Modeling for Apple-Slice Drying in Carbon Dioxide Gas
Abstract
1. Introduction
2. Modeling
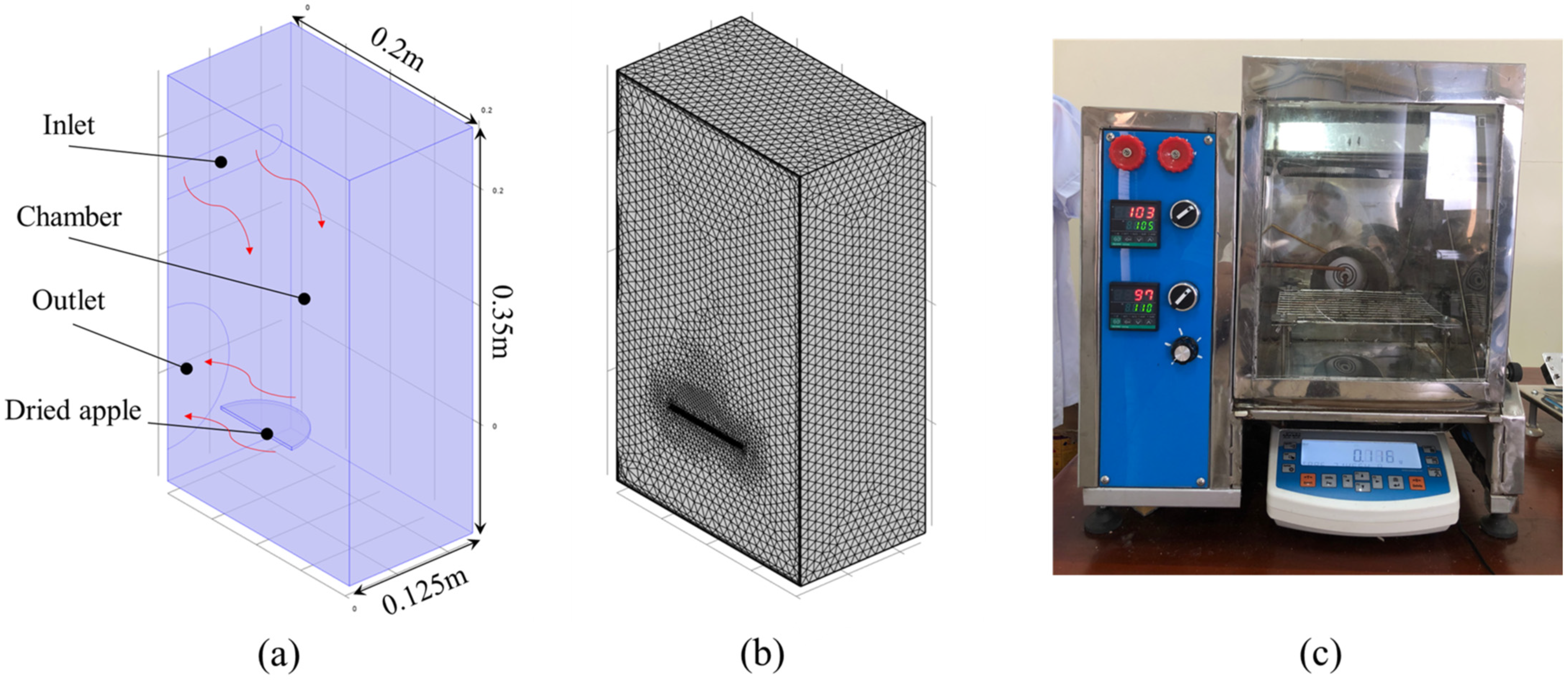
2.1. Problem Description
2.2. Mathematical Model
2.2.1. Model for Porous Media
2.2.2. Model for Gas Phase
2.2.3. Model Implementation
3. Results
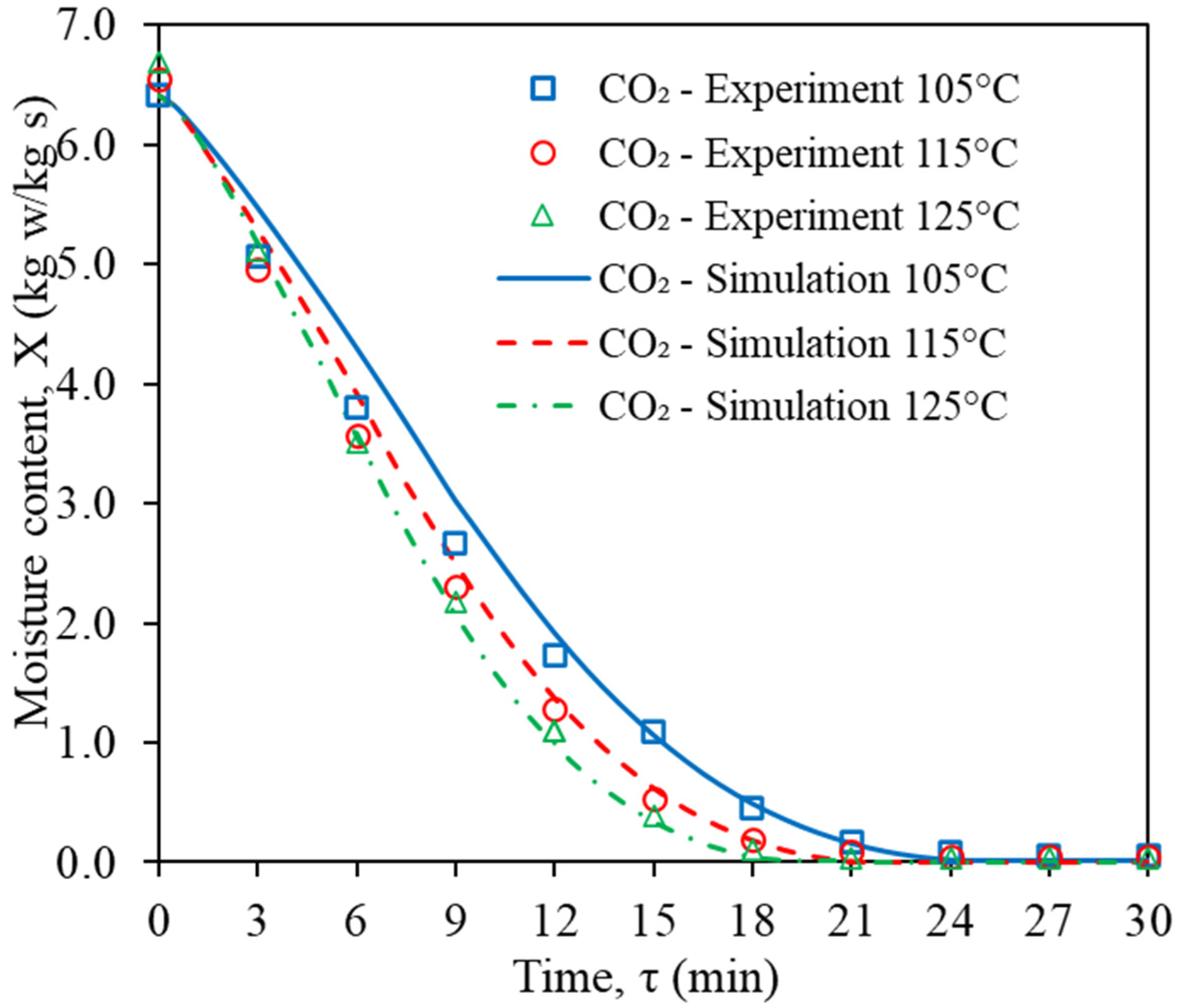
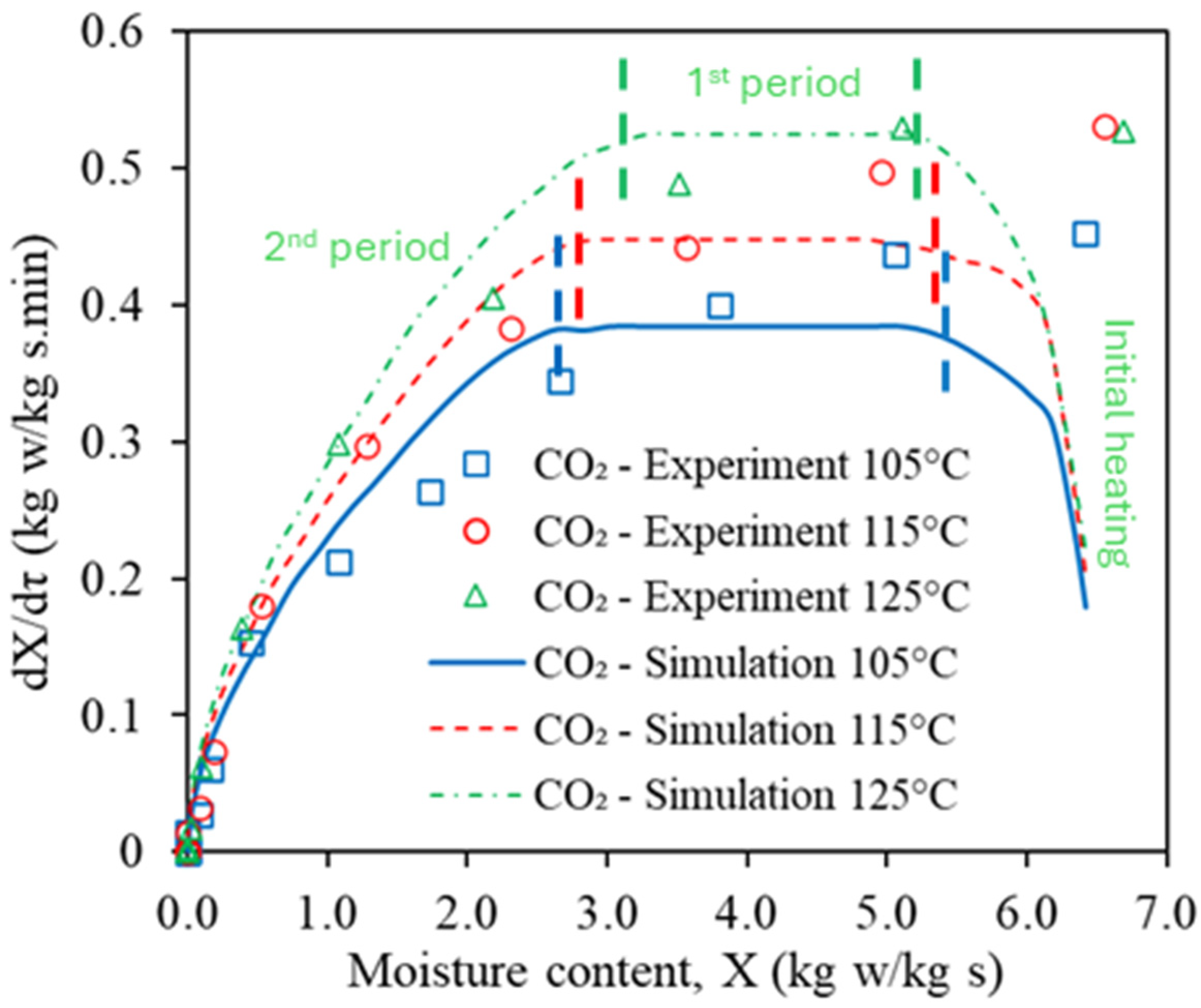
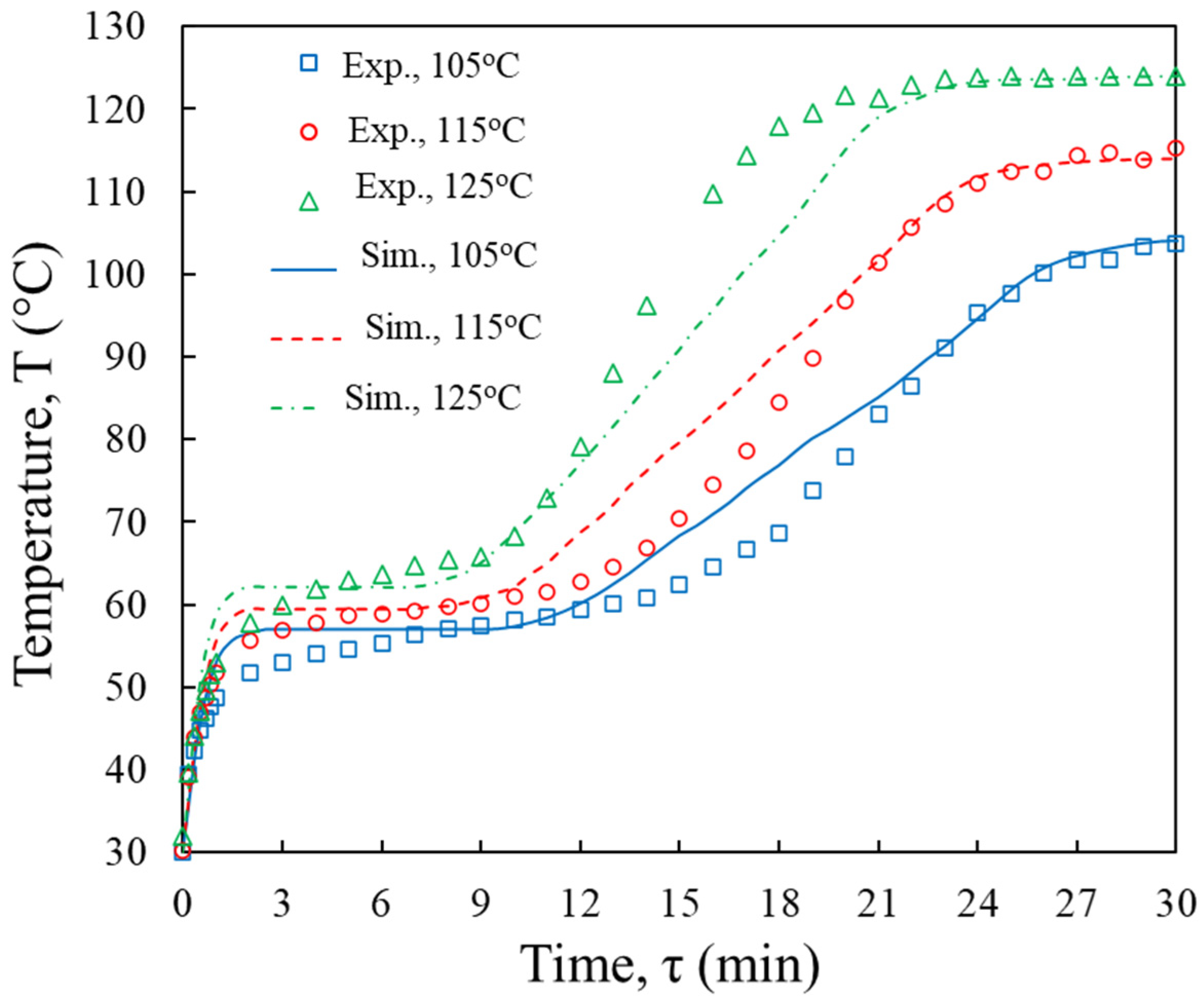
3.1. Model Validation
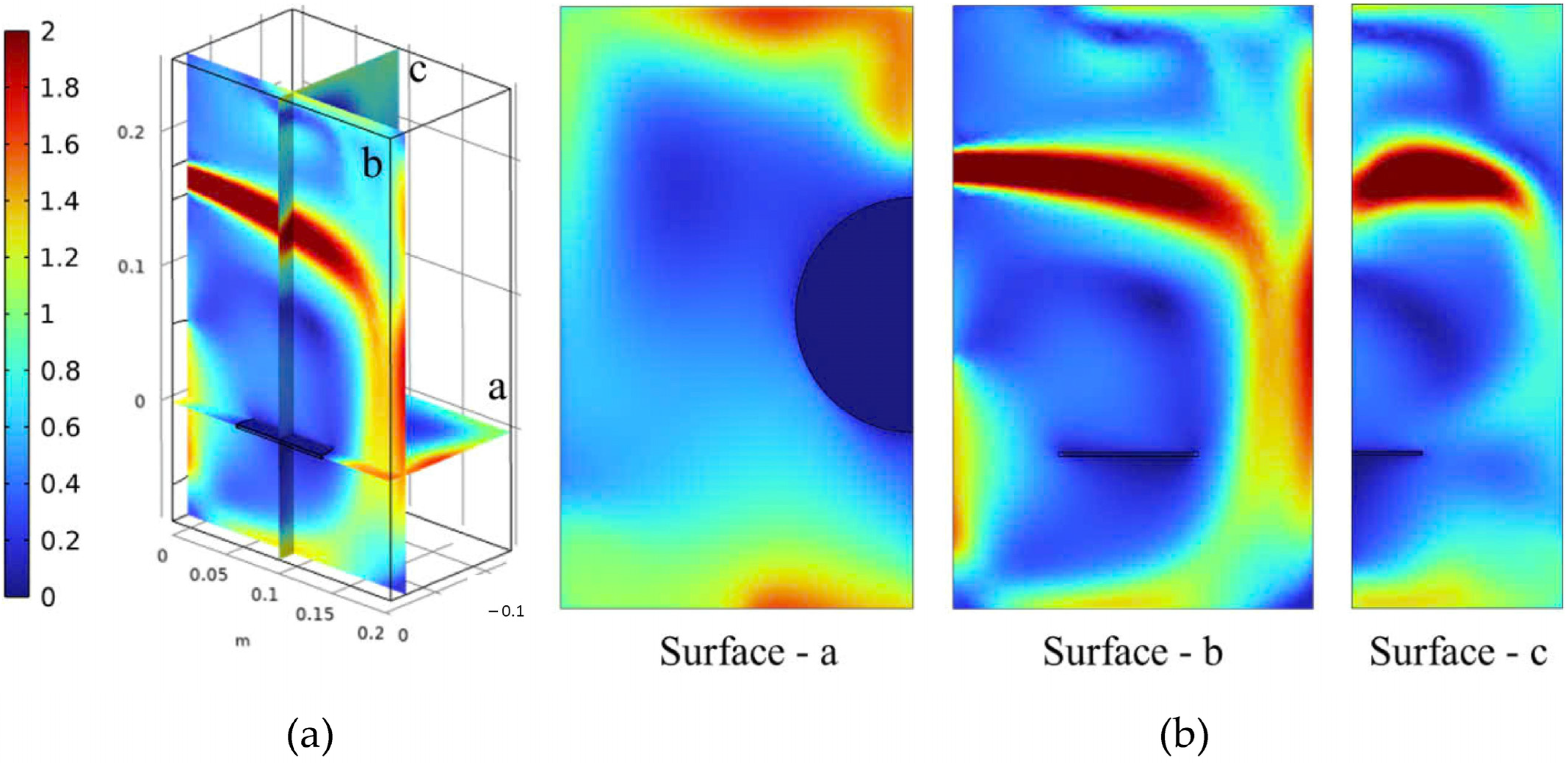
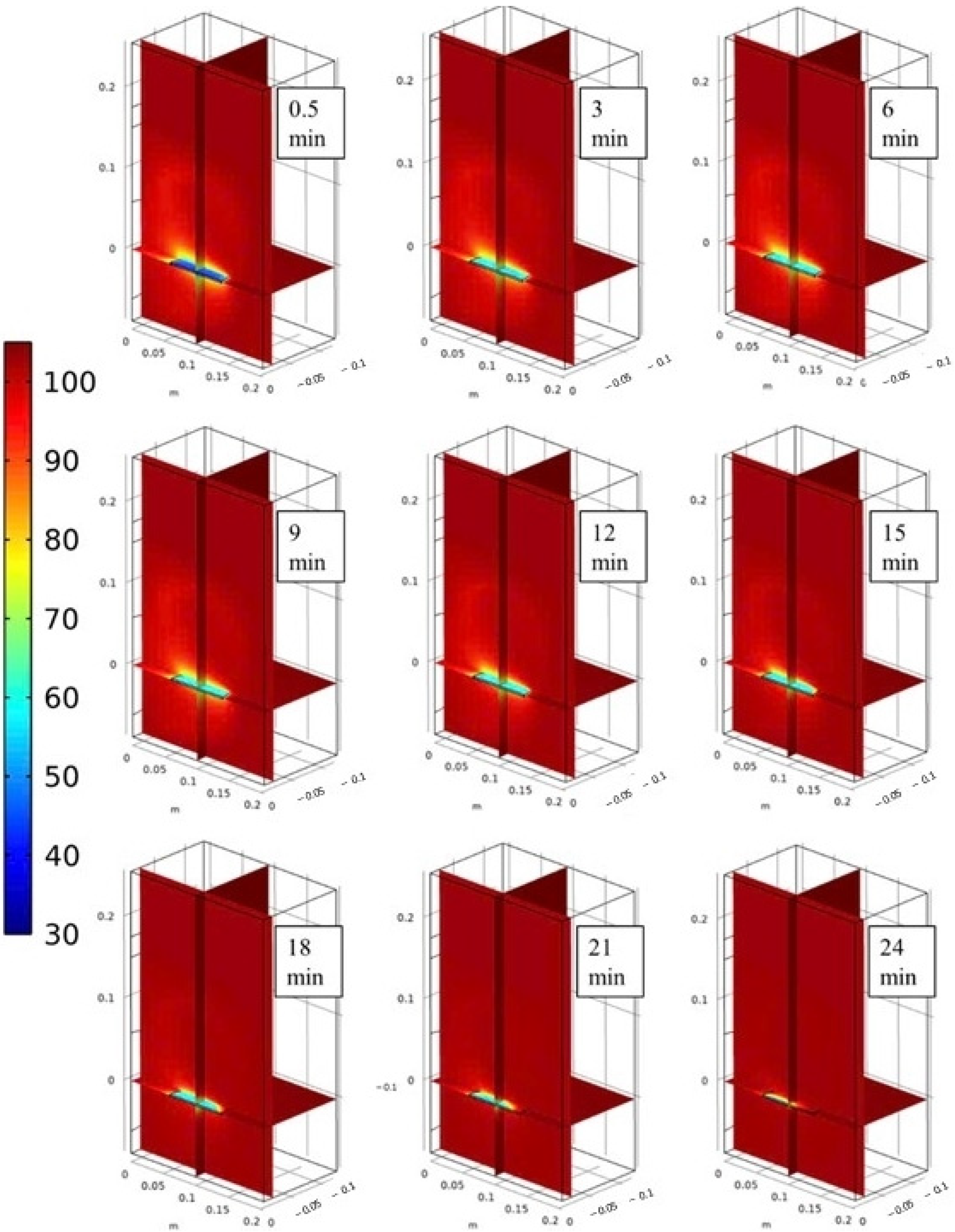
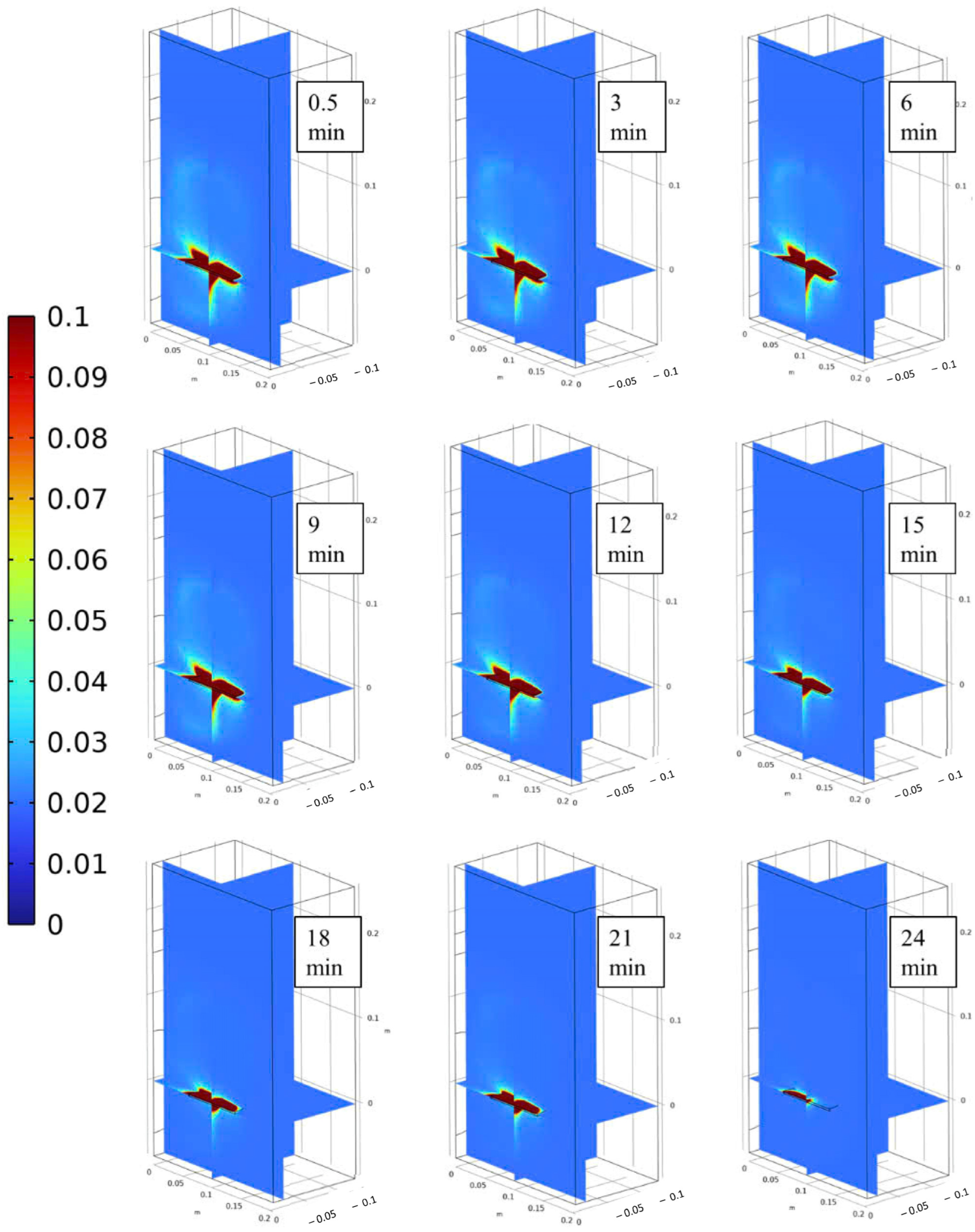
3.2. Velocity, Temperature, and Moisture Distributions of Gas
3.3. Temperature and Moisture Content Distributions of Dried Sample
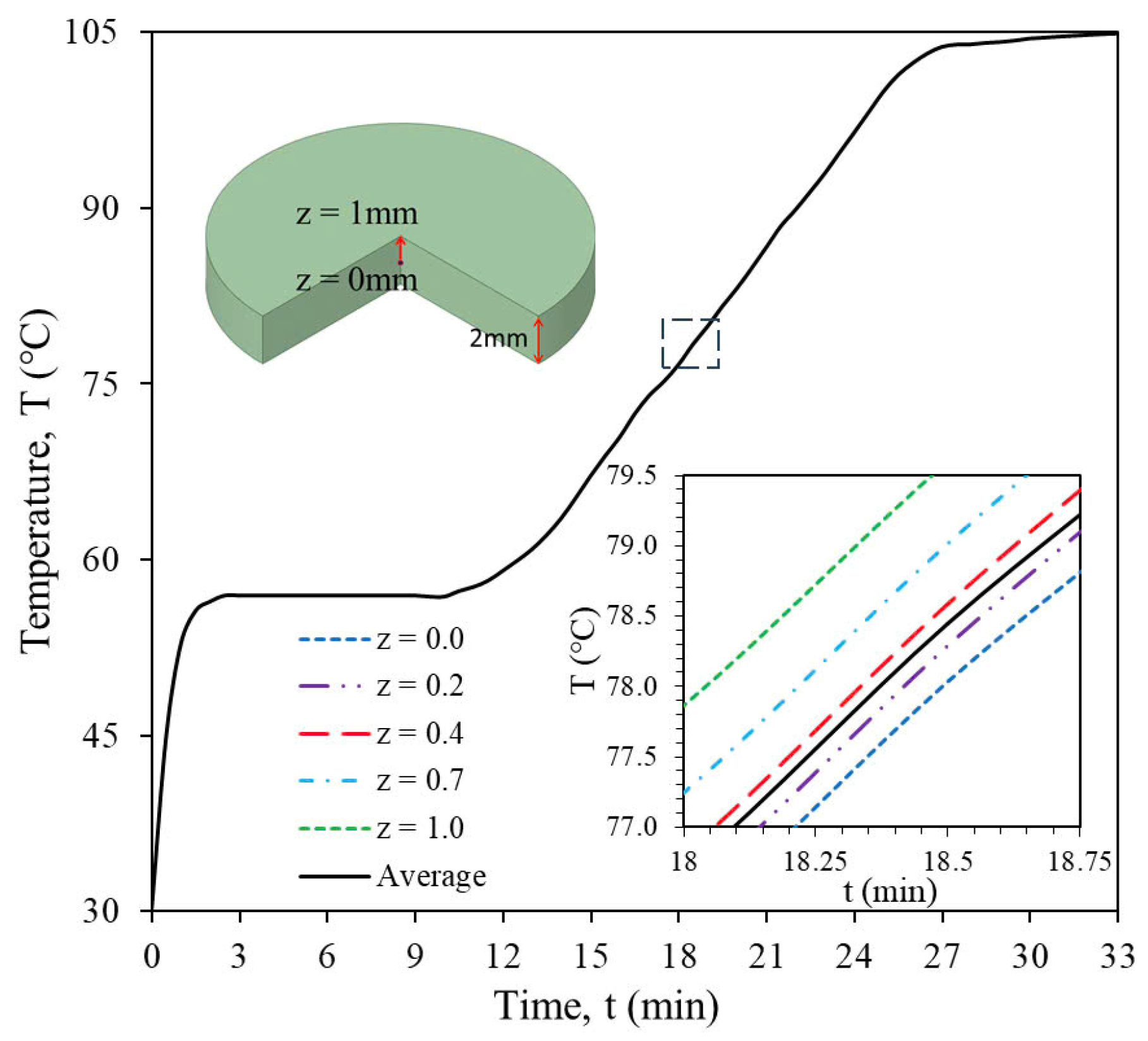
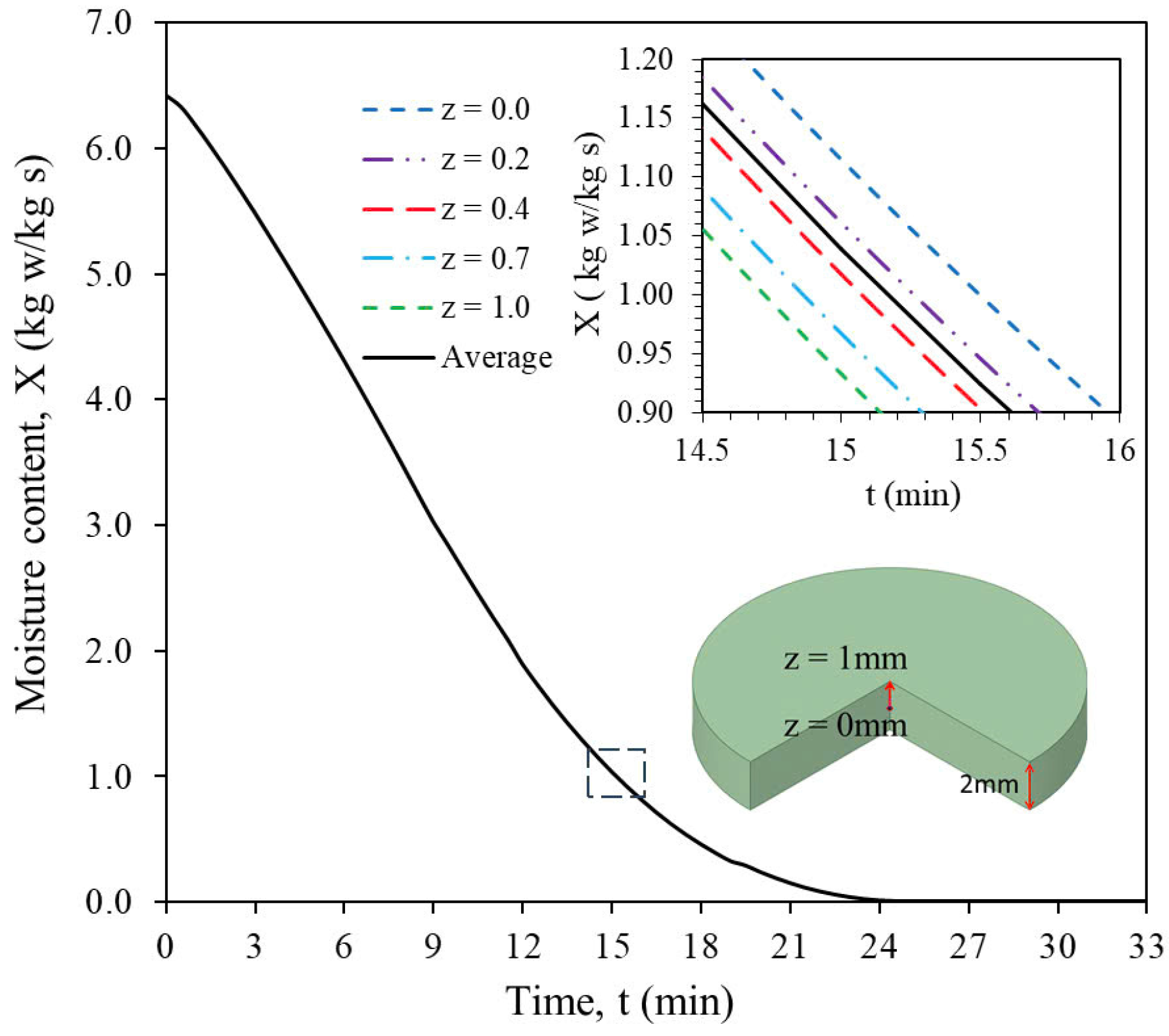
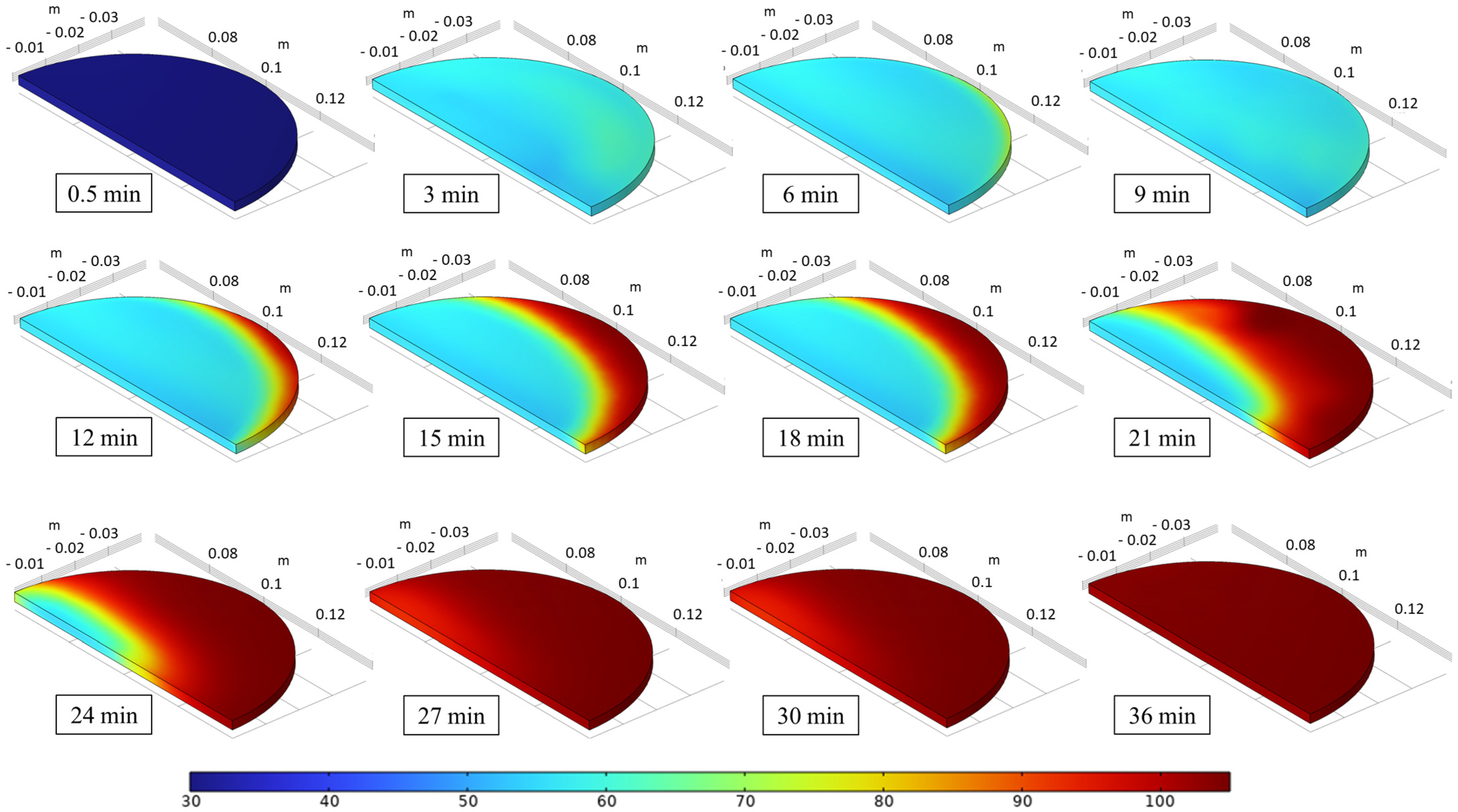
3.3.1. Temperature and Moisture Content Distributions
3.3.2. Temperature and Moisture Content Distributions of the Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ci | Mass concentration of species i in gas (kg/m3) |
| cw | Water mass concentration (kg/m3) |
| cv | Vapor mass concentration (kg/m3) |
| cg | Gas mass concentration (kg/m3) |
| cp,eff | Effective specific heat capacity (J/kgK) |
| cp,w, cp,g, cp.s | Specific heat capacity of water, gas, and solid (J/kgK) |
| Dc, Deff,g, Dvg | Capillary diffusivity, effective gas diffusivity, and vapor gas binary diffusivity (m2/s) |
| hg, hw | Gas enthalpy and water enthalpy (J) |
| hfg | Latent heat evaporation (J/kg) |
| Kevap | Evaporation constant |
| kw | Intrinsic permeability of water (m2) |
| kr,w | Relative permeability of water (m2) |
| kg | Intrinsic permeability of gas (m2) |
| kr,g | Relative permeability of gas (m2) |
| keff | Effective thermal conductivity (W/mK) |
| kth,g, kth,w, kth,s | Thermal conductivity of gas, water, and solid (W/mK) |
| Mv, Mg | Vapor molecular weight and gas molecular weight (kg/mol) |
| P | Total pressure (Pa) |
| pv, pg | Partial pressure of vapor and gas (Pa) |
| pv,eq | Equilibrium vapor pressure (Pa) |
| pv,sat | Saturation vapor pressure (Pa) |
| pamb | Ambient pressure (Pa) |
| R | Universal gas constant (J/molK) |
| Revap | Evaporation rate (kg/m3s) |
| Sw, Sg | Saturation of water and gas, respectively |
| Sw0, Sv0, Sg0 | Initial saturation of water, vapor, and gas |
| T | Product temperature (°C) |
| Tg | Gas temperature (°C) |
| u | Gas velocity (m/s) |
| Volume of gas, water, and solid (m3) | |
| X | Moisture content (kg w/kg s) |
| z | Coordinate (mm) |
| Apparent porosity | |
| Viscosity of water and gas (Pa·s) | |
| Mass fraction of vapor and gas | |
| Density of water and gas (kg/m3) | |
| Effective density (kg/m3) | |
| Time (s) |
References
- Hawlader, M.; Perera, C.O.; Tian, M. Properties of modified atmosphere heat pump dried foods. J. Food Eng. 2006, 74, 392–401. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Law, C.L. Drying Technology: Trends and Applications in Postharvest Processing. Food Bioprocess. Technol. 2010, 3, 843–852. [Google Scholar] [CrossRef]
- Alwazeer, D.; Örs, B. Reducing atmosphere drying as a novel drying technique for preserving the sensorial and nutritional notes of foods. J. Food Sci. Technol. 2019, 56, 3790–3800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Miao, S.; Chong, C.; Sun, Y. Effect of a Modified Atmosphere on Drying and Quality Characteristics of Carrots. Food Bioprocess. Technol. 2014, 7, 2549–2559. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Perera, C.O.; Tian, M. Comparison of the Retention of 6-Gingerol in Drying of Ginger Under Modified Atmosphere Heat Pump Drying and other Drying Methods. Dry. Technol. 2006, 24, 51–56. [Google Scholar] [CrossRef]
- Cam, I.B.; Basunal Gulmez, H.; Eroglu, E.; Topuz, A. Strawberry drying: Development of a closed-cycle modified atmosphere drying system for food products and the performance evaluation of a case study. Dry. Technol. 2018, 36, 1460–1473. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Miao, S.; Wu, J.-Y.; Liu, J.-X. Drying and Quality Characteristics of Flos Lonicerae in Modified Atmosphere with Heat Pump System. J. Food Process Eng. 2014, 37, 37–45. [Google Scholar] [CrossRef]
- O’Neill, M.B.; Rahman, M.S.; Perera, C.O.; Smith, B.; Melton, L.D. Color and density of apple cubes dried in air and modified atmosphere. Int. J. Food Prop. 1998, 1, 197–205. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Perera, C.O.; Tian, M.; Yeo, K.L. Drying of Guava and Papaya: Impact of Different Drying Methods. Dry. Technol. 2006, 24, 77–87. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Kinetics of L -Ascorbic Acid Degradation in Pineapple Drying under Ethanolic Atmosphere. Dry. Technol. 2009, 27, 947–954. [Google Scholar] [CrossRef]
- Braga, A.M.P.; Silva, M.A.; Pedroso, M.P.; Augusto, F.; Barata, L.E.S. Volatile Composition Changes of Pineapple during Drying in Modified and Controlled Atmosphere. Int. J. Food Eng. 2010, 6, 1–12. [Google Scholar] [CrossRef]
- Khanlari, Y.; Aroujalian, A.; Fazel, S.; Fathizadeh, M. An Experimental Work and Mathematical Modeling on Kinetic Drying of Tomato Pulp Under Different Modified Atmosphere Conditions. Int. J. Food Prop. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Nadhari, W.N.A.W.; Hashim, R.; Sulaiman, O.; Jumhuri, N. Drying kinetics of oil palm trunk waste in control atmosphere and open air convection drying. Int. J. Heat. Mass. Transf. 2014, 68, 14–20. [Google Scholar] [CrossRef]
- Jannot, Y.; Talla, A.; Nganhou, J.; Puiggali, J.-R. Modeling of Banana Convective Drying by the Drying Characteristic Curve (DCC) Method. Dry. Technol. 2004, 22, 1949–1968. [Google Scholar] [CrossRef]
- Akpinar, E.K. Determination of suitable thin layer drying curve model for some vegetables and fruits. J. Food Eng. 2006, 73, 75–84. [Google Scholar] [CrossRef]
- Chen, X.D.; Putranto, A. Reaction Engineering Approach (REA) to Modeling Drying Problems: Recent Development and Implementations. Dry. Technol. 2015, 33, 1899–1910. [Google Scholar] [CrossRef]
- Compaore, A.; Dissa, A.O.; Rogaume, Y.; Putranto, A.; Chen, X.D.; Mangindaan, D.; Zoulalian, A.; Rémond, R.; Tiendrebeogo, E. Application of the reaction engineering approach (REA) for modeling of the convective drying of onion. Dry. Technol. 2017, 35, 500–508. [Google Scholar] [CrossRef]
- Vega-Castro, O.; Osorio-Arias, J.; Duarte-Correa, Y.; Jaques, A.; Ramírez, C.; Núñez, H.; Simpson, R. Critical Analysis of the Use of Semiempirical Models on the Dehydration of Thin-Layer Foods Based on Two Study Cases. Arab. J. Sci. Eng. 2023, 48, 15851–15863. [Google Scholar] [CrossRef]
- Anabel, F.; Celia, R.; Germán, M.; Rosa, R. Determination of effective moisture diffusivity and thermodynamic properties variation of regional wastes under different atmospheres. Case Stud. Therm. Eng. 2018, 12, 248–257. [Google Scholar] [CrossRef]
- Vu, H.T.; Tsotsas, E. Mass and Heat Transport Models for Analysis of the Drying Process in Porous Media: A Review and Numerical Implementation. Int. J. Chem. Eng. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Elustondo, D.; Elustondo, M.P.; Urbicain, M.J. Mathematical modeling of moisture evaporation from foodstuffs exposed to subatmospheric pressure superheated steam. J. Food Eng. 2001, 49, 15–24. [Google Scholar] [CrossRef]
- Kumar, C.; Joardder, M.U.; Farrell, T.W.; Millar, G.J.; Karim, A. A porous media transport model for apple drying. Biosyst. Eng. 2018, 176, 12–25. [Google Scholar] [CrossRef]
- Agarla, M.; Napoletano, P.; Schettini, R. Quasi Real-Time Apple Defect Segmentation Using Deep Learning. Sensors 2023, 23, 7893. [Google Scholar] [CrossRef] [PubMed]
- Demiray, E.; Yazar, J.G.; Aktok, Ö.; Çulluk, B.; Çalışkan Koç, G.; Pandiselvam, R. The Effect of Drying Temperature and Thickness on the Drying Kinetic, Antioxidant Activity, Phenolic Compounds, and Color Values of Apple Slices. J. Food Qual. 2023, 2023, 1–12. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Le, K.H.; Tsotsas, E.; Kharaghani, A. Continuum-scale modeling of superheated steam drying of cellular plant porous media. Int. J. Heat. Mass. Transf. 2018, 124, 1033–1044. [Google Scholar] [CrossRef]
- Heinen, M.; Vrabec, J.; Fischer, J. Communication: Evaporation: Influence of heat transport in the liquid on the interface temperature and the particle flux. J. Chem. Phys. 2016, 145, 81101. [Google Scholar] [CrossRef] [PubMed]
- Isaac, O.; Thiemer, K. Biochemische Untersuchungen von Kamilleninhaltsstoffen. III. In-vitro-Versuche über die antipeptische Wirkung des (-)-alpha-Bisabolols. Arzneimittelforschung 1975, 25, 1352–1354. [Google Scholar]
- Le, K.H.; Hampel, N.; Kharaghani, A.; Bück, A.; Tsotsas, E. Superheated steam drying of single wood particles: A characteristic drying curve model deduced from continuum model simulations and assessed by experiments. Dry. Technol. 2018, 36, 1866–1881. [Google Scholar] [CrossRef]

| Parameter | Amount | Unit |
|---|---|---|
| Water density | kg/m3 | |
| Water heat capacity | kJ/kgK | |
| Water thermal conductivity | W/mK | |
| Water dynamic viscosity | kg/m·s | |
| Vapor heat capacity | kJ/kgK | |
| Vapor thermal conductivity | W/mK | |
| Vapor dynamic viscosity | kg/m·s | |
| Carbon dioxide density | kg/m3 | |
| Carbon dioxide heat capacity | kJ/kgK | |
| Carbon dioxide thermal conductivity | W/mK | |
| Carbon dioxide dynamic viscosity | kg/m·s |
| Parameters | Formula |
|---|---|
| Apple tissue intrinsic permeability | |
| Gas intrinsic permeability | |
| Permeabilities of water and gas for apple | |
| Gas diffusivity | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.C.; Le, Q.T.; Tran, T.T.H. Modeling for Apple-Slice Drying in Carbon Dioxide Gas. Agriculture 2024, 14, 1642. https://doi.org/10.3390/agriculture14091642
Do TC, Le QT, Tran TTH. Modeling for Apple-Slice Drying in Carbon Dioxide Gas. Agriculture. 2024; 14(9):1642. https://doi.org/10.3390/agriculture14091642
Chicago/Turabian StyleDo, Tien Cong, Quoc Tuan Le, and Thi Thu Hang Tran. 2024. "Modeling for Apple-Slice Drying in Carbon Dioxide Gas" Agriculture 14, no. 9: 1642. https://doi.org/10.3390/agriculture14091642
APA StyleDo, T. C., Le, Q. T., & Tran, T. T. H. (2024). Modeling for Apple-Slice Drying in Carbon Dioxide Gas. Agriculture, 14(9), 1642. https://doi.org/10.3390/agriculture14091642






