Apocarotenoids as Stress Signaling Molecules in Plants
Abstract
1. Introduction
2. Carotenoids
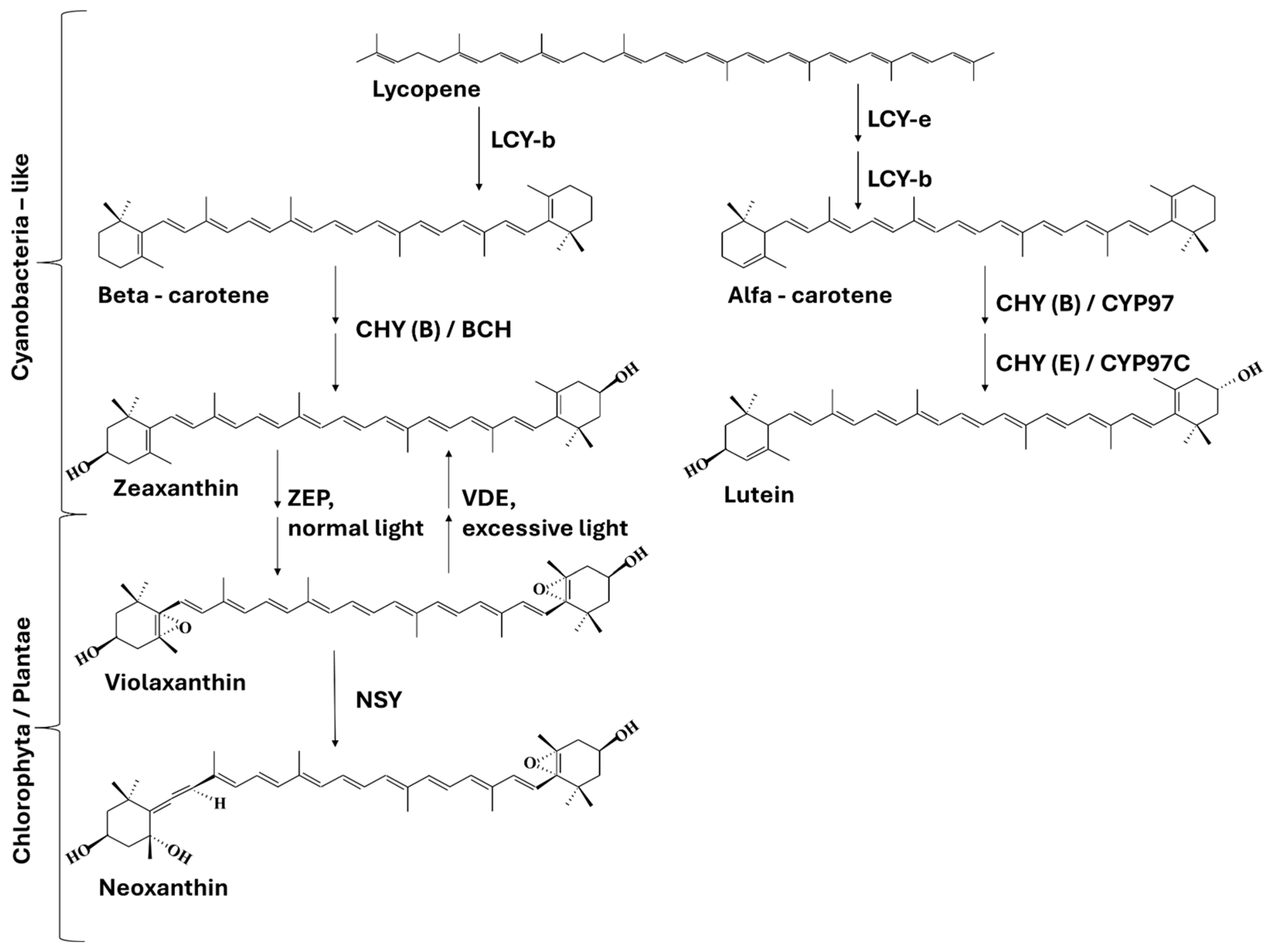
3. Apocarotenoids
4. Are ROS-Induced Apocarotenoids Singlet Oxygen Sensors?
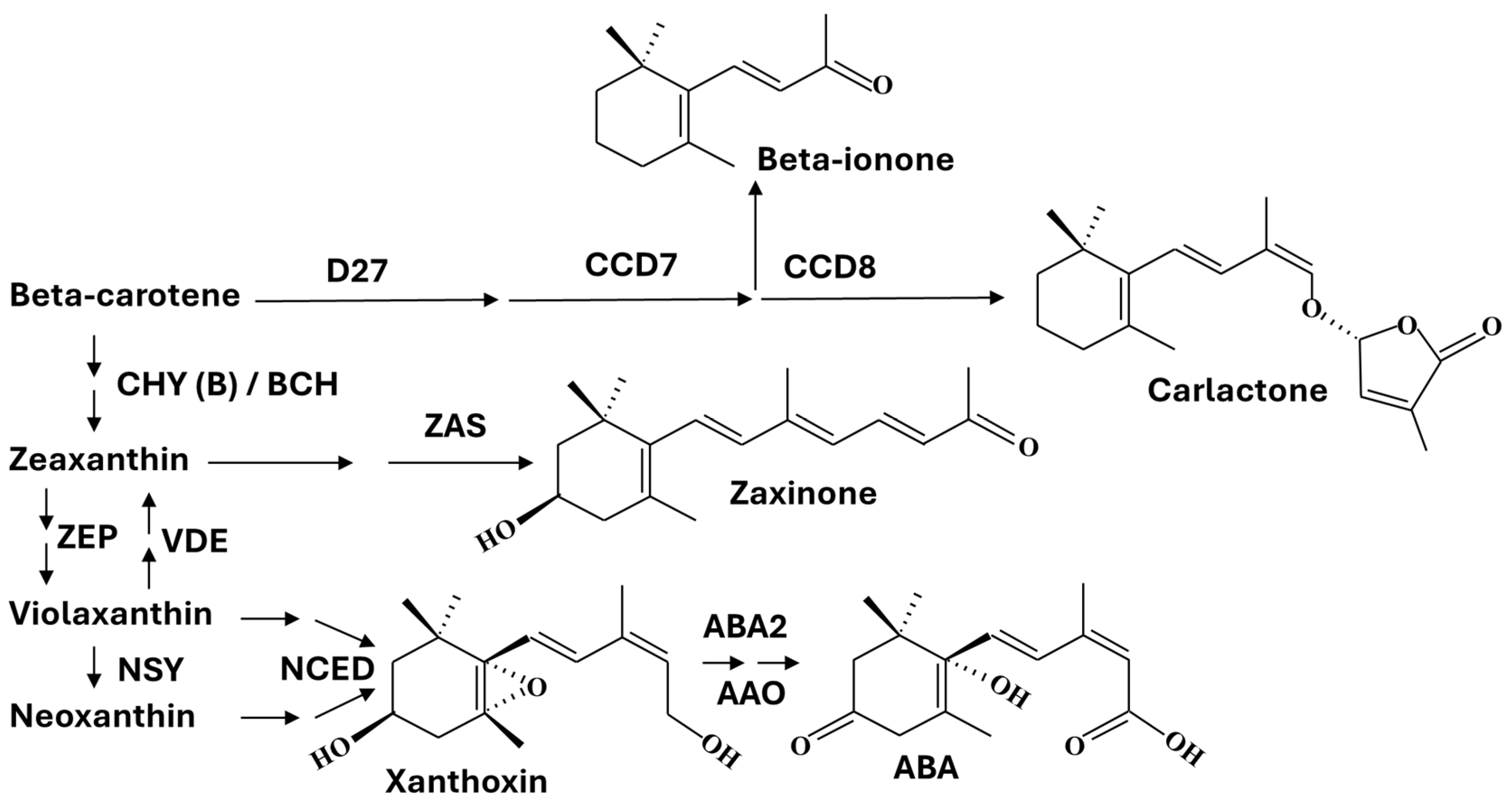
5. Origin of Apocarotenoids
6. Signaling of β-Carotene-Derived Apocarotenoids
6.1. β-cyclocitral (APO7, β-cc, Bcc, β-cyc)
β-cyclocitral and Salicylic Acid
6.2. β-Cyclogeranic Acid (β-Cyclocitric Acid, β-cca, Bcca, APO7-OOH)
6.3. β-Ionone (β-I, APO9)
6.4. Dihydroactinidiolide (dhA)
6.5. β-Apo-11-carotenoids
6.6. Anchorene and Iso-Anchorene (12,12′-Diapocarotene-12,12′-dial)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhäuser: Basel, Switzerland, 2012; ISBN 978-3-0348-7836-4. [Google Scholar]
- Sandmann, G. Diversity and origin of carotenoid biosynthesis: Its history of coevolution towards plant photosynthesis. New Phytol. 2021, 232, 479–493. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Ashenafi, E.L.; Nyman, M.C.; Shelley, J.T.; Mattson, N.S. Spectral properties and stability of selected carotenoid and chlorophyll compounds in different solvent systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Pineau, B.; Dainese, P.; Marquardt, J. Carotenoid-binding proteins of photosystem II. Eur. J. Biochem. 1993, 212, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Bressan, M.; Bassi, R. Biogenesis of light harvesting proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 861–871. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Mohamed, H.E.; van de Meene, A.M.L.; Roberson, R.W.; Vermaas, W.F.J. Myxoxanthophyll Is Required for Normal Cell Wall Structure and Thylakoid Organization in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2005, 187, 6883–6892. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. CMLS 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Pinnola, A.; Bassi, R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018, 46, 467–482. [Google Scholar] [CrossRef]
- Telfer, A. What is beta-carotene doing in the photosystem II reaction centre? Philos. Trans. R Soc. Lond. B Biol. Sci. 2002, 357, 1431–1470. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Kish, E.; Méndez-Hernández, D.D.; WongCarter, K.; Pillai, S.; Kodis, G.; Niklas, J.; Poluektov, O.G.; Gust, D.; Moore, T.A.; et al. Triplet–triplet energy transfer in artificial and natural photosynthetic antennas. Proc. Natl. Acad. Sci. USA 2017, 114, E5513–E5521. [Google Scholar] [CrossRef] [PubMed]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2013, 16, 78–90. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. Arab. Book/Am. Soc. Plant Biol. 2012, 10, e0158. [Google Scholar] [CrossRef]
- Tian, L.; Magallanes-Lundback, M.; Musetti, V.; DellaPenna, D. Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 2003, 15, 1320–1332. [Google Scholar] [CrossRef]
- Badejo, A. Elevated carotenoids in staple crops: The biosynthesis, challenges and measures for target delivery. J. Genet. Eng. Biotechnol. 2018, 16, 553–562. [Google Scholar] [CrossRef]
- Karniel, U.; Adler Berke, N.; Mann, V.; Hirschberg, J. Perturbations in the Carotenoid Biosynthesis Pathway in Tomato Fruit Reactivate the Leaf-Specific Phytoene Synthase 2. Front. Plant Sci. 2022, 13, 844748. [Google Scholar] [CrossRef]
- Sommerburg, O.; Langhans, C.-D.; Arnhold, J.; Leichsenring, M.; Salerno, C.; Crifò, C.; Hoffmann, G.F.; Debatin, K.-M.; Siems, W.G. β-Carotene cleavage products after oxidation mediated by hypochlorous acid—A model for neutrophil-derived degradation. Free. Radic. Biol. Med. 2003, 35, 1480–1490. [Google Scholar] [CrossRef]
- Stratton, S.P.; Schaefer, W.H.; Liebler, D.C. Isolation and identification of singlet oxygen oxidation products of. beta.-carotene. Chem. Res. Toxicol. 1993, 6, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.W.; Mogg, T.J.; Riley, W.W.; Nickerson, J.G. β-Carotene oxidation products—Function and safety. Food Chem. Toxicol. 2021, 152, 112207. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, H.; Arif, Y.; Alam, P.; Hayat, S. Apocarotenoids biosynthesis, signaling regulation, crosstalk with phytohormone, and its role in stress tolerance. Environ. Exp. Bot. 2023, 210, 105337. [Google Scholar] [CrossRef]
- Simkin, A.J. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants 2021, 10, 2321. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Klingler, J.P.; Batelli, G.; Zhu, J.-K. ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp. Bot. 2010, 61, 3199–3210. [Google Scholar] [CrossRef]
- Seto, Y.; Yasui, R.; Kameoka, H.; Tamiru, M.; Cao, M.; Terauchi, R.; Sakurada, A.; Hirano, R.; Kisugi, T.; Hanada, A.; et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019, 10, 191. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-Carotene to Carlactone, a Strigolactone-Like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef]
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.-P.; Guo, X.; et al. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019, 10, 810. [Google Scholar] [CrossRef]
- Moreno, J.C.; Mi, J.; Alagoz, Y.; Al-Babili, S. Plant apocarotenoids: From retrograde signaling to interspecific communication. Plant J. 2021, 105, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Hameed, U.S.; Balakrishna, A.; Ablazov, A.; Liew, K.X.; Jamil, M.; Mi, J.; Alashoor, K.; de Saint Germain, A.; Arold, S.T.; et al. DWARF14 and KARRIKIN INSENSITIVE2 mediate signaling of the apocarotenoid zaxinone in Arabidopsis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ablazov, A.; Votta, C.; Fiorilli, V.; Wang, J.Y.; Aljedaani, F.; Jamil, M.; Balakrishna, A.; Balestrini, R.; Liew, K.X.; Rajan, C.; et al. ZAXINONE SYNTHASE 2 regulates growth and arbuscular mycorrhizal symbiosis in rice. Plant Physiol. 2023, 191, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Shumbe, L.; Bott, R.; Havaux, M. Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Mol. Plant 2014, 7, 1248–1251. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Beaugelin, I.; Havaux, M. Tanned or Sunburned: How Excessive Light Triggers Plant Cell Death. Mol. Plant 2020, 13, 1545–1555. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Havaux, M. Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. 2019, 223, 1776–1783. [Google Scholar] [CrossRef]
- Jia, K.-P.; Dickinson, A.J.; Mi, J.; Cui, G.; Xiao, T.T.; Kharbatia, N.M.; Guo, X.; Sugiono, E.; Aranda, M.; Blilou, I.; et al. Anchorene is a carotenoid-derived regulatory metabolite required for anchor root formation in Arabidopsis. Sci. Adv. 2019, 5, eaaw6787. [Google Scholar] [CrossRef]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids Involved in Plant Development and Stress Response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. 2021, 105, 335–350. [Google Scholar] [CrossRef]
- Wu, W.; Cao, S.; Shi, L.; Chen, W.; Yin, X.; Yang, Z. Abscisic acid biosynthesis, metabolism and signaling in ripening fruit. Front. Plant Sci. 2023, 14, 1279031. [Google Scholar] [CrossRef] [PubMed]
- Sadali, N.M.; Sowden, R.G.; Ling, Q.; Jarvis, R.P. Differentiation of chromoplasts and other plastids in plants. Plant Cell Rep. 2019, 38, 803–818. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 Mutant Reveals a Critical Role of β-Carotene in Photosystem I Photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef]
- Hideg, É.; Barta, C.; Kálai, T.; Vass, I.; Hideg, K.; Asada, K. Detection of Singlet Oxygen and Superoxide with Fluorescent Sensors in Leaves Under Stress by Photoinhibition or UV Radiation. Plant Cell Physiol. 2002, 43, 1154–1164. [Google Scholar] [CrossRef]
- Fiedor, J.; Fiedor, L.; Haeßner, R.; Scheer, H. Cyclic endoperoxides of β-carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta (BBA) Bioenerg. 2005, 1709, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dogra, V.; Kim, C. Singlet Oxygen Metabolism: From Genesis to Signaling. Front. Plant Sci. 2020, 10, 1640. [Google Scholar] [CrossRef]
- Mattila, H.; Tyystjärvi, E. Photosystem II does not convert nascent oxygen to the poisonous singlet form. bioRxiv 2019. [Google Scholar] [CrossRef]
- Telfer, A.; Dhami, S.; Bishop, S.M.; Phillips, D.; Barber, J. .beta.-Carotene Quenches Singlet Oxygen Formed by Isolated Photosystem II Reaction Centers. Biochemistry 1994, 33, 14469–14474. [Google Scholar] [CrossRef]
- Gall, A.; Berera, R.; Alexandre, M.T.A.; Pascal, A.A.; Bordes, L.; Mendes-Pinto, M.M.; Andrianambinintsoa, S.; Stoitchkova, K.V.; Marin, A.; Valkunas, L.; et al. Molecular Adaptation of Photoprotection: Triplet States in Light-Harvesting Proteins. Biophys. J. 2011, 101, 934–942. [Google Scholar] [CrossRef]
- Mozzo, M.; Dall’Osto, L.; Hienerwadel, R.; Bassi, R.; Croce, R. Photoprotection in the Antenna Complexes of Photosystem II: ROLE OF INDIVIDUAL XANTHOPHYLLS IN CHLOROPHYLL TRIPLET QUENCHING. J. Biol. Chem. 2008, 283, 6184–6192. [Google Scholar] [CrossRef]
- Ramel, F.; Ksas, B.; Akkari, E.; Mialoundama, A.S.; Monnet, F.; Krieger-Liszkay, A.; Ravanat, J.-L.; Mueller, M.J.; Bouvier, F.; Havaux, M. Light-Induced Acclimation of the Arabidopsis chlorina1 Mutant to Singlet Oxygen. Plant Cell 2013, 25, 1445–1462. [Google Scholar] [CrossRef] [PubMed]
- Setif, P.; Hervo, G.; Mathis, P. Flash-induced absorption changes in Photosystem I, Radical pair or triplet state formation? Biochim. Biophys. Acta (BBA) Bioenerg. 1981, 638, 257–267. [Google Scholar] [CrossRef]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef] [PubMed]
- Zbyradowski, M.; Duda, M.; Wisniewska-Becker, A.; Heriyanto; Rajwa, W.; Fiedor, J.; Cvetkovic, D.; Pilch, M.; Fiedor, L. Triplet-driven chemical reactivity of β-carotene and its biological implications. Nat. Commun. 2022, 13, 2474. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Russell, G.A. Deuterium-isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of PEroxy Radicals. J. Am. Chem. Soc. 1957, 79, 3871–3877. [Google Scholar] [CrossRef]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef]
- Xu, P.; Chukhutsina, V.U.; Nawrocki, W.J.; Schansker, G.; Bielczynski, L.W.; Lu, Y.; Karcher, D.; Bock, R.; Croce, R. Photosynthesis without β-carotene. eLife 2020, 9, e58984. [Google Scholar] [CrossRef]
- Mi, J.; Jia, K.-P.; Balakrishna, A.; Wang, J.Y.; Al-Babili, S. An LC-MS profiling method reveals a route for apocarotene glycosylation and shows its induction by high light stress in Arabidopsis. Analyst 2018, 144, 1197–1204. [Google Scholar] [CrossRef]
- Li, F.-L.; Chen, X.; Luo, H.-M.; Meiners, S.J.; Kong, C.-H. Root-secreted (−)-loliolide modulates both belowground defense and aboveground flowering in Arabidopsis and tobacco. J. Exp. Bot. 2023, 74, 964–975. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Mizokami, Y.; Légeret, B.; Havaux, M. The Apocarotenoid β-Cyclocitric Acid Elicits Drought Tolerance in Plants. iScience 2019, 19, 461–473. [Google Scholar] [CrossRef]
- Ahrazem, O.; Gómez-Gómez, L.; Rodrigo, M.J.; Avalos, J.; Limón, M.C. Carotenoid Cleavage Oxygenases from Microbes and Photosynthetic Organisms: Features and Functions. Int. J. Mol. Sci. 2016, 17, 1781. [Google Scholar] [CrossRef] [PubMed]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Rambla, J.L.; Fernández-de-Carmen, A.; Trapero-Mozos, A.; Ahrazem, O.; Orzáez, D.; Granell, A.; Gómez-Gómez, L. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol. Biol. 2014, 86, 555–569. [Google Scholar] [CrossRef]
- Lätari, K.; Wüst, F.; Hübner, M.; Schaub, P.; Beisel, K.G.; Matsubara, S.; Beyer, P.; Welsch, R. Tissue-Specific Apocarotenoid Glycosylation Contributes to Carotenoid Homeostasis in Arabidopsis Leaves. Plant Physiol. 2015, 168, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Molnár, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef]
- Taniguchi, S.; Takeda, A.; Kiryu, M.; Gomi, K. Jasmonic Acid-Induced β-Cyclocitral Confers Resistance to Bacterial Blight and Negatively Affects Abscisic Acid Biosynthesis in Rice. Int. J. Mol. Sci. 2023, 24, 1704. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, A. Identification of the carotenoid cleavage dioxygenase genes and functional analysis reveal DoCCD1 is potentially involved in beta-ionone formation in Dendrobium officinale. Front. Plant Sci. 2022, 13, 967819. [Google Scholar] [CrossRef]
- Matilla, A.J.; Carrillo-Barral, N.; Rodríguez-Gacio, M.d.C. An Update on the Role of NCED and CYP707A ABA Metabolism Genes in Seed Dormancy Induction and the Response to After-Ripening and Nitrate. J. Plant Growth Regul. 2015, 34, 274–293. [Google Scholar] [CrossRef]
- Gao, L.; Gonda, I.; Sun, H.; Ma, Q.; Bao, K.; Tieman, D.M.; Burzynski-Chang, E.A.; Fish, T.L.; Stromberg, K.A.; Sacks, G.L.; et al. The tomato pan-genome uncovers new genes and a rare allele regulating fruit flavor. Nat. Genet. 2019, 51, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tieman, D.; Jones, J.B.; Taylor, M.G.; Schmelz, E.; Huffaker, A.; Bies, D.; Chen, K.; Klee, H.J. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J. Exp. Bot. 2014, 65, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Klein, B.P.; King, D.; Grossman, S. Cooxidation reactions of lipoxygenase in plant systems. Adv. Free. Radic. Biol. Med. 1985, 1, 309–343. [Google Scholar] [CrossRef]
- Ksas, B.; Havaux, M. Determination of ROS-Induced Lipid Peroxidation by HPLC-Based Quantification of Hydroxy Polyunsaturated Fatty Acids. In Reactive Oxygen Species in Plants: Methods and Protocols; Mhamdi, A., Ed.; Springer: New York, NY, USA, 2022; pp. 181–189. ISBN 978-1-0716-2469-2. [Google Scholar]
- Montillet, J.-L.; Cacas, J.-L.; Garnier, L.; Montané, M.-H.; Douki, T.; Bessoule, J.-J.; Polkowska-Kowalczyk, L.; Maciejewska, U.; Agnel, J.-P.; Vial, A.; et al. The upstream oxylipin profile of Arabidopsis thaliana: A tool to scan for oxidative stresses. Plant J. 2004, 40, 439–451. [Google Scholar] [CrossRef]
- Koschmieder, J.; Wüst, F.; Schaub, P.; Álvarez, D.; Trautmann, D.; Krischke, M.; Rustenholz, C.; Mano, J.; Mueller, M.J.; Bartels, D.; et al. Plant apocarotenoid metabolism utilizes defense mechanisms against reactive carbonyl species and xenobiotics. Plant Physiol. 2021, 185, 331–351. [Google Scholar] [CrossRef]
- Tomita, K.; Hasegawa, M.; Arii, S.; Tsuji, K.; Bober, B.; Harada, K. Characteristic oxidation behavior of β-cyclocitral from the cyanobacterium Microcystis. Environ. Sci. Pollut. Res. 2016, 23, 11998–12006. [Google Scholar] [CrossRef]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wan, P.; Sun, S.; Li, J.; Chen, M. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol. Biol. 2004, 54, 519–532. [Google Scholar] [CrossRef]
- Morohashi, K.; Minami, M.; Takase, H.; Hotta, Y.; Hiratsuka, K. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J. Biol. Chem. 2003, 278, 20865–20873. [Google Scholar] [CrossRef]
- Fode, B.; Siemsen, T.; Thurow, C.; Weigel, R.; Gatz, C. The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 2008, 20, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Pysh, L.D.; Wysocka-Diller, J.W.; Camilleri, C.; Bouchez, D.; Benfey, P.N. The GRAS gene family in Arabidopsis: Sequence characterization and basic expression analysis of the SCARECROW-LIKE genes. Plant J. 1999, 18, 111–119. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Ksas, B.; Havaux, M. Decoding β-Cyclocitral-Mediated Retrograde Signaling Reveals the Role of a Detoxification Response in Plant Tolerance to Photooxidative Stress. Plant Cell 2018, 30, 2495–2511. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Wagner, D.; Przybyla, D.; Op den Camp, R.; Kim, C.; Landgraf, F.; Lee, K.P.; Würsch, M.; Laloi, C.; Nater, M.; Hideg, E.; et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 2004, 306, 1183–1185. [Google Scholar] [CrossRef]
- Gadjev, I.; Vanderauwera, S.; Gechev, T.S.; Laloi, C.; Minkov, I.N.; Shulaev, V.; Apel, K.; Inzé, D.; Mittler, R.; Van Breusegem, F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006, 141, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef]
- Cresta, A.; D’Alessandro, S. Arabidopsis ANAC102, Chloroplastic or Nucleocytosolic Localization? Genes 2023, 14, 438. [Google Scholar] [CrossRef]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012, 59, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Purkar, V.; Mitra, S. β-Cyclocitral, a Master Regulator of Multiple Stress-Responsive Genes in Solanum lycopersicum L. Plants. Plants 2021, 10, 2465. [Google Scholar] [CrossRef]
- Köster, J.; Thurow, C.; Kruse, K.; Meier, A.; Iven, T.; Feussner, I.; Gatz, C. Xenobiotic-and jasmonic acid-inducible signal transduction pathways have become interdependent at the Arabidopsis CYP81D11 promoter. Plant Physiol. 2012, 159, 391–402. [Google Scholar] [CrossRef]
- Shumbe, L.; D’Alessandro, S.; Shao, N.; Chevalier, A.; Ksas, B.; Bock, R.; Havaux, M. METHYLENE BLUE SENSITIVITY 1 (MBS1) is required for acclimation of Arabidopsis to singlet oxygen and acts downstream of β-cyclocitral. Plant Cell Environ. 2017, 40, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Duan, G.Y.; Bock, R. A mediator of singlet oxygen responses in Chlamydomonas reinhardtii and Arabidopsis identified by a luciferase-based genetic screen in algal cells. Plant Cell 2013, 25, 4209–4226. [Google Scholar] [CrossRef]
- Lv, F.; Zhou, J.; Zeng, L.; Xing, D. β-cyclocitral upregulates salicylic acid signalling to enhance excess light acclimation in Arabidopsis. J. Exp. Bot. 2015, 66, 4719–4732. [Google Scholar] [CrossRef]
- Beaugelin, I.; Chevalier, A.; D’Alessandro, S.; Ksas, B.; Novák, O.; Strnad, M.; Forzani, C.; Hirt, H.; Havaux, M.; Monnet, F. OXI1 and DAD Regulate Light-Induced Cell Death Antagonistically through Jasmonate and Salicylate Levels. Plant Physiol. 2019, 180, 1691–1708. [Google Scholar] [CrossRef]
- Huang, L.-J.; Li, N.; Thurow, C.; Wirtz, M.; Hell, R.; Gatz, C. Ectopically expressed glutaredoxin ROXY19 negatively regulates the detoxification pathway in Arabidopsis thaliana. BMC Plant Biol. 2016, 16, 200. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Urzúa, T.; Jelenska, J.; Sbarbaro, C.; Seguel, A.; Duarte, Y.; Greenberg, J.T.; Holuigue, L.; Blanco-Herrera, F.; Herrera-Vásquez, A. The TGA Transcription Factors from Clade II Negatively Regulate the Salicylic Acid Accumulation in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 11631. [Google Scholar] [CrossRef]
- Zhu, K.; Feng, Y.; Huang, Y.; Zhang, D.; Ateeq, M.; Zheng, X.; Al-Babili, S.; Li, G.; Liu, J. β-Cyclocitric acid enhances drought tolerance in peach (Prunus persica) seedlings. Tree Physiol. 2023, 43, 1933–1949. [Google Scholar] [CrossRef]
- Deshpande, S.; Manoharan, R.; Mitra, S. Exogenous β-cyclocitral treatment primes tomato plants against drought by inducing tolerance traits, independent of abscisic acid. Plant Biol. 2021, 23, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Ksas, B.; Legeret, B.; Caffarri, S.; Havaux, M. A cytochrome P450 involved in apocarotenoid signaling enhances plant photosynthetic capacity and photooxidative stress tolerance. bioRxiv 2024. [Google Scholar] [CrossRef]
- Braat, J.; Jaonina, M.; David, P.; Leschevin, M.; Légeret, B.; D’Alessandro, S.; Beisson, F.; Havaux, M. The response of Arabidopsis to the apocarotenoid β-cyclocitric acid reveals a role for SIAMESE-RELATED 5 in root development and drought tolerance. PNAS Nexus 2023, 2, pgad353. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef]
- Qi, Z.; Tong, X.; Zhang, X.; Lin, H.; Bu, S.; Zhao, L. One-pot synthesis of dihydro-β-ionone from carotenoids using carotenoid cleavage dioxygenase and enoate reductase. Bioprocess Biosyst. Eng. 2022, 45, 891–900. [Google Scholar] [CrossRef]
- Felemban, A.; Moreno, J.C.; Mi, J.; Ali, S.; Sham, A.; AbuQamar, S.F.; Al-Babili, S. The apocarotenoid β-ionone regulates the transcriptome of Arabidopsis thaliana and increases its resistance against Botrytis cinerea. Plant J. 2024, 117, 541–560. [Google Scholar] [CrossRef]
- Votta, C.; Wang, J.Y.; Cavallini, N.; Savorani, F.; Capparotto, A.; Liew, K.X.; Giovannetti, M.; Lanfranco, L.; Al-Babili, S.; Fiorilli, V. Integration of rice apocarotenoid profile and expression pattern of Carotenoid Cleavage Dioxygenases reveals a positive effect of β-ionone on mycorrhization. Plant Physiol. Biochem. 2024, 207, 108366. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vallino, M.; Biselli, C.; Faccio, A.; Bagnaresi, P.; Bonfante, P. Host and non-host roots in rice: Cellular and molecular approaches reveal differential responses to arbuscular mycorrhizal fungi. Front. Plant Sci. 2015, 6, 636. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. An endogenous growth inhibitor, 3-hydroxy-β-ionone. I. Its role in light-induced growth inhibition of hypocotyls of Phaseolus vulgaris. Physiol. Plant. 1992, 86, 583–586. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Yamamoto, M.; Tamura, K.; Teruya, T.; Suenaga, K.; Fujii, Y. Isolation and identification of potent allelopathic substances in rattail fescue. Plant Growth Regul. 2010, 60, 127–131. [Google Scholar] [CrossRef]
- Ida, N.; Iwasaki, A.; Teruya, T.; Suenaga, K.; Kato-Noguchi, H. Tree Fern Cyathea lepifera May Survive by Its Phytotoxic Property. Plants 2020, 9, 46. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic Activity and Characterization of Allelopathic Substances from Elaeocarpus floribundus Blume Leaves for the Development of Bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential. Plants 2023, 12, 1577. [Google Scholar] [CrossRef]
- Stevens, K.L.; Merrill, G.B. Growth inhibitors from spikerush. J. Agric. Food Chem. 1980, 28, 644–646. [Google Scholar] [CrossRef]
- Stevens, K.L.; Merrill, G.B. Dihydroactinidiolide—A potent growth inhibitor from Eleocharis coloradoensis (spikerush). Experientia 1981, 37, 1133. [Google Scholar] [CrossRef]
- Franken, H.; Mathieson, T.; Childs, D.; Sweetman, G.M.A.; Werner, T.; Tögel, I.; Doce, C.; Gade, S.; Bantscheff, M.; Drewes, G.; et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat. Protoc. 2015, 10, 1567–1593. [Google Scholar] [CrossRef]
- Jia, K.-P.; Mi, J.; Ali, S.; Ohyanagi, H.; Moreno, J.C.; Ablazov, A.; Balakrishna, A.; Berqdar, L.; Fiore, A.; Diretto, G.; et al. An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol. Plant 2022, 15, 151–166. [Google Scholar] [CrossRef]
- Ke, D.; Xie, Y.; Li, H.; Hu, L.; He, Y.; Guo, C.; Zhai, Y.; Guo, J.; Li, K.; Chu, Z.; et al. Anchorene, a carotenoid-derived growth regulator, modulates auxin homeostasis by suppressing GH3-mediated auxin conjugation. J. Integr. Plant Biol. 2024, 66, 2490–2504. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.-P.; Mi, J.; Ablazov, A.; Ali, S.; Yang, Y.; Balakrishna, A.; Berqdar, L.; Feng, Q.; Blilou, I.; Al-Babili, S. Iso-anchorene is an endogenous metabolite that inhibits primary root growth in Arabidopsis. Plant J. 2021, 107, 54–66. [Google Scholar] [CrossRef] [PubMed]
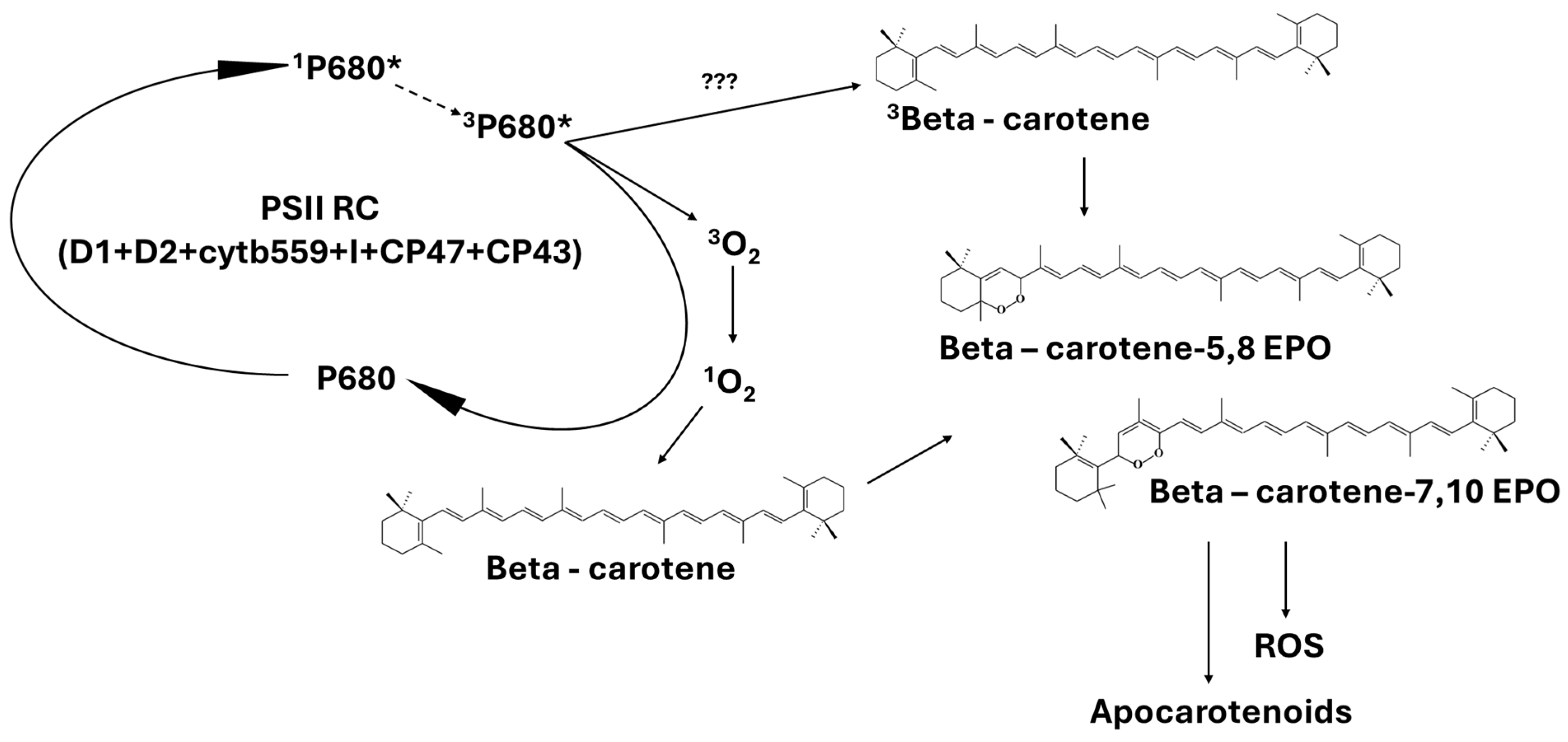


| Name | Symbol | Cleavage Site | Precursor |
|---|---|---|---|
| β-cyclocitral hydroxy- β-cyclocitral | APO7 OH-APO7 | C7/8 C7/8 | β-carotene zeaxanthin |
| β-ionone | APO9 | C9/10 | β-carotene |
| hydroxy-β-ionone | OH-APO9 | C9/10 | zeaxanthin |
| β-apo-11-carotenal | APO11 | C11/12 | β-carotene |
| hydroxy-β-apo-11-carotenal | OH-APO11 | C11/12 | zeaxanthin |
| β-apo-13-carotenone (d’orenone) | APO13 | C13/14 | β-carotene |
| hydroxy-β-apo-13-carotenone (zaxinone) | OH-APO13 | C13/14 | zeaxanthin |
| β-apo-15-carotenal (retinal) | APO15 | C15/15′ | β-carotene |
| hydroxy-β-apo-15-carotenal | OH-APO15 | C15/15′ | zeaxanthin |
| β-apo-14′-carotenal | APO14′ | C13′/14′ | β-carotene |
| hydroxy-β-apo-14′-carotenal | OH-APO14′ | C13′/14′ | zeaxanthin |
| β-apo-12′-carotenal | APO12′ | C11′/12′ | β-carotene |
| hydroxy-β-apo-12′-carotenal | OH-APO12′ | C11′/12′ | zeaxanthin |
| β-apo-10′-carotenal | APO10′ | C9′/10′ | β-carotene |
| hydroxy-β-apo-10′-carotenal | OH-APO10′ | C9′/10′ | zeaxanthin |
| β-apo-8′-carotenal | APO8′ | C7′/8′ | β-carotene |
| hydroxy-β-apo-8′-carotenal | OH-APO8′ | C7′/8′ | zeaxanthin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnà, M.; Korwin Krukowski, P.; Tosato, E.; D’Alessandro, S. Apocarotenoids as Stress Signaling Molecules in Plants. Agriculture 2025, 15, 926. https://doi.org/10.3390/agriculture15090926
Carnà M, Korwin Krukowski P, Tosato E, D’Alessandro S. Apocarotenoids as Stress Signaling Molecules in Plants. Agriculture. 2025; 15(9):926. https://doi.org/10.3390/agriculture15090926
Chicago/Turabian StyleCarnà, Maurizio, Paolo Korwin Krukowski, Edoardo Tosato, and Stefano D’Alessandro. 2025. "Apocarotenoids as Stress Signaling Molecules in Plants" Agriculture 15, no. 9: 926. https://doi.org/10.3390/agriculture15090926
APA StyleCarnà, M., Korwin Krukowski, P., Tosato, E., & D’Alessandro, S. (2025). Apocarotenoids as Stress Signaling Molecules in Plants. Agriculture, 15(9), 926. https://doi.org/10.3390/agriculture15090926





