequester cadmium by trichomes, which reduces the need for Cd chelation by PC [37,38]. In this study, transcriptome analysis revealed that the roots of tall fescue respond to Sb stress mainly through phenylpropanoid biosynthesis, phenylalanine metabolism, flavonoid biosynthesis, and PPAR signaling pathways. In leaves, glutathione metabolism, β-alanine metabolism, ascorbate, and aldarate metabolism play an important role in Sb stress. Meanwhile, cytochrome P450 also deserved better attention because the associated DEGs in the KEGG analysis were significantly enriched in both roots and leaves. It was observed that those regulated genes or pathways were expressed in a tissue-specific manner during the response to Sb. In addition, DEGs in the roots of tall fescue treated with Sb were significantly higher than those in the leaves. Therefore, it proved that there are different Sb defense mechanisms in the leaf and root.
When plants are subjected to abiotic stresses such as HMs, they produce excess reactive oxygen species (ROS), which cause damage to proteins, lipids, carbohydrates, and DNA and ultimately lead to cell death [39]. In our results, there are three significant pathways with a lignin synthesis correlation, which are phenylpropanoid biosynthesis, phenylalanine metabolism, and flavonoid biosynthesis. The DEGs (e.g., CYP73A and 4CL) related to lignin synthesis were up-regulated in these three pathways, which suggested that Sb treatment could induce lignin biosynthesis. In addition, the DEGs (e.g., CYP75A, CYP98A, and HCT) related to flavonoid synthesis were up-regulated in flavonoid biosynthesis pathways. When plants are subjected to HM stress, flavonoids and lignin can chelate heavy metal ions in the cytoplasm [40,41]. Flavonoids also have the function of clearing reactive oxygen species in cells and protecting plant cells from oxidative stress damage [42]. Similarly, Sedum plumbizincicola and Citrus grandis have also been found to tolerate HM stress by affecting the synthesis of lignin and flavonoids to strengthen the chelation of heavy metal ions and the removal of reactive oxygen species by flavonoids [43,44]. In summary, the root of tall fescue regulates lignin and flavonoid synthesis mainly through 4CL and Cytochrome P450 family genes to decrease the toxicity of Sb to tall fescue.
Glutathione plays an important part in the stress of cadmium [45], copper [46], lead [47], and other heavy metals. In this study, GST, which regulates the synthesis of R-S-glutathione, was identified. Glutathione (GSH), a tripeptide compound containing sulfhydryl groups, is a small non-enzymatic antioxidant molecule that exists primarily in a reductive form [48]. When plants are stressed by heavy metals, they can work with the antioxidant oxidase system to remove harmful free radicals and maintain normal physiological activities [49]. On the other hand, GSH is converted into PC under the action of PC synthase, which is then mainly bound to heavy metal ions through thiol groups (-SH) of PC to form high molecular weight complexes, which are thus intercepted in vacuoles to prevent heavy metals from harming other tissues of the plant [50,51]. Similarly, it was reported that Arabidopsis participated in Sb detoxification through glutathione [37]. Betaine plays an important role in plant resistance to heavy metal stress, which is known as an osmoprotector because it protects plants from dehydration [52]. However, studies have shown that betaine is not likely to regulate the stress response by adjusting osmotic pressure but to reduce stress damage by enhancing the photosynthetic efficiency and the integrity of the cell membrane when exposed to stress conditions [52]. In this study, all DEGs related to the glycine, serine, and threonine metabolic pathways were up-regulated, and some of the genes were involved in regulating betaine synthesis (AGXT2, ALDH7A1). In addition, betaine, as a zwitterion, can interact with the hydrophilic and hydrophobic domains of protein complexes and membranes, thereby maintaining the structural and functional integrity of these molecules and protecting them from the detrimental effects of ROS [53,54]. Betaine mitigates the deadly effects of heavy metals mainly by increasing antioxidant enzyme activities under stress conditions [55]. β-alanine is a non-proteinogenic amino acid and is a precursor of Coenzyme A (CoA) and acyl-carrier protein [56]. It can be converted to β-alanine betaine, which enhances the osmotic potential of plant cells and thus enhances tolerance to stress [57]. In the present study, the β-alanine metabolism-related gene, ALDH7A1, was found to be up-regulated in leaves. Recently, the studies on the β-alanine involved stress response in plants mainly focused on temperature and water stress [58,59], but the studies on its role in heavy metal stress response were relatively few. In brief, the results of this study suggested that tall fescue leaves respond to Sb stress mainly through the antioxidant and chelation effects of glutathione, the protective effects of betaine on the photosynthetic system, and the osmotic regulation of β-alanine.
Cytochrome P450 monooxygenases (P450s; encoded by the CYP genes) are found in all organisms and catalyze various oxidative transformations of substrates [60]. Studies have shown that cytochrome P450 plays a major role in protecting plants from heavy metal stresses [61]. In this study, CYP73A gene expression involved in lignin and flavonoids was up-regulated in tall fescue root. It was reported that cytochrome P450s was up-regulated in the root of rice (Oryza sativa), which regulates the synthesis of flavonoids and other secondary metabolites under chromium and cadmium stresses [62]. Therefore, CYP genes were mainly involved in the synthesis of secondary metabolites in plant roots to regulate the response to heavy metal stress. CYPs also participate in the metabolic clearance of heavy metals with glutathione-S-transferase (GST) [63]. In this study, GST was enriched in cytochrome P450-related pathways, and its expression was up-regulated. These results indicate that the CYP gene is of great significance in the Sb resistance of tall fescue, and its specific regulatory mechanism can be further studied.
The metabolic processes and genes related to Sb stress response in tall fescue were explored in this study, which provided theoretical knowledge for improving Sb tolerance in tall fescue by transgenic and gene editing technologies. In addition, it also provided theoretical support for the bioremediation of Sb-contaminated soil.
5. Conclusions
In conclusion, the response of tall fescue roots and leaves to Sb stress involves different metabolic processes and gene regulation. Transcriptomic analysis showed that the root of tall fescue may decrease Sb toxicity mainly through Sb chelation and ROS clearance by lignin and flavonoids. The DEGs involved were CYP73A, 4CL, CYP75A, CYP98A, and HCT. Leaves may respond to Sb stress mainly through photosynthetic system protection, osmoregulation, oxidation resistance, and metal chelation by glutathione, betaine, and β-alanine. The related DEGs were GST, AGXT2, and ALDH7A1. This study will help elucidate the mechanism of Sb accumulation and detoxification in herbaceous plants and provide candidate genes for breeding Sb-resistant varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14091504/s1, Table S1: Number of unigenes that are annotated to the KEGG database; Table S2: All GO enrichment pathway of DEGs in the root; Table S3: List of candidate genes of tall fescue response to antimony stress; Table S4: N50 statistics of transcriptome assembly; Table S5: Transcriptome completeness: BUSCO results; Table S6: Annotation summary of unigenes across multiple databases.
Author Contributions
X.L.: methodology, software, writing, editing, data analysis, and result verification. J.F.: funding acquisition, methodology, and supervision. F.W.: methodology and review. Y.X.: methodology and review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 32171672 and 31702165) and the Project of Forestry Science and Technology Innovation and Promotion of Jiangsu (Grant No. LYKJ[2021]09).
Data Availability Statement
High-throughput sequencing data that support the findings of this study have been deposited in the NCBI Sequence Read Archive database under Accession Number CRA016645. https://bigd.big.ac.cn/gsa/browse/CRA016645 (accessed on 23 August 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lai, Z.; He, M.; Lin, C.; Ouyang, W.; Liu, X. Interactions of antimony with biomolecules and its effects on human health. Ecotoxicol. Environ. Saf. 2022, 233, 113317. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2021, 158, 106908. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Z.; Chu, W.; Feng, R. Speciation of antimony and arsenic in the soils and plants in an old antimony mine. Environ. Exp. Bot. 2015, 109, 31–39. [Google Scholar] [CrossRef]
- Wu, F.; Liu, X.; Qu, G.; Ning, P.; Jin, C.; Cui, Q.; Ren, Y.; He, M.; Yang, Y.; Li, J. Solidification and stabilization of harmful elements in antimony tailings and synergistic utilization of multiple solid wastes. Cem. Concr. Compos. 2022, 133, 104718. [Google Scholar] [CrossRef]
- Huan, D. Application and research on collaborative disposal of antimony contaminated soil by cement kiln. Cem. Eng. 2023, 6, 6–8. [Google Scholar]
- Aniruddha, S.; Md Abdullah Al, M.; Deen Mohammad, D.; Kallol, D.; Rakhi, N.; Most Waheda Rahman, A.; Abu Reza Md Towfiqul, I.; Tofazzal, I. Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review. Chemosphere 2023, 332, 138861. [Google Scholar]
- Xi, J.; Wu, L.; Fu, R.; Chen, L. Comparison of biotoxicity evalution methods of antimony in soil environment. Administrat. Tech. Environ. Monit. 2021, 33, 9–13. [Google Scholar]
- Agata, B.; Jadwiga, W.; Mirosław, K.; Jan, K. The role of dactylis glomerata and diesel oil in the formation of microbiome and soil enzyme activity. Sensors 2020, 20, 3362. [Google Scholar] [CrossRef]
- Xue, L.; Gao, M.; Shi, S.; Wei, Y.; Jiang, Z.; Liu, J. Physiological response and accumlation characters of four anti-pollution woody plants under antimony stress. Ecol. Environ. Sci. 2014, 23, 1344–1350. [Google Scholar]
- Liu, Z.; Ma, W.; Tong, F.; Wang, J. Effects of antimony stress on growth and physiology of 10 genotypes of catalpa bungei. Forests 2021, 12, 1036. [Google Scholar] [CrossRef]
- Tong, F.P.; Long, Y.; Yi, J.X.; Li, G.; Shi, W.F.; Yi, A. Characteristics of heavy metal accumulation in broussonetia papyrifera in an antimony mine. Chin. Agric. Sci. Bull. 2011, 9, 701–705. [Google Scholar]
- Prasad, M.N.V.; Sajwan, K.S.; Naidu, R. Stabilization, Remediation, and integrated management of metal-contaminated ecosystems by grasses (Poaceae). In Trace Elements in the Environment; CRC Press: Boca Raton, FL, USA, 2005; pp. 405–424. [Google Scholar]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Hunag, Z.; Jiang, M.; Li, X. Effect of 24-epibrassinolide on reactive oxygen species and antioxidative defense systems in tall fescue plants under lead stress. Environ. Res. Sect. B 2020, 187, 109831. [Google Scholar] [CrossRef]
- Ma, P.; Li, Y.; Wang, Z.; Wang, X. Proteomic analysis of fescue arundinacea under low phosphorus stress. Chin. J. Grassl. 2023, 45, 6–14. [Google Scholar]
- Manohar, C.; Padmaja, N.; Christopher, L.S.; Randy, D.D. Differential gene expression in tall fescue tissues in response to water deficit. Plant Genome 2022, 15, e20199. [Google Scholar]
- Long, J.; Dong, M.; Zhou, Z.; Bao, S.; Xu, Z.; Miao, Y. Advances in research on the stress resistance of fescue in graminease. China Feed 2022, 13, 105–112. [Google Scholar]
- Jiang, H.; Sun, X.; Wu, C.; Cao, W. Effects of light and seeding rates on the growth and turf quality of tall fescue (Fescue arundinecea). Acta Pratacul. Sin. 2000, 9, 63–67. [Google Scholar]
- Xu, P. Studies on Cadmium Tolerance and Detoxification in Tall Fescue and Kentucky Bluecgrass; Shanghai Jiao Tong University: Shanghai, China, 2016. [Google Scholar]
- Xiong, Z.M.; Yang, J.H.; Wang, L.A. Responses of three turfgrasses to soil Pb and Cd stresses. J. Yangzhou Univ. Agric. Life Sci. Ed. 2019, 40, 117–121. [Google Scholar]
- Huang, M.; Ai, H.; Xu, X.; Chen, K.; Niu, H.; Zhu, H.; Sun, J.; Du, D.; Chen, L. Nitric oxide alleviates toxicity of hexavalent chromium on tall fescue and improves performance of photosystem II. Environ. Res. Sect. B 2018, 164, 32–40. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, C. Study on the absorption and transportation of heavy metals in soil by the plants in Daqingshan. Anhui Nongye Kexue 2021, 49, 90–92. [Google Scholar]
- Hu, Z.; Zhao, F.; Yan, B. Application of plant ecological remediation technology in heavy metal contaminated soil in urban park greenbelt. Huanjing Kexue Yu Guanli 2023, 48, 139–142. [Google Scholar]
- Sun, N.; Yan, G.X.; Wang, L.F.; Zhang, Y.; Xing, Y.; Wang, J. Research of heavy metal removal rate by phytoremediation ofdeserted mine in Beijing. Urban Geol. 2016, 11, 7–14. [Google Scholar]
- Kavita, G.; Neeti, S. RNA-seq for revealing the function of the transcriptome. In Bioinformatics: Methods and Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 105–129. [Google Scholar]
- Zhang, K. Study on Tolerance Mechanism of Hypertolerant Plant Conyza canadensis to Cadmium; Fujian Agriculture and Forestry University: Fuzhou, China, 2010. [Google Scholar]
- Shi, H.Z. Physiological and Molecular Response of Horticultural Plant Tagetes erecta L. to Cadmium Stress; Sichuan Agricutural University: Yaan, China, 2023. [Google Scholar]
- Hu, J.N. Enhancement of Resistance Mechanism and Repair Efficiency of Phragmites Australis under Copper Stress; Liaoning University: Shenyang, China, 2022. [Google Scholar]
- Qiao, L.; Zhang, Z.; Chen, L.; Sun, H.; Li, M.; Li, L.; Ma, J. Detection of chlorophyll content in maize canopy from UAV imagery. IFAC-Pap. 2019, 52, 330–335. [Google Scholar]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase-activity and isoenzymes in embryogenic and non-embryogenic shamouti orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Regev, A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Y.; Sun, G.; Zhang, L.; Li, Z.; Sommar, J.; Yao, H.; Feng, X. Characteristics, accumulation, and potential health risks of antimony in atmospheric particulate matter. ACS Omega 2021, 6, 9460–9470. [Google Scholar] [CrossRef]
- Chirappurathu Sukumaran-Nair, V.; Rajpal, S.; Miroslava, V.; Marek, V. Antimony toxicity in soils and plants, and mechanisms of its alleviation. Environ. Exp. Bot. 2022, 202, 104996. [Google Scholar]
- Cao, Z.Z.; Lin, X.Y.; Yang, Y.J.; Guan, M.Y.; Xu, P.; Chen, M.X. Gene identification and transcriptome analysis of low cadmium accumulation rice mutant (lcd1) in response to cadmium stress using MutMap and RNA-seq. BMC Plant Biol. 2019, 19, 250. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, C.; Peng, F.; Wang, R.; Xiao, X.; Zeng, J.; Kang, H.; Fan, X.; Sha, L.; et al. Transcriptomic profiles reveal the interactions of Cd/Zn in dwarf polish wheat (Triticum polonicum L.) roots. Front. Physiol. 2017, 8, 168. [Google Scholar] [CrossRef]
- Marijke, J.; Els, K.; Henk, S.; Mattijs, B.; Luis, E.H.; Robert, C.; Tony, R.; Sacha, B.; Jaco, V.; Ann, C. Differential response of arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiol. Biochem. 2014, 83, 1–9. [Google Scholar]
- Cui, B.; Liu, C.; Hu, C.; Liang, S. Transcriptomic sequencing analysis on key genes and pathways regulating cadmium (Cd) in ryegrass (Lolium & nbsp; perenne L.) under different cadmium concentrations. Toxics 2022, 10, 734. [Google Scholar]
- Muhammad, S.; Bertrand, P.; Camille, D.; Muhammad, N.; Muhammad, A.; Eric, P. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Kidd, P.S.; Llugany, M.; Poschenrieder, C.H.; Gunse, B.; Barcelo, J. The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J. Exp. Bot. 2001, 52, 1339–1352. [Google Scholar]
- Su, N.; Ling, F.; Xing, A.; Zhao, H.; Zhu, Y.; Wang, Y.; Deng, X.; Wang, C.; Xu, X.; Hu, Z.; et al. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020, 175, 104059. [Google Scholar] [CrossRef]
- Kolahi, M.; Kazemi, E.M.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol. Biochem. 2020, 146, 71–89. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, W.; He, X.; Wu, L.; Bi, D.; Deng, Z.; He, Z.; Wu, C.; Zhuo, R. Integrative analysis of transcriptome and proteome provides insights into adaptation to cadmium stress in Sedum plumbizincicola. Ecotoxicol. Environ. Saf. 2021, 230, 113149. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Huang, Z.R.; Huang, W.L.; Huang, W.T.; Chen, H.H.; Yang, L.-T.; Ye, X.; Chen, L.-S. Physiological and molecular adaptations of Citrus grandis roots to long-term copper excess revealed by physiology, metabolome and transcriptome. Environ. Exp. Bot. 2022, 203, 105049. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Zhang, D.; Fang, B.; YangJin, T.; Zou, J.; Chen, Y.; Su, N.; Cui, J. Enhanced vacuole compartmentalization of cadmium in root cells contributes to glutathione-induced reduction of cadmium translocation from roots to shoots in pakchoi (Brassica Chinensis L.). Ecotoxicol. Environ. Saf. 2021, 208, 111616. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Mostofa, M.G.; Polash, M.A.S.; Mahamud, A.G.M.S.U.; Afrin, S.; Hossain, M.A.; Hossain, M.A.; Murata, Y.; Tran, L.-S.P. Comparative effects of ascobin and glutathione on copper homeostasis and oxidative stress metabolism in mitigation of copper toxicity in rice. Plant Biol. 2021, 23, 162–169. [Google Scholar] [CrossRef]
- Paula Pícoli, D.; Mariana Bisarro Dos, R.; Willian Robert, G.; Flora Troina, M.; Diego Luis, R.; Lusânia Maria Greggi, A.; Bruno Lemos, B.; Denise, G.; Rui Manuel, R.; Fernando, B.; et al. Adaptive epigenetic response of glutathione (GSH)-related genes against lead (Pb)-induced toxicity, in individuals chronically exposed to the metal. Chemosphere 2020, 269, 128758. [Google Scholar]
- Sarvajeet Singh, G.; Narendra, T. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 2007, 164, 1489–1498. [Google Scholar] [CrossRef]
- Fabio, F.N.; Clarissa, L.; Barbara, C.; Pierre, F.; Jean-Claude, D.; Gian Attilio, S. Heavy metal stress and sulfate uptake in maize roots. Plant Physiol. 2006, 141, 1138–1148. [Google Scholar]
- Hu, S.; Lau, K.W.; Wu, M. Cadmium sequestration in Chlamydomonas reinhardtii. Plant Sci. 2001, 161, 987–996. [Google Scholar] [CrossRef]
- Shafaqat, A.; Zohaib, A.; Mahmoud, F.S.; Muhammad, R.; İlkay, Y.; Bushra Ahmed, A.; Ashwag, S.; Mirza, H.; Dimitris, K. Glycine betaine accumulation, significance and interests for heavy metal yolerance in plants. Plants 2020, 9, 896. [Google Scholar]
- Chen, T.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mohammad Muzahidul, I.; Md Anamul, H.; Eiji, O.; Mst Nasrin Akhter, B.; Yasuaki, S.; Yoshimasa, N.; Yoshiyuki, M. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar]
- Anutthaman, P.; Michael, A.S.; André, O.H. The synthesis and role of β-Alanine in Plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar]
- Abdelhakim, L.O.A.; Mendanha, T.; Palma, C.F.F.; Vrobel, O.; Štefelová, N.; Ćavar Zeljković, S.; Tarkowski, P.; De Diego, N.; Wollenweber, B.; Rosenqvist, E.; et al. Elevated CO2 improves the physiology but not the final yield in spring wheat genotypes subjected to heat and drought stress during anthesis. Front. Plant Sci. 2022, 13, 824476. [Google Scholar] [CrossRef] [PubMed]
- Cen, W.; Zhao, W.; Ma, M.; Lu, S.; Liu, J.; Cao, Y.; Zeng, Z.; Wei, H.; Wang, S.; Li, R.; et al. The wild rice locus cts-12 mediates aba-dependent stomatal opening modulation to limit water loss under severe chilling stress. Front. Plant Sci. 2020, 11, 575699. [Google Scholar] [CrossRef] [PubMed]
- Devnarain, N.; Crampton, B.G.; Olivier, N.; Van der Westhuyzen, C.; Becker, J.V.; O’Kennedy, M.M. Transcriptomic analysis of a Sorghum bicolor landrace identifies a role for beta-alanine betaine biosynthesis in drought tolerance. S. Afr. J. Bot. 2019, 127, 244–255. [Google Scholar] [CrossRef]
- David, R.N. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta 2018, 1866, 141–154. [Google Scholar]
- Rai, A.; Singh, R.; Shirke, P.A.; Tripathi, R.D.; Trivedi, P.K.; Chakrabarty, D. Expression of rice CTP 450-Like Gene (Os08g01480) in arabidopsis modulates regulatory network leading to heavy metal and other abiotic stress tolerance. PLoS ONE 2015, 10, e0138574. [Google Scholar] [CrossRef] [PubMed]
- Sonali, D.; Manju, S.; Prashant, M.; Deepika, L.; Sumit Kumar, B.; Mehar, H.A.; Prabodh Kumar, T.; Rudro Deo, T.; Debasis, C. Heavy metals induce oxidative stress and genome-wide modulation in transcriptome of rice root. Funct. Integr. Genom. 2014, 14, 401–417. [Google Scholar]
- Ippei, O.; Hiromi, N.; Satoshi, M.; Naoko, K.N. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2009, 325, 97–108. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).