Dietary Passion Fruit Seed Oil Supplementation for Health and Performance of Laying Hens
Abstract
:1. Introduction
2. Materials and Methods
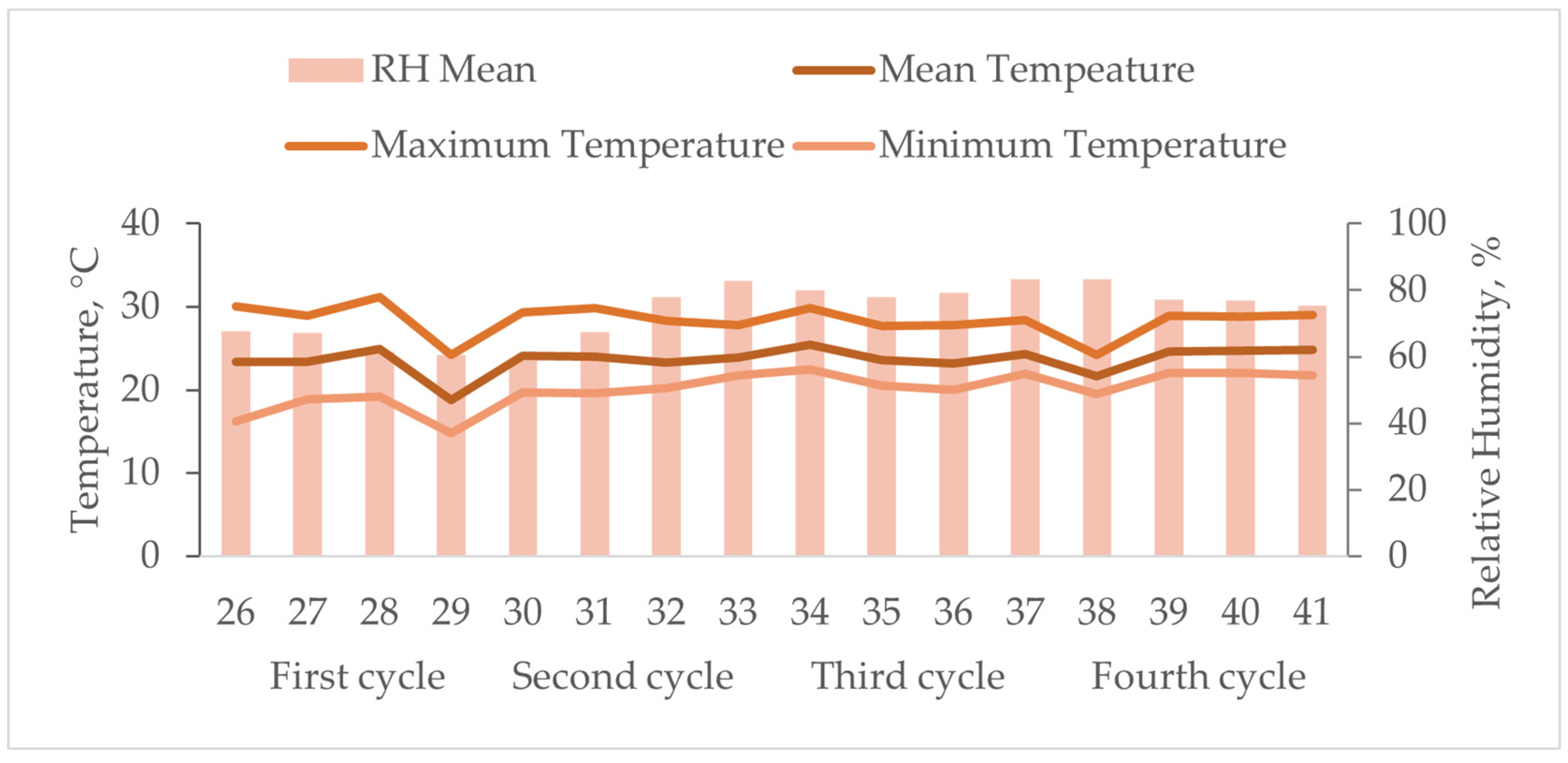
2.1. Experimental Design and Diets
2.2. Effects of PFSO on Performance
2.3. Effects of PFSO on Egg Quality
2.4. Internal Organs
2.5. Lipid Oxidation in Plasma
2.6. Antioxidant Enzyme Activity in the Liver
2.7. RNA Extraction and Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Performance
3.2. Egg Quality
3.3. Relative Internal Organ Weights
3.4. Lipid Oxidation in Plasma and Antioxidant Activity in the Liver
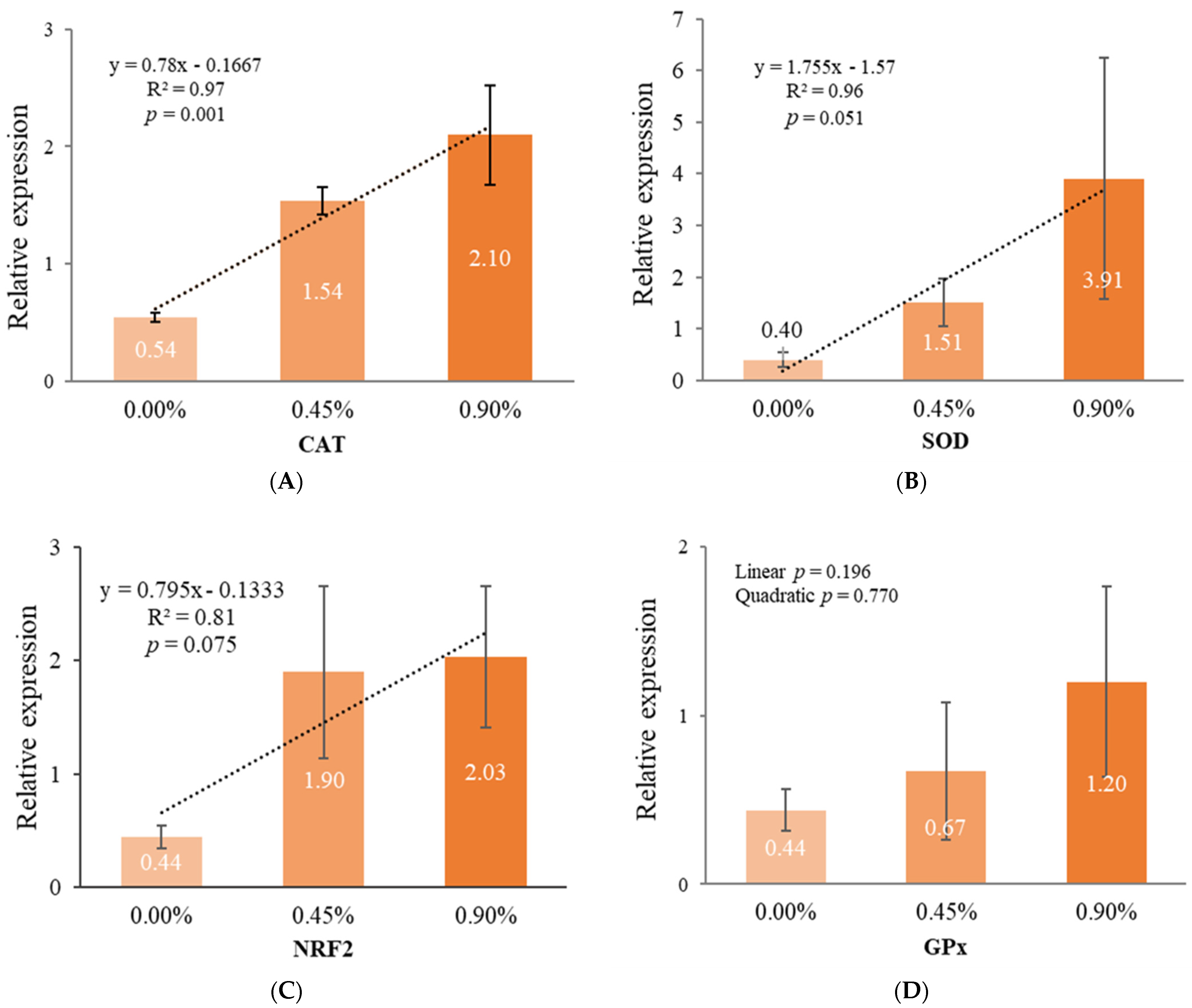
3.5. Gene Expression in the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | carotenoids |
| CAT | catalase |
| GPx | glutathione peroxidase |
| HCl | Hydrochloric acid |
| HU | Haugh unit |
| IBTEC | Biotechnology Institute |
| MDA | malonaldehyde |
| NRF2 | erythroid nuclear factor 2 |
| PFSO | passion fruit seed oil |
| RH | relative humidity |
| SOD | superoxide dismutase |
| SWUSA | shell weight per surface area |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
| TCA | Trichloroacetic acid |
| UNESP | São Paulo State University |
References
- Malacrida, C.R.; Jorge, N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): Physical and chemical characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 fatty acids, inflammation, and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.C.; Shinagawa, F.B.; Araujo, E.S.; Costa, A.M.; Mancini-Filho, J. Chemical composition and antioxidant capacity of brazilian passiflora seed oils. J. Food Sci. 2015, 80, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, L.H.; Granero, L.; Poletto, M.; Da Luz, P.A.; Lala, B.; Delbem, N.L.C.; Sobral, N.C.; Brito, E.P.; Denadai, J.C.; Andrighetto, C.; et al. Chemical, physical, and oxidative characteristics of broilers meat supplemented with passion fruit seed oil. Int. J. Innov. Educ. Res. 2021, 9, 69–83. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive Compounds in food waste: A review on the transformation of food waste to animal feed. Foods 2020, 9, 291. [Google Scholar] [CrossRef]
- Cruvinel, J.M.; Groff-Urayama, P.M.; Oura, C.Y.; de Lima-Krenchinski, F.K.; dos Santos, T.S.; de Souza, B.A.; Kadri, S.M.; Correa, C.R.; Sartori, J.R.; Pezzato, A.C. Pequi Oil (Caryocar brasiliense Camb.) Attenuates the Adverse Effects of Cyclical Heat Stress and Modulates the Oxidative Stress-Related Genes in Broiler Chickens. Animals 2023, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, F.; Khajali, F. Flavonoid antioxidants in chicken meat production: Potential application and future trends. Worlds Poult. Sci. J. 2021, 77, 347–361. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative stress as a mediator of life history trade-offs: Mechanisms, measurements, and interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef]
- Celi, P.; Selle, P.H.; Cowieson, A.J. Effects of organic selenium supplementation on growth performance, nutrient utilisation, oxidative stress and selenium tissue concentrations in broiler chickens. Anim. Prod. Sci. 2013, 54, 966–971. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Meng, C.; Sun, Y.; Safdar, A.; Pasha, R.H.; Munir, M.; Ding, C. Oxidative stress in poultry: Lessons from the viral infections. Oxidative Med. Cell. Long. 2018, 2018, 5123147. [Google Scholar] [CrossRef]
- Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; Xia, W.G.; Zheng, C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: A review. Int. J. Poult. Sci. 2016, 15, 81. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, T.; Li, L.; Zhang, J.; Jiang, Y.; Gao, F. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim. Biosci. 2022, 35, 1616. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhou, Q.; Lan, F.; Li, J.; Yang, N.; Sun, C. Microbial composition of egg component and its association with hatchability of laying hens. Front. Microbiol. 2022, 13, 943097. [Google Scholar] [CrossRef]
- Zanetti, L.H. Óleo de Semente de Maracujá (Passiflora edulis) na Alimentação de Frangos de Corte. Ph.D. Thesis, São Paulo State University, São Paulo, Brazil, 2018; 100p. [Google Scholar]
- Assunção, A.S.; Martins, R.A.; Vieira, J.C.S.; Rocha, L.C.; Lima-Krenchinski, F.K.; Buzalaf, M.A.R.; Sartori, J.R.; Padilha, P.M. Shotgun proteomics reveals changes in the Pectoralis major muscle of broilers supplemented with passion fruit seed oil under cyclic heat stress conditions. Food Res. Int. 2023, 167, 112731. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Lu, S.; Tian, Y.Z.; Wang, W.Y.; Ling, T.J.; Liu, R.T.; Novel, A. A Novel Multifunctional Compound Camellikaempferoside B Decreases Aβ Production, Interferes with Aβ Aggregation, and Prohibits Aβ-Mediated Neurotoxicity and Neuroinflammation. ACS Chem. Neurosci. 2016, 7, 505–518. [Google Scholar] [CrossRef]
- dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; Rios, A.d.O. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Donzele, J.L.; Gomes, P.C.; Oliveira, R.F.; Lopes, D.C.; Ferreira, A.S.; Barreto, S.L.T. Tabelas Brasileiras para aves e Suínos: Composição de Alimentos e Exigências Nutricionais, 4th ed.; Departamento de Zootecnia: Viçosa, Brazil, 2017. [Google Scholar]
- Lohmann LSL-Lite. Manual de Manejo: Alojamento em Gaiolas. 2020. Available online: https://lohmann-breeders.com/media/2020/08/LOHMANN_MG_LSL-Lite_Portuguese.pdf (accessed on 5 August 2022).
- Sleigh, R.W. Egg Science and Technology, 4th ed.; Stadelman, W.J., Cotterill, O.J., Eds.; Routledge: New York, NY, USA, 1996; Volume 7, p. 341. [Google Scholar]
- Montenegro, A.T.; Garcia, E.A.; Molino, A.B.; Cruvinel, J.M.; Ouros, C.C.; Alves, K.S. Methods to Evaluate the Eggshell Quality of Table Eggs. Braz. J. Poult. Sci. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Haugh, R.R. The Haugh unit for measuring egg quality. U.S. Egg Poult. Mag. 1937, 43, 552–555. [Google Scholar]
- Abdallah, G.A.; Harms, R.H.; El-Husseiny, O. Various methods of measuring shell quality in relation to percentage of cracked eggs. Poult. Sci. 1993, 72, 2038–2043. [Google Scholar] [CrossRef]
- Vyncke, B.W. Direct determination of the thiobarbituric acid value in trichloroacetic acid extracts of fish as a measure of oxidative rancidity. Fett. Sci. Technol. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assay and applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zhang, J.F.; Bai, K.W.; Su, W.P.; Wang, A.A.; Zhang, L.L.; Huang, K.H.; Wang, T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018, 97, 1209–1219. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Sukker, H.; Ababneh, M.M. Effect of termal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019, 98, 991–1001. [Google Scholar] [CrossRef]
- SAS—Statistical Analysis System. SAS OnDemand for Academics, Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2023.
- Sahin, K.; Orhan, C.; Tuzcu, M.; Shain, N.; Hayirli, A.; Bilgili, S.; Kucuk, O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016, 95, 1088–1095. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure Activity Relationship of Carotenoid Derivatives in Activation of the Electrophile/Antioxidant Response Element Transcription System. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Lima-Krenchinski, F.K. Efeitos do Óleo da Semente de Maracujá nos Sistemas Imune e Antioxidante de Frangos de Corte. Master’s Thesis, São Paulo State University, São Paulo, Brazil, 2021; 137p. [Google Scholar]
- Marques, I.C.S. Pomada a Base de Óleo de Semente de Maracujá no Tratamento de Lesões Cutâneas em Coelhos. Master’s Thesis, São Paulo State University, São Paulo, Brazil, 2012; 76p. [Google Scholar]
- Rocha-Filho, P.A.; Camargo, M.F.P.; Ferrari, M.; Maruno, M. Influence of Lavander Essential Oil Addition on Passion Fruit Oil Nanoemulsions: Stability and In Vivo Study. J. Nanomed. Nanotechnol. 2014, 5, 198. [Google Scholar] [CrossRef]
- Marques, I.C.S. Óleo de Semente de Maracujá no Reparo de Feridas Cutâneas em Equinos e Ratos Wistar. Ph.D. Thesis, São Paulo State University, São Paulo, Brazil, 2016; 77p. [Google Scholar]
- Alvarenga, A.C.M. Efeitos do Óleo da Semente do Maracujá na Psoríase Experimental. Master’s Thesis, São Paulo State University, São Paulo, Brazil, 2018; 77p. [Google Scholar]
- Ding, X.M.; Mu, Y.D.; Zhang, K.Y.; Wang, J.P.; Bai, S.P.; Zeng, Q.F.; Peng, H.W. Vitamin E improves antioxidant status but not lipid metabolism in laying hens fed a aged corn-containing diet. Anim. Biosci. 2021, 34, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Júnior, J.V.R.; Araújo, G.R.; Da Cruz Pádua, B.; de Brito Magalhães, C.L.; Chaves, M.M.; Pedrosa, M.L.; Silva, M.E.; Costa, D.C. Annatto extract and β-carotene enhances antioxidant status and regulate gene expression in neutrophils of diabetic rats. Free Radic. Res. 2012, 46, 329–338. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, J.R.; Yu, Z.G.; Zhao, J.; Mo, F.; Jiang, S.X. Effects of ionophores on liver CYP1A and 3A in male broilers. J. Vet. Pharmacol. Ther. 2010, 33, 551–557. [Google Scholar] [CrossRef]
- Nagano, S.; Unuma, K.; Aki, T.; Uemura, K. N-acetylcysteine alleviates arsenic trioxide-induced reductions in hepatic catalase gene expression both in vitro and in vivo. Leg. Med. 2024, 69, 102458. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, L.; Ge, P.; Wang, L.; Liu, L.; Ye, J.; Xu, H.; Wang, L.; Song, L. CgmiR307 involved in the regulation of Nrf2-dependent oxidative response in the Pacific oyster Crassostrea gigas under high-temperature stress. Dev. Comp. Immunol. 2025, 162, 105306. [Google Scholar] [CrossRef]
- Wen, C.; Wang, L.C.; Zhou, Y.M.; Jiang, Z.Y.; Wang, T. Effect of enzyme preparation on egg production, nutrient retention, digestive enzyme activities and pancreatic enzyme messenger RNA expression of late-phase laying hens. Anim. Feed Sci. Technol. 2012, 172, 180–186. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Jiao, Y.; Wang, Q.; Deng, Y.; Du, X. The role of Pm-miR-184-3p in regulating the immune response in the pearl oyster Pinctada fucata martensii. Fish Shellfish. Immunol. 2024, 150, 109658. [Google Scholar] [CrossRef]
- Whitehead, C.C. Nutritional and metabolic aspects of fatty liver disease in poultry. Tijdschr. Diergeneeskd. 1979, 1, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, J.; Li, F.; Zheng, J.; Xu, G. Effect of Oils in Feed on the Production Performance and Egg Quality of Laying Hens. Animals 2021, 11, 3482. [Google Scholar] [CrossRef] [PubMed]
- van Eck, L.M.; Entinga, H.; Carvalhidoa, I.J.; Chen, H.; Kwakkel, R.P. Lipid metabolism and body composition in long-termproducing hens. Worlds Poult. Sci. J. 2023, 79, 243–264. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidants in poultry nutrition and reproduction: An update. Antioxidants 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Noguchi, N. Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic. Res. 2020, 55, 352–363. [Google Scholar] [CrossRef]
- Reza, A.; Bakhshalinejad, R.; Shafiee, M. Effect of dietary zinc and α-tocopheryl acetate on broiler performance, immune responses, antioxidant enzyme activities, minerals and vitamin concentration in blood and tissues of broilers. Anim. Feed Sci. Technol. 2016, 221, 12–26. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Min, Y.; Sun, T.; Niu, Z.; Liu, F. Vitamin C and vitamin E supplementation alleviates oxidative stress induced by dexamethasone and improves fertility of breeder roosters. Anim. Reprod. Sci. 2016, 171, 1–6. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.H.; Zheng, X.C.; Zhang, H.; Zhang, J.F.; Zhang, L.L.; Zhou, Y.M.; Wang, T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017, 96, 1159–1166. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Wang, S.; Wen, C.; Zhou, Y. Effects of dietary natural vitamin E supplementation on laying performance, egg quality, serum biochemical indices, tocopherol deposition and antioxidant capacity of laying hens. Ital. J. Anim. Sci. 2021, 20, 2254–2262. [Google Scholar] [CrossRef]
- Panda, A.K.; Ramarao, S.V.; Raju, M.V.; Chatterjee, R.N. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions. Br. Poult. Sci. 2008, 49, 592–599. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, M.; Huang, X.; Zheng, X.; Song, Z.; Zhang, L.; Wang, T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2018, 98, 187–193. [Google Scholar] [CrossRef]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult. Sci. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Norum, K.R. Dietary fat and blood lipids. Nutr. Rev. 1992, 50, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Takam, P.N.; Djikeng, F.T.; Kuate, D.; Kengne, A.P.N.; Tsafack, H.D.; Makamwé, I.; Oben, J.E. Passiflora edulis seed oil from west Cameroon: Chemical characterization and assessment of its hypolipidemic effect in high-fat diet–induced rats. Food Sci. Nutr. 2019, 7, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Denny, R.W. Chemistry of singlet oxygen quenching by β-carotene. J. Am. Chem. Soc. 1968, 90, 6233–6235. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M.; Amaya-Farfan, J. Fontes Brasileiras de Carotenóides:Tabela Brasileira de Composição de Carotenóides Em Alimentos; Ministério do Meio Ambiente: Brasilia, Brazil, 2008.
- Vilchez, C.; Touchburn, S.P.; Chavez, E.R.; Chan, C.W. Effect of feeding palmitic, oleic, and linoleic acids to Japanese quail hens (Coturnix coturnix japonica): 1. Reproductive performance and tissue fatty acids. Poult. Sci. 1991, 70, 2484–2493. [Google Scholar] [CrossRef]
- Grobas, S.; Méndez, J.; Lázaro, R.; de Blas, C.; Mateo, G.G. Influence of source and percentage of fat added to diet on performance and fatty acid composition of egg yolks of two strains of laying hens. Poult. Sci. 2001, 80, 1171–1179. [Google Scholar] [CrossRef]
- Keshavarz, K.; Nakajima, S. The Effect of Dietary Manipulations of Energy, Protein, and Fat During the Growing and Laying Periods on Early Egg Weight and Egg Components. Poult. Sci. 1995, 74, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Balnave, D. Essential fatty Acids in Poultry Nutrition. Worlds Poult. Sci. J. 1970, 26, 442–460. [Google Scholar] [CrossRef] [PubMed]
- March, B.E.; Macmillan, C. Linoleic Acid as a Mediator of Egg Size. Poult. Sci. 1990, 69, 634–639. [Google Scholar] [CrossRef]
- Bollengier-Lee, S.; Mitchell, M.A.; Utomo, D.B.; Williams, P.E.; Whitehead, C.C. Influence of high dietary vitamin E supplementation on egg production and plasma characteristics in hens subjected to heat stress. Br. Poult. Sci. 1998, 39, 106–112. [Google Scholar] [CrossRef]
- USDA. Egg-Grading Manual; USDA: Washington, DC, USA, 2000; 56p.
- Gerber, N. Factors Affecting Egg Quality in the Commercial Laying Hen: A Review; Poultry industry Association of New Zealand: Auckland, New Zealand, 2006. [Google Scholar]
- Wheeler, P.; Peterson, D.W.; Michaels, G.D. Fatty Acid Distribution in Egg Yolk as Influenced by Type and Level of Dietary Fat. J. Nutr. 1959, 69, 253–260. [Google Scholar] [CrossRef]
- Lelis, G.R.; Da Silva, M.D.; Tavernari, F.C.; Albino, L.F.T.; Rostagno, H.S. Performance of layers fed diets containing different oils. Braz. J. Poult. Sci. 2009, 11, 235–240. [Google Scholar] [CrossRef]
- Ceylan, N.; Ciftçi, I.; Mizrak, C.; Kahraman, Z.; Efil, H. Influence of different dietary oil sources on performance and fatty acids profile of egg yolk in laying hens. J. Anim. Feed Sci. 2011, 20, 71–83. [Google Scholar] [CrossRef]
- Reddy, R.V.; Lightsey, S.F.; Maurice, D.V. Research Note: Effect of Feeding Garlic Oil on Performance and Egg Yolk Cholesterol Concentration. Poult. Sci. 1991, 70, 2006–2009. [Google Scholar] [CrossRef]
- Costa, F.G.P.; de Souza, J.G.; da Silva, J.H.V.; Rabello, C.B.-V.; de Castro Goulart, C.; da Cunha Lima Neto, R. Influência do óleo de linhaça sobre o desempenho e a qualidade dos ovos de poedeiras semipesadas. Rev. Bras. Zootec. 2008, 37, 861–868. [Google Scholar] [CrossRef]
- Zaazaa, A.; Sabbah, M.; Omar, J. Effects of oil source on egg quality and yolk fatty acid profile of layer hens. Braz. J. Poult. Sci. 2022, 24, 1–8. [Google Scholar] [CrossRef]
- Amad, A.A.; Manner, K.; Wendler, K.R.; Neumann, K.; Zentek, J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011, 90, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, H.J.; Kim, I.H. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest. Sci. 2014, 160, 82–88. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant Compounds [3] | |
| Parameter | Quantity |
| Total carotenoids, mg of β-carotene/100 g of oil | 75.63 |
| Total phenolic compounds, g GAE/100 g of oil | 1.47 |
| Fatty acid profile [4] | |
| Name | Quantity, % |
| Myristic acid | 0.09 ± 0.01 |
| Palmitic acid | 10.99 ± 0.03 |
| Palmitoleic acid | 0.17 ± 0.01 |
| Margaric acid | 0.07 ± 0.00 |
| Stearic acid | 2.89 ± 0.02 |
| Oleic acid | 14.89 ± 0.06 |
| Vaccinium acid | 0.96 ± 0.04 |
| Linoleic acid | 69.14 ± 0.01 |
| Linolenic acid | 0.39 ± 0.01 |
| Eicosanoic acid | 0.12 ± 0.02 |
| Eicosenoic acid | 0.11 ± 0.04 |
| Docosanoic acid | 0.06 ± 0.01 |
| Erucic acid | 0.05 ± 0.01 |
| Lignoceric acid | 0.06 ± 0.00 |
| Saturated | 14.28 ± 0.01 |
| Monounsaturated | 16.19 ± 0.02 |
| Polyunsaturated | 69.53 ± 0.02 |
| Ingredients, % | Passion Fruit Seed Oil | ||
|---|---|---|---|
| 0.00% | 0.45% | 0.90% | |
| Corn | 58.49 | 58.49 | 58.49 |
| Soybean meal | 28.90 | 28.90 | 28.90 |
| Soy oil | 2.00 | 1.52 | 1.04 |
| Passion fruit seed oil | 0.00 | 0.45 | 0.90 |
| Limestone (fine) | 1.60 | 1.60 | 1.60 |
| Limestone (coarse) | 5.40 | 5.40 | 5.40 |
| Dicalcium phosphate | 0.45 | 0.45 | 0.45 |
| Sodium chloride | 0.08 | 0.08 | 0.08 |
| DL-Methionine | 0.08 | 0.08 | 0.08 |
| Supplements 1 | 3.00 | 3.00 | 3.00 |
| Inert | 0.00 | 0.03 | 0.06 |
| Nutrient composition | |||
| Metabolizable energy, kcal/kg | 2804 | 2804 | 2804 |
| Crude protein, % | 17.51 | 17.51 | 17.51 |
| Total Methionine, % | 0.410 | 0.410 | 0.410 |
| Total Methionine + Cystine, % | 0.699 | 0.699 | 0.699 |
| Total Lysine, % | 0.966 | 0.966 | 0.966 |
| Total Tryptophan, % | 0.212 | 0.212 | 0.212 |
| Total Threonine, % | 0.670 | 0.670 | 0.670 |
| Total Isoleucine, % | 0.772 | 0.772 | 0.772 |
| Digestible Lysine, % | 0.840 | 0.840 | 0.840 |
| Digestible Methionine, % | 0.390 | 0.390 | 0.390 |
| Digestible Methionine + Cystine, % | 0.632 | 0.632 | 0.632 |
| Digestible Tryptophane, % | 0.201 | 0.201 | 0.201 |
| Digestible Threonine, % | 0.594 | 0.594 | 0.594 |
| Sodium, % | 0.173 | 0.173 | 0.173 |
| Calcium, % | 3.688 | 3.688 | 3.688 |
| Total Phosphorus, % | 0.641 | 0.641 | 0.641 |
| Digestible Phosphorus, % | 0.418 | 0.418 | 0.418 |
| Choline, mg/kg | 0.110 | 0.110 | 0.110 |
| Gene 1 | Primers Sequence (5′–3′) 2 | Base Pairs | References | |
|---|---|---|---|---|
| β-actin | F: R: | TGCTGTGTTCCCATCTATCG TTGGTGACAATACCGTGTTCA | 136 | [30] |
| GPx | F: R: | GACCAACCCGCAGTACATCA GAGGTGCGGGCTTTCCTTTA | 205 | [30] |
| NRF2 | F: R: | GATGTCACCCTGCCCTTAG CTGCCACCATGTTATTCC | 215 | [30] |
| SOD2 | F: R: | CTGACCTGCCTTACGACTATG CGCCTCTTTGTATTTCTCCTCT | 131 | [31] |
| CAT | F: R: | GAAGCAGAGAGGTTCCCATTTA CATACGCCATCTGTTCTACCTC | 142 | [31] |
| Item | Passion Fruit Seed Oil | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| 0.00% | 0.45% | 0.90% | Linear | Quadratic | ||
| Egg production, % | 99.14 | 99.04 | 99.24 | 0.107 | 0.537 | 0.284 |
| Viable eggs, % | 98.79 | 99.05 | 98.47 | 0.253 | 0.426 | 0.395 |
| Total egg weight, kg | 54.25 | 53.40 | 53.95 | 0.525 | 0.690 | 0.285 |
| Average egg weight, g/hen/day | 61.25 | 60.79 | 60.85 | 0.543 | 0.603 | 0.701 |
| Daily egg mass, g/hen/day | 60.63 | 60.03 | 60.30 | 0.540 | 0.660 | 0.519 |
| Daily feed intake, g/hen/day | 115.37 | 115.99 | 116.14 | 1.191 | 0.646 | 0.877 |
| Feed conversion ratio, kg/kg | 1.933 | 1.983 | 1.958 | 0.021 | 0.412 | 0.152 |
| Body weight gain, g | 208.73 | 218.70 | 171.13 | 23.990 | 0.279 | 0.339 |
| Item | Passion Fruit Seed Oil | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| 0.00% | 0.45% | 0.90% | Linear | Quadratic | ||
| Egg weight, g | 62.76 | 61.88 | 62.61 | 0.464 | 0.825 | 0.171 |
| Albumen, % | 63.45 | 63.15 | 63.05 | 0.280 | 0.314 | 0.771 |
| Yolk, % | 26.59 | 26.84 | 27.00 | 0.244 | 0.237 | 0.882 |
| Eggshell, % | 9.96 | 10.02 | 9.96 | 0.061 | 0.965 | 0.464 |
| Yolk fan color | 7.36 | 7.35 | 7.34 | 0.051 | 0.821 | 0.977 |
| Yolk digital color | 7.49 | 7.39 | 7.38 | 0.053 | 0.148 | 0.490 |
| Yolk index | 0.44 | 0.44 | 0.43 | 0.003 | 0.378 | 0.399 |
| Haugh unit | 93.19 | 92.26 | 91.78 | 0.497 | 0.054 | 0.709 |
| Albumen diameter, mm | 68.89 | 68.73 | 69.42 | 0.440 | 0.396 | 0.438 |
| Albumen height, mm | 8.85 | 8.62 | 8.57 | 0.094 | 0.046 a | 0.459 |
| Eggshell strength, N | 46.10 | 46.19 | 47.46 | 0.923 | 0.303 | 0.611 |
| Eggshell deformity, mm | 0.88 | 0.88 | 0.92 | 0.038 | 0.480 | 0.728 |
| Eggshell thickness, mm | 0.399 | 0.400 | 0.400 | 0.002 | 0.754 | 0.965 |
| Specific gravity, g/mL | 1.096 | 1.096 | 1.096 | 0.000 | 0.465 | 0.890 |
| SWUSA, mg/cm2 2 | 84.70 | 84.79 | 84.58 | 0.477 | 0.853 | 0.802 |
| Item | Passion Fruit Seed Oil | SEM 1 | p-Value | |||
|---|---|---|---|---|---|---|
| 0.00% | 0.45% | 0.90% | Linear | Quadratic | ||
| Body weight, g | 1768.25 | 1806.50 | 1726.63 | 51.82 | 0.575 | 0.363 |
| Oviduct and intestine lengths | ||||||
| Oviduct, cm | 71.00 | 71.63 | 70.88 | 1.894 | 0.962 | 0.770 |
| Intestines, cm | 167.50 | 168.50 | 174.13 | 3.809 | 0.224 | 0.625 |
| Relative internal organ weights | ||||||
| Liver, % | 2.02 | 2.15 | 2.11 | 0.092 | 0.500 | 0.471 |
| Spleen, % | 0.09 | 0.09 | 0.09 | 0.004 | 0.367 | 0.190 |
| Pancreas, % | 0.19 | 0.21 | 0.20 | 0.011 | 0.746 | 0.264 |
| Gizzard, % | 1.69 | 1.77 | 1.79 | 0.089 | 0.391 | 0.820 |
| Abdominal Fat, % | 4.79 | 5.42 | 4.35 | 0.376 | 0.440 | 0.081 a |
| Ovary, % | 3.25 | 3.45 | 3.11 | 0.129 | 0.495 | 0.098 b |
| Oviduct, % | 4.36 | 4.55 | 4.50 | 0.199 | 0.631 | 0.632 |
| Intestines, % | 4.31 | 4.36 | 4.54 | 0.155 | 0.314 | 0.723 |
| Description 1 | Passion Fruit Seed Oil | SEM 2 | p-Value | |||
|---|---|---|---|---|---|---|
| 0.00% | 0.45% | 0.90% | Linear | Quadratic | ||
| Plasma | ||||||
| MDA (mg/L) | 0.0984 | 0.0747 | 0.0643 | 0.00887 | 0.012 a | 0.549 |
| Liver | ||||||
| SOD (U/mg) | 71.36 | 70.18 | 68.49 | 1.88 | 0.302 | 0.920 |
| GPx (U/mg) | 2.90 | 2.72 | 2.52 | 0.31 | 0.384 | 0.975 |
| CAT (U/mg) | 3.34 | 2.96 | 2.97 | 0.13 | 0.078 b | 0.270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, L.G.; Rodrigues, P.A.D.; Lima, G.A.d.; Castillo, E.O.F.; Silva, J.A.d.; Lopes, J.d.L.; Lang, A.L.; Kadri, S.M.; Pezzato, A.C.; Sartori, J.R. Dietary Passion Fruit Seed Oil Supplementation for Health and Performance of Laying Hens. Agriculture 2025, 15, 864. https://doi.org/10.3390/agriculture15080864
Cordeiro LG, Rodrigues PAD, Lima GAd, Castillo EOF, Silva JAd, Lopes JdL, Lang AL, Kadri SM, Pezzato AC, Sartori JR. Dietary Passion Fruit Seed Oil Supplementation for Health and Performance of Laying Hens. Agriculture. 2025; 15(8):864. https://doi.org/10.3390/agriculture15080864
Chicago/Turabian StyleCordeiro, Laís Garcia, Paola Aparecida Damázio Rodrigues, Gabrieli Andressa de Lima, Elis Omar Figueroa Castillo, Joyce Andrade da Silva, Júlia de Lima Lopes, Anna Luísa Lang, Samir Moura Kadri, Antônio Celso Pezzato, and José Roberto Sartori. 2025. "Dietary Passion Fruit Seed Oil Supplementation for Health and Performance of Laying Hens" Agriculture 15, no. 8: 864. https://doi.org/10.3390/agriculture15080864
APA StyleCordeiro, L. G., Rodrigues, P. A. D., Lima, G. A. d., Castillo, E. O. F., Silva, J. A. d., Lopes, J. d. L., Lang, A. L., Kadri, S. M., Pezzato, A. C., & Sartori, J. R. (2025). Dietary Passion Fruit Seed Oil Supplementation for Health and Performance of Laying Hens. Agriculture, 15(8), 864. https://doi.org/10.3390/agriculture15080864









