Non-Destructive PTR-ToF-MS Profiling of Red Delicious and Granny Smith Apple Volatilomes During Ripening
Abstract
:1. Introduction
2. Results
2.1. Quality Parameters at Harvest
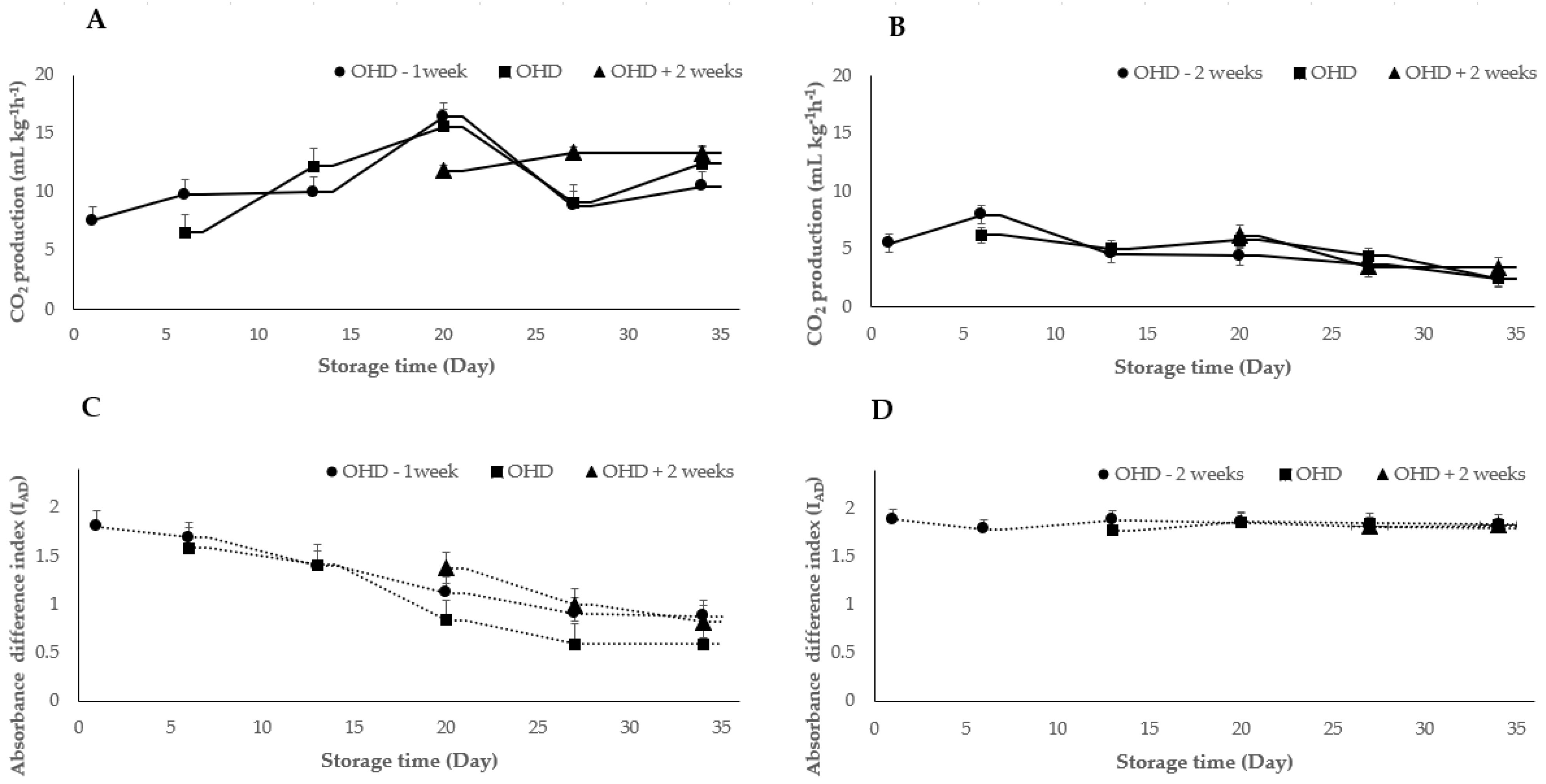
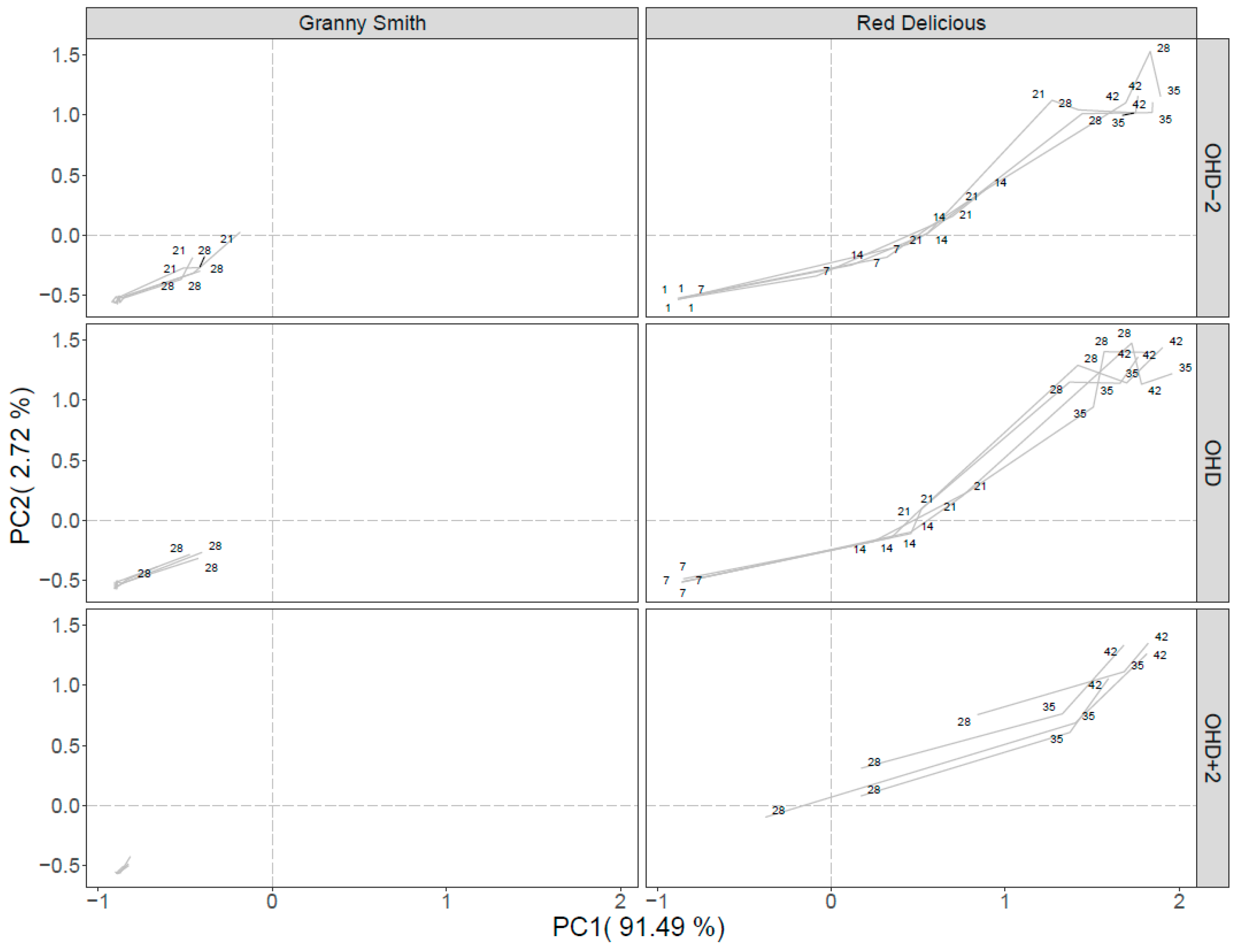
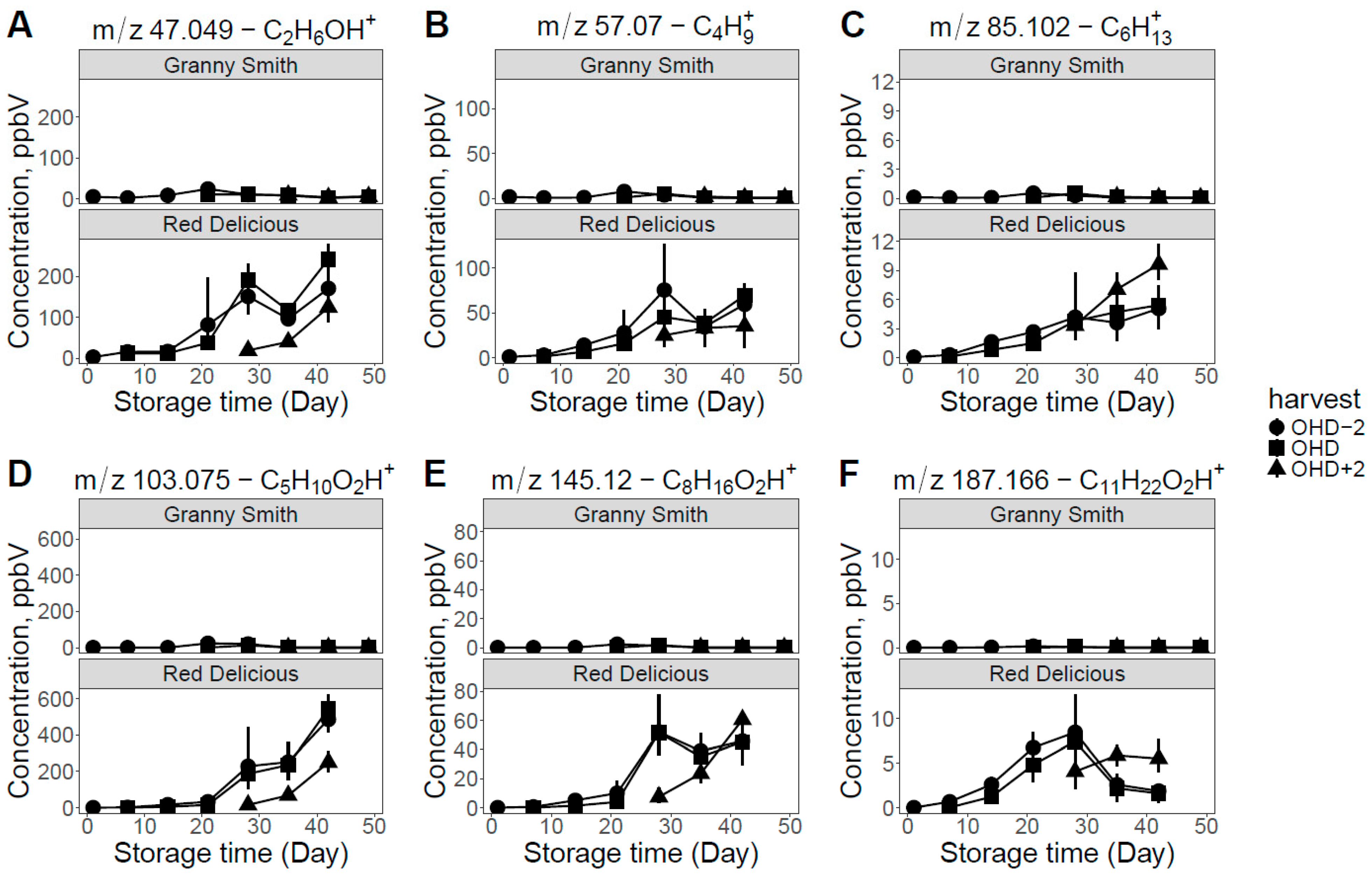
2.2. VOCs Analysis in Relation to CO2 Production and IAD Value
3. Discussion
4. Materials and Methods
4.1. Fruit Sampling and Quality Parameters at Harvest
4.2. Non-Destructive VOCs Analysis by PTR-ToF-MS
4.3. CO2 Analysis with Li-COR (LI-850)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinding, C.; Saint-Eve, A.; Thomas-Danguin, T. Multimodal sensory interactions. In Flavor; Woodhead Publishing: Cambridge, UK, 2023; pp. 205–231. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Nijssen, L.M.; van Ingen-Visscher, C.A.; Donders, J.J.H. Volatile Compounds in Food (VCF) Database; Version 13.1; TNO Triskelion: Zeist, The Netherlands, 2011; Available online: http://www.vcf-online.nl/VcfHome.Cfm (accessed on 7 February 2022).
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavor volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Plotto, A.; McDaniel, M.R.; Mattheis, J.P. Characterization of changes in ’Gala’apple aroma during storage using Osme analysis, a gas chromatography-olfactometry technique. J. Am. Soc. Hort Sci. 1999, 124, 416–423. [Google Scholar] [CrossRef]
- Komthong, P.; Hayakawa, S.; Katoh, T.; Igura, N.; Shimoda, M. Determination of potent odorants in apple by headspace gas dilution analysis. LWT-Food Sci. Technol. 2006, 39, 472–478. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Aroma volatiles: Biosynthesis and mechanisms of modulation during fruit ripening. Adv. Bot. Res. 2009, 50, 1–37. [Google Scholar]
- Fellman, J.K.; Miller, T.W.; Mattinson, D.S.; Mattheis, J.P. Factors that influence biosynthesis of volatile flavor compounds in apple fruits. HortScience 2000, 35, 1026–1033. [Google Scholar] [CrossRef]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- De Pooter, H.; Van Acker, M.R.; Schamp, N.M. Aldehyde metabolism and the aroma quality of stored Golden Delicious apples. Phytochemistry 1986, 26, 89–92. [Google Scholar] [CrossRef]
- Hongsoongnern, P.; Chambers, E. A lexicon for texture and flavor characteristics of fresh and processed tomatoes. J. Sens. Stud. 2008, 23, 583–599. [Google Scholar] [CrossRef]
- Bartley, I.M.; Hindley, S.J. Alcohol dehydrogenase of apple. J. Exp. Bot. 1980, 31, 449–459. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Lu, H.; Liu, J.; Song, C.; Xu, Z.; Yang, H.; Shang, X.; Feng, T. Comparative Aroma Profile Analysis and Development of a Sensory Aroma Lexicon of Seven Different Varieties of Flammulina velutipes. Front. Nutr. 2022, 9, 827825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- St-Pierre, B.; De Luca, V. Origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. Recent. Adv. Phytochem. 2000, 34, 285–315. [Google Scholar]
- Olivas, G. Quality attributes during maturation of ‘Golden Delicious’ and ‘Red Delicious’ apples grown in two geographical regions with different environmental conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12241. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 20, 53. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Buchanan, D.A.; Fellman, J.K. Change in apple fruit volatiles after storage in atmospheres inducing anaerobic metabolism. J. Agric. Food Chem. 1992, 39, 1602–1605. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375. [Google Scholar] [CrossRef] [PubMed]
- Fellman, J.K.; Rudell, D.R.; Mattinson, D.S.; Mattheis, J.P. Relationship of harvest maturity to flavor regeneration after CA storage of “Delicious” apples. Postharvest Biol. Technol. 2003, 27, 39–51. [Google Scholar] [CrossRef]
- Zanella, A.; Rossi, O. Post-harvest retention of apple fruit firmness by 1-methylcyclopropene (1-MCP) treatment or dynamic CA storage with chlorophyll fluorescence (DCA-CF). Eur. J. Hortic. Sci. 2015, 80, 11–17. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Silsby, K.J. Predicting Harvest Date Windows for Apples; Cornell Cooperative Extension: Ithaca, NY, USA, 1992. [Google Scholar]
- Knee, M.; Smith, S.M.; Johnson, D.S. Comparison of methods for estimating the onset of the respiration climacteric in unpicked apples. J. Hortic. Sci. 1983, 58, 521–526. [Google Scholar] [CrossRef]
- Zanella, A.; Stürz, S.; Panarese, A.; Rossi, O. The potential of alternative methods for determining the optimum harvest date of apple fruit. Acta Hortic. 2015, 1079, 373–381. [Google Scholar] [CrossRef]
- Biasioli, F.; Gasperi, F.; Yeretzian, C.; Märk, T.D. PTR-MS monitoring of VOCs and BVOCs in food science and technology. TrAC Trends Anal. Chem. 2011, 30, 968–977. [Google Scholar] [CrossRef]
- Mazzucotelli, M.; Farneti, B.; Khomenko, I.; Gonzalez-Estanol, K.; Pedrotti, M.; Fragasso, M.G.; Capozzi, V.; Biasioli, F. Proton transfer reaction mass spectrometry: A green alternative for food volatilome profiling. Green Anal. Chem. 2022, 3, 100041. [Google Scholar] [CrossRef]
- Soukoulis, C.; Cappellin, L.; Aprea, E.; Costa, F.; Viola, R.; Märk, T.D.; Biasioli, F. PTR-ToF-MS, a novel, rapid, high sensitivity and non-invasive tool to monitor volatile compound release during fruit post-harvest storage: The case study of apple ripening. Food Bioprocess. Technol. 2013, 6, 2831–2843. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2015, 11, 838–850. [Google Scholar] [CrossRef]
- Cappellin, L.; Costa, F.; Aprea, E.; Betta, E.; Gasperi, F.; Biasioli, F. Double clustering of PTR-ToF-MS data enables the mapping of QTLs related to apple fruit volatilome. Sci. Hortic. 2015, 197, 24–32. [Google Scholar] [CrossRef]
- Baldi, P.; Buti, M.; Gualandri, V.; Khomenko, I.; Farneti, B.; Biasioli, F.; Malnoy, M. Transcriptomic and volatilomic profiles reveal Neofabraea vagabunda infection-induced changes in susceptible and resistant apples during storage. Postharvest Biol. Technol. 2024, 212, 112889. [Google Scholar] [CrossRef]
- Neri, F.; Cappellin, L.; Aprea, E.; Biasioli, F.; Gasperi, F.; Spadoni, A.; Baraldi, E. Interplay of apple volatile organic compounds with Neofabraea vagabunda and other post-harvest pathogens. Plant Pathol. 2019, 68, 1508–1524. [Google Scholar] [CrossRef]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Knee, M. Anthocyanin, Carotenoid, and Chlorophyll Changes in the Pell of Cox’s Orange Pippin Apples during Ripening on and off the Tree. J. Exp. Bot. 1972, 23, 184–196. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Update on the biochemistry of chlorophyll breakdown. Plant Mol. Biol. 2013, 82, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, P.M.A.; Mostofil, Y.; Wiersma, P.; Hampson, C. Evaluation of Non-Destructive Instruments for Assessing Apple Maturity and Quality: 2011 Results; Agriculture and Agri-Food Canada and the Okanagan Plant Improvement Corporation (PICO) Report; Agriculture and Agri-Food Canada (AAFC): Ottawa, ON, Canada, 2012.
- Betemps, D.L.; Fachinello, J.C.; Galarça, S.P.; Portela, N.M.; Remorini, D.; Massai, R.; Agati, G. Non-destructive evaluation of ripening and quality traits in apples using a multiparametric fluorescence sensor. J. Sci. Food Agric. 2012, 92, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Graus, M.; Müller, M.; Hansel, A. High resolution PTR-TOF: Quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass. Spectrom. 2011, 21, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Materić, D.; Lanza, M.; Sulzer, P.; Herbig, J.; Bruhn, D.; Turner, C.; Gauci, V. Monoterpene separation by coupling proton transfer reaction time-of-flight mass spectrometry with fast GC. Anal. Bioanal. Chem. 2015, 407, 7757–7763. [Google Scholar] [CrossRef]
- Ellis, A.M.; Mayhew, C.A. Proton Transfer Reaction Mass Spectrometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Galliard, T. Aspects of lipid metabolism in higher plants—II. The identification and quantitative analysis of lipids from the pulp of pre-and post-climacteric apples. Phytochemistry 1968, 7, 1915–1922. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Mattivi, F. Is there room for improving the nutraceutical composition of apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Fellman, J.K.; Patterson, M.E. Effect of methyl jasmonate on ethylene and volatile production by summerred apples depends on fruit developmental stage. J. Agric. Food Chem. 1997, 45, 208–211. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. The effect of harvest date on aroma compound production from ‘Golden Delicious’ apple fruit and relationship to respiration and ethylene production. Postharvest Biol. Technol. 1996, 8, 259–269. [Google Scholar] [CrossRef]
- Brackman, A.; Streif, J. Ethylene, CO2 and aroma volatiles production by apple cultivars. In Proceedings of the International Symposium on Postharvest Treatment of Horticultural Crops, Kecskemét, Hungary, 30 August–3 September 1993; Volume 368, pp. 51–58. [Google Scholar]
- Rudell, D.R.; Mattinson, D.S.; Fellman, J.K.; Mattheis, J.P. The Progression of Ethylene Production and Respiration in the Tissues of Ripening Fuji Apple Fruit. HortScience 2000, 35, 1300–1303. [Google Scholar] [CrossRef]
- Zanella, A.; Werth, E. Physico-chemical parameters related to quality in apples. Riv. Di Fruttic. E Di Ortofloric. 2005, 67, 54–58. [Google Scholar]
- Sadar, N.; Zanella, A. A study on the potential of IAD as a surrogate index of quality and storability in cv. ‘Gala’ apple fruit. Agronomy 2019, 9, 642. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Mannapperuma, J.D.; Singh, R.P.; Montero, M.E. Simultaneous gas diffusion and chemical reaction in foods stored in modified atmospheres. J. Food Eng. 1991, 14, 167–183. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Fabris, A.; Schuhfried, E.; Soukoulis, C.; Märk, T.D.; Gasperi, F. Improved mass accuracy in PTR-TOF-MS: Another step towards better compound identification in PTR-MS. Int. J. Mass Spectrom. 2010, 290, 60–63. [Google Scholar] [CrossRef]
- Farneti, B.; Busatto, N.; Khomenko, I.; Cappellin, L.; Gutierrez, S.; Spinelli, F.; Costa, F. Untargeted metabolomics investigation of volatile compounds involved in the development of apple superficial scald by PTR-ToF–MS. Metabolomics 2015, 11, 341–349. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in ‘Red Delicious’ and ‘Granny Smith’ apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef]
| Cultivar | Harvest | Date | Starch (LB Scale 1–5) | Firmness (N) | TTA (MAeq g/L) | TSS (°Brix) | IEC (ppm) | IAD |
|---|---|---|---|---|---|---|---|---|
| Red Delicious | OHD − 1 week | 22.08.22 | 1.77 ± 0.04 | 76.69 ± 0.11 a | 3.43 ± 0.09 a | 8.73 ± 0.05 a | n.a. | 1.80 ± 0.01 |
| OHD | 30.08.22 | 2.54 ± 0.08 | 68.70 ± 0.08 a | 3.33 ± 0.03 a | 9.79 ± 0.08 a | 4.53 ± 1.15 | 1.54 ± 0.02 | |
| OHD + 2 weeks | 13.09.22 | 4.21 ± 0.11 | 63.10 ± 0.09 a | 2.87 ± 0.03 a | 10.38± 0.23 a | 7.48 ± 2.20 | 1.35 ± 0.03 | |
| Granny Smith | OHD − 2 weeks | 01.09.22 | 1.73 ± 0.02 | 82.55 ± 0.11 b | 9.13 ± 0.47 b | 9.08 ± 0.05 b | 0.06 ± 0.03 | 1.81 ± 0.01 |
| OHD | 13.09.22 | 1.86 ± 0.10 | 77.97 ± 0.10 b | 8.87 ± 0.30 b | 8.48 ± 0.53 b | 0.14 ± 0.05 | 1.83 ± 0.02 | |
| OHD + 2 weeks | 27.09.22 | 2.45 ± 0.08 | 68.05 ± 0.27 b | 7.4 ± 0.31 b | 9.57 ± 0.31 b | 0.15 ± 0.04 | 1.88 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panarese, A.; Farneti, B.; Zanella, A.; Khomenko, I. Non-Destructive PTR-ToF-MS Profiling of Red Delicious and Granny Smith Apple Volatilomes During Ripening. Agriculture 2025, 15, 638. https://doi.org/10.3390/agriculture15060638
Panarese A, Farneti B, Zanella A, Khomenko I. Non-Destructive PTR-ToF-MS Profiling of Red Delicious and Granny Smith Apple Volatilomes During Ripening. Agriculture. 2025; 15(6):638. https://doi.org/10.3390/agriculture15060638
Chicago/Turabian StylePanarese, Alessia, Brian Farneti, Angelo Zanella, and Iuliia Khomenko. 2025. "Non-Destructive PTR-ToF-MS Profiling of Red Delicious and Granny Smith Apple Volatilomes During Ripening" Agriculture 15, no. 6: 638. https://doi.org/10.3390/agriculture15060638
APA StylePanarese, A., Farneti, B., Zanella, A., & Khomenko, I. (2025). Non-Destructive PTR-ToF-MS Profiling of Red Delicious and Granny Smith Apple Volatilomes During Ripening. Agriculture, 15(6), 638. https://doi.org/10.3390/agriculture15060638







