Physiological Response of European Sea Bass (Dicentrarchus labrax) Juveniles to an Acute Stress Challenge: The Impact of Partial and Total Dietary Fishmeal Replacement by an Insect Meal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients and Experimental Diets
2.2. Ethical Issues
2.3. Acute Stress Challenge and Fish Sampling
2.4. Haematological Parameters
2.5. Plasma Metabolites and Innate Immunity-Related Parameters
2.6. Hepatic Oxidative Stress and Antioxidant Capacity
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
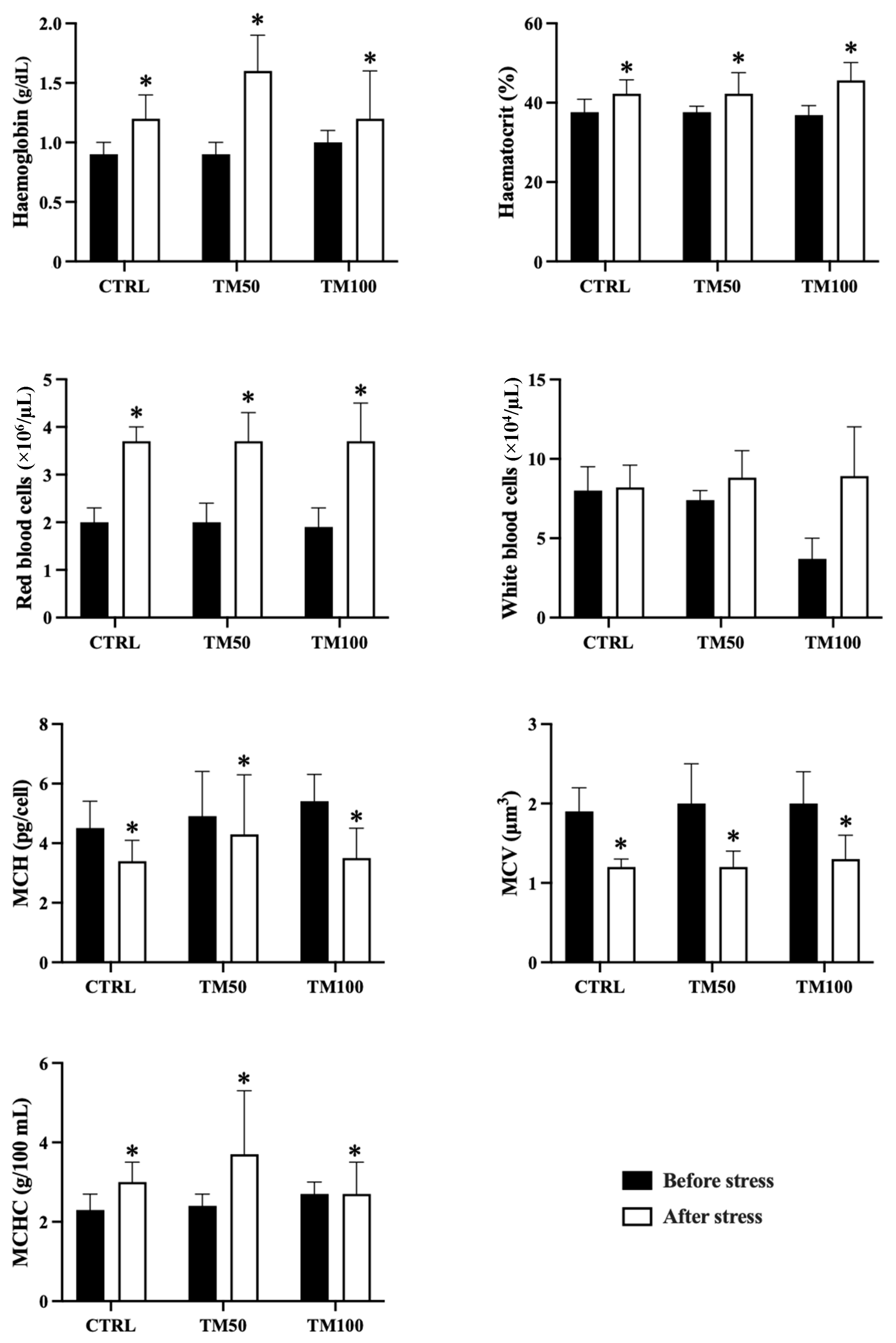
3.2. Haematological Profile
3.3. Plasma Metabolites and Innate Immunity-Related Parameters
3.4. Hepatic Oxidative Stress and Antioxidant Capacity
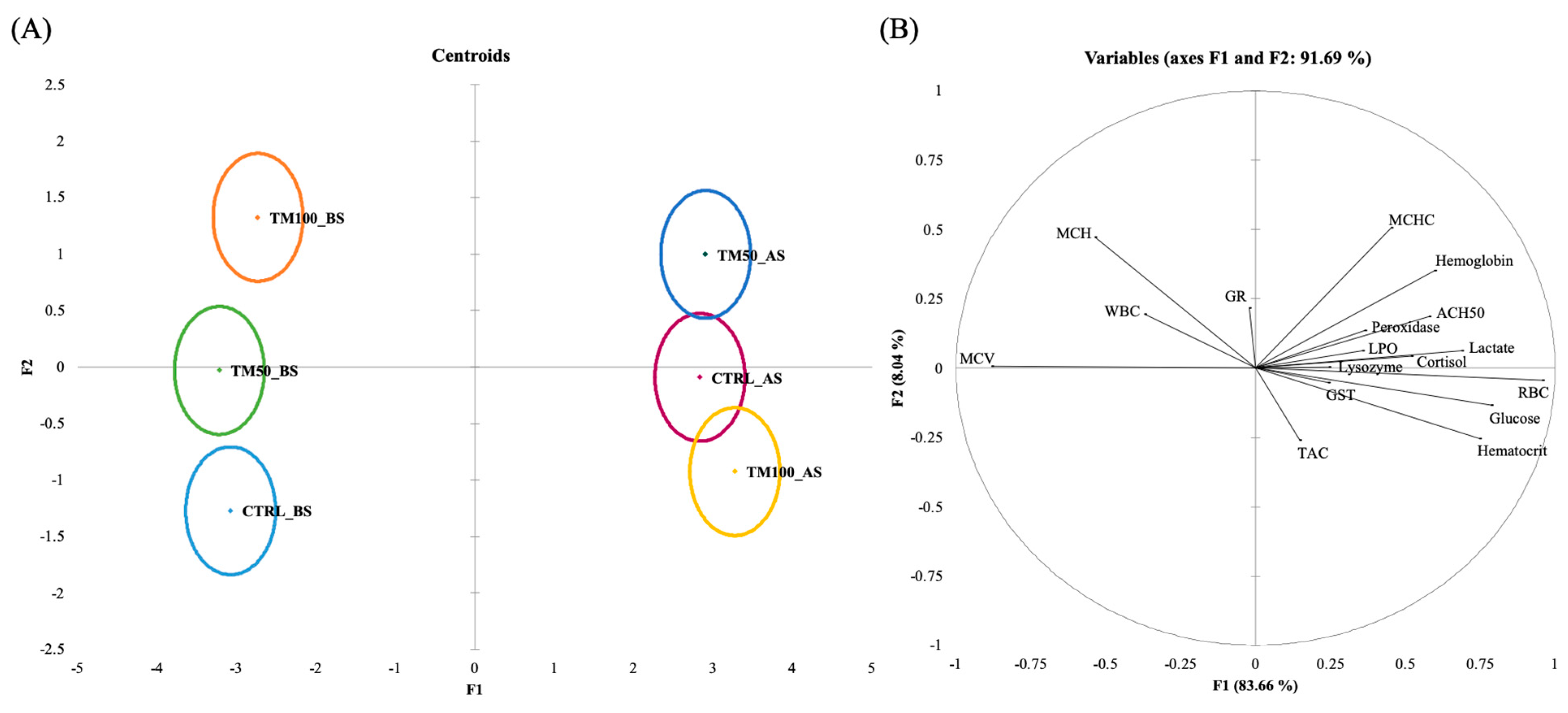
3.5. Discriminant Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Roma, Italy, 2022. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B.; Fracalossi, D.M.; Hua, K.; Izquierdo, M.; Mai, K.; Øverland, M.; Robb, D.; Roubach, R.; Schrama, J.; Small, B.; et al. Harvesting the benefits of nutritional research to address global challenges in the 21st century. J. World Aquac. Soc. 2023, 54, 343–363. [Google Scholar] [CrossRef]
- Glencross, B.; Ling, X.; Gatlin, D.; Kaushik, S.; Øverland, M.; Newton, R.; Valente, L.M.P. A SWOT analysis of the use of marine, grain, terrestrial-animal and novel protein ingredients in aquaculture feeds. Rev. Fish. Sci. Aquac. 2024, 1–39. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017, 60, 92–116. [Google Scholar]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Singh, S.K.; Pawar, L.; Thomas, A.J.; Debbarma, R.; Biswas, P.; Ningombam, A.; Devi, A.G.; Waikhom, G.; Patel, A.B.; Meena, D.K.; et al. The current state of research and potential applications of insects for resource recovery and aquaculture feed. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative proteins for fish diets: Implications beyond growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Martos-Sitcha, J.A.; Mancera, J.M.; Prunet, P.; Magnoni, L.J. Welfare and stressors in fish: Challenges facing aquaculture. Front. Physiol. 2020, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Biology of Stress in Fish; Schreck, C.B., Tort, L., Farrell, A., Brauner, C., Eds.; Academic Press: London, UK, 2016; pp. 2–34. [Google Scholar]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Zarantoniello, M.; de Oliveira, A.A.; Sahin, T.; Freddi, L.; Torregiani, M.; Tucciarone, I.; Chemello, G.; Cardinaletti, G.; Gatto, E.; Parisi, G.; et al. Enhancing rearing of European seabass (Dicentrarchus labrax) in aquaponic systems: Investigating the effects of enriched black soldier fly (Hermetia illucens) prepupae meal on fish welfare and quality traits. Animals 2023, 13, 1921. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Fish and Shrimp, 1st ed.; Press, T.N.A., Ed.; The National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Basto, A.; Marques, A.; Silva, A.S.; Sá, T.; Sousa, V.; Oliveira, M.B.P.P.; Aires, T.; Valente, L.M.P. Nutritional, organoleptic and sensory quality of market-sized European sea bass (Dicentrarchus labrax) fed defatted Tenebrio molitor larvae meal as main protein source. Aquaculture 2022, 566, 739210. [Google Scholar] [CrossRef]
- Basto, A.; Valente, L.M.P.; Soengas, J.L.; Conde-Sieira, M. Partial and total fishmeal replacement by defatted Tenebrio molitor larvae meal do not alter short- and mid-term regulation of food intake in European sea bass (Dicentrarchus labrax). Aquaculture 2022, 560, 738604. [Google Scholar] [CrossRef]
- Basto, A.; Valente, L.M.P.; Sousa, V.; Conde-Sieira, M.; Soengas, J.L. Total fishmeal replacement by defatted Tenebrio molitor larvae meal induces alterations in intermediary metabolism of European sea bass (Dicentrarchus labrax). J. Anim. Sci. 2023, 101, skad040. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Azeredo, R.; Diaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, R.; Machado, M.; Afonso, A.; Fierro-Castro, C.; Reyes-Lopez, F.E.; Tort, L.; Gesto, M.; Conde-Sieira, M.; Miguez, J.M.; Soengas, J.L.; et al. Neuroendocrine and immune responses undertake different fates following tryptophan or methionine dietary treatment: Tales from a teleost model. Front. Immunol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Manning, M.J. Seasonal trends in serum lysozyme activity and total protein concentration in dab (Limanda limanda L.) sampled from Lyme Bay, U.K. Fish Shellfish Immunol. 1996, 6, 473–482. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O.; Tort, L. Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet. Immunol. Immunopathol. 1995, 45, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.; Pereira, R.; Domínguez, D.; Pereira, M.; Pereira, C.; Pintado, M.; Velasco, C. Stress response of European seabass (Dicentrarchus labrax) fed plant-based diets supplemented with swine blood hydrolysates. Aquac. Rep. 2023, 30, 101600. [Google Scholar] [CrossRef]
- Pereira, R.; Costa, M.; Velasco, C.; Cunha, L.M.; Lima, R.C.; Baião, L.F.; Batista, S.; Marques, A.; Sá, T.; Campos, D.A.; et al. Comparative analysis between synthetic vitamin E and natural antioxidant sources from tomato, carrot and coriander in diets for market-sized Dicentrarchus labrax. Antioxidants 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aqua. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Cook, K.V.; Lennox, R.J.; Hinch, S.G.; Cooke, S.J. Fish out of water: How much air is too much? Fisheries 2015, 40, 452–461. [Google Scholar] [CrossRef]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish. Immunol. 2021, 2, 100010. [Google Scholar] [CrossRef]
- Gai, F.; Cusimano, G.M.; Maricchiolo, G.; Caccamo, L.; Caimi, C.; Macchi, E.; Meola, M.; Perdichizzi, A.; Tartarisco, G.; Gasco, L.; et al. Defatted black soldier fly meal in diet for grow-out gilthead seabream (Sparus aurata L. 1758): Effects on growth performance, gill cortisol level, digestive enzyme activities, and intestinal histological structure. Aqua. Res. 2023, 2023, 3465335. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Caccamo, L.; Pansera, L.; Oteri, M.; Chiofalo, B.; Maricchiolo, G. Influence of Hermetia illucens larvae meal dietary inclusion on growth performance, gut histological traits and stress parameters in Sparus aurata. Animals 2023, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish—Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Fazio, F.; Ferrantelli, V.; Fortino, G.; Arfuso, F.; Giangrosso, G.; Faggio, C. The influence of acute handling stress on some blood parameters in cultured sea bream (Sparus aurata Linnaeus, 1758). Ital. J. Food Saf. 2015, 4, 4174. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Khallaf, M.A.; Abdel-Latif, H.M.R. Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 2020, 522, 735136. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of fish meal by black soldier fly (Hermetia illucens) larvae meal: Effects on growth, haematology, and skin mucus immunity of Nile Tilapia, Oreochromis niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Khieokhajonkhet, A.; Thammang, S.; Aeksiri, N.; Kaneko, G.; Tatsapong, P.; Phromkunthong, W. Fish meal replacement by Brachytrupes portentosusas for Oreochromis niloticus: Effect on growth, feed utilization, fatty acid profiles, hematology, and histological changes. Anim. Feed Sci. Technol. 2024, 308, 115873. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Melenchón, F.; de Mercado, E.; Pula, H.J.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenço, H.M.; Pessoa, M.F.; Lagos, L.; Weththasinghe, P.; et al. Fishmeal dietary replacement up to 50%: A comparative study of two insect meals for rainbow trout (Oncorhynchus mykiss). Animals 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sun, C.; Liu, B.; Xu, P. Oxidative stress in aquatic organisms. Antioxidants 2023, 12, 1223. [Google Scholar] [CrossRef]
- Saikia, S.K.; Chowdhury, S. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M.; Hawkyard, M.; Sealey, W.M.; Bradshaw, D.; Meesala, K.M.; Bouchard, D.A. Effects of fishmeal substitution with mealworm meals (Tenebrio molitor and Alphitobius diaperinus) on the growth, physiobiochemical response, digesta microbiome, and immune genes expression of Atlantic salmon (Salmo salar). Aquac. Nutr. 2024, 2024, 6618117. [Google Scholar] [CrossRef] [PubMed]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Riera Romo, M.; Perez-Martinez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Foysal, M.J.; Fotedar, R.; Tay, C.Y.; Gupta, S.K. Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 2019, 7, e6891. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Siddik, M.A.B.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 16703. [Google Scholar] [CrossRef] [PubMed]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Abdel-Tawwab, M.; Khalil, R.H.; Metwally, A.A.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.A. Black soldier fly (Hermetia illucens) larvae meal in diets of European seabass: Effects on antioxidative capacity, non-specific immunity, transcriptomic responses, and resistance to the challenge with Vibrio alginolyticus. Fish Shellfish Immunol. 2021, 111, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Rajan, D.K.; Ganesan, A.R.; Divya, D.; Johansen, J.; Zhang, S. Chitin, chitosan and chitooligosaccharides as potential growth promoters and immunostimulants in aquaculture: A comprehensive review. Int. J. Biol. Macromol. 2023, 251, 126285. [Google Scholar] [CrossRef]


| dTM | CTRL | TM50 | TM100 | |
|---|---|---|---|---|
| Ingredients (%) | ||||
| Fishmeal 1 | 40 | 20 | - | |
| Defatted Tenebrio molitor larvae meal | - | 20.5 | 40.4 | |
| Soy protein concentrate 2 | 10.5 | 10.5 | 10.5 | |
| Soybean meal 3 | 13 | 13 | 13 | |
| Rapeseed meal 4 | 5 | 5 | 5 | |
| Wheat meal 5 | 16.2 | 15.2 | 14.3 | |
| Fish oil 6 | 14.0 | 13.3 | 12.5 | |
| Vitamin and mineral premix 7 | 1 | 1 | 1 | |
| Vitamin C | 0.1 | 0.1 | 0.1 | |
| Vitamin E | 0.1 | 0.1 | 0.1 | |
| Monocalcium phosphate | - | 1.0 | 2.0 | |
| L-Lysine | - | - | 0.2 | |
| L-Threonine | - | - | 0.2 | |
| L-Tryptophan | - | - | 0.1 | |
| DL-Methionine | 0.1 | 0.2 | 0.3 | |
| Proximate composition (% dry matter (DM) or kJ/kg DM) | ||||
| Dry matter | 97.8 | 93.1 | 92.6 | 92.5 |
| Crude protein | 71.0 | 46.9 | 47.3 | 47.2 |
| Crude fat | 12.1 | 19.7 | 19.8 | 19.0 |
| Gross energy | 24.3 | 23.2 | 23.5 | 24.0 |
| Ash | 4.8 | 10.2 | 8.1 | 6.3 |
| Phosphorus | 0.8 | 1.2 | 1.2 | 1.0 |
| Before Stress | After Stress | Two-Way ANOVA p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL | TM50 | TM100 | CTRL | TM50 | TM100 | Diet | Stress | Diet × Stress | |||
| SOD | 78.5 ± 13.2 b | 80.4 ± 12.8 ab | 90.5 ± 1.8 a | 72.2 ± 13.7 b | 83.9 ± 20.3 ab | 87.1 ± 1.8 a | 0.02 | 0.46 | 0.45 | ||
| CAT | 8.1 ± 2.5 | 6.7 ± 3.4 | 8.6 ± 2.7 | 8.7 ± 1.3 | 9.5 ± 2.7 | 8.5 ± 2.3 | 0.81 | 0.10 | 0.14 | ||
| GPx | 196.8 ± 11.7 | 205.8 ± 14.4 | 200.2 ± 3.2 | 197.0 ± 5.6 | 191.3 ± 2.8 | 190.6 ± 2.7 | 0.67 | 0.06 | 0.06 | ||
| GST | 54.0 ± 9.9 | 50.7 ± 8.0 | 56.1 ± 13.6 | 56.1 ± 6.9 | 56.3 ± 1.5 | 61.7 ± 15.5 | 0.26 | 0.11 | 0.84 | ||
| GR | 20.5 ± 5.1 | 22.8 ± 6.6 | 23.8 ± 8.4 | 21.6 ± 2.0 | 22.3 ± 4.0 | 22.2 ± 4.6 | 0.47 | 0.84 | 0.71 | ||
| LPO | 10.9 ± 4.1 | 9.2 ± 3.9 | 11.0 ± 2.0 | 9.9 ± 3.9 | 13.4 ± 7.4 | 14.8 ± 5.3 | 0.23 | 0.06 | 0.14 | ||
| TAC | 0.21 ± 0.05 | 0.18 ± 0.05 | 0.21 ± 0.05 | 0.23 ± 0.05 | 0.20 ± 0.05 | 0.20 ± 0.04 | 0.15 | 0.23 | 0.45 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basto, A.; Peixoto, D.; Machado, M.; Costas, B.; Murta, D.; Valente, L.M.P. Physiological Response of European Sea Bass (Dicentrarchus labrax) Juveniles to an Acute Stress Challenge: The Impact of Partial and Total Dietary Fishmeal Replacement by an Insect Meal. J. Mar. Sci. Eng. 2024, 12, 815. https://doi.org/10.3390/jmse12050815
Basto A, Peixoto D, Machado M, Costas B, Murta D, Valente LMP. Physiological Response of European Sea Bass (Dicentrarchus labrax) Juveniles to an Acute Stress Challenge: The Impact of Partial and Total Dietary Fishmeal Replacement by an Insect Meal. Journal of Marine Science and Engineering. 2024; 12(5):815. https://doi.org/10.3390/jmse12050815
Chicago/Turabian StyleBasto, Ana, Diogo Peixoto, Marina Machado, Benjamin Costas, Daniel Murta, and Luisa M. P. Valente. 2024. "Physiological Response of European Sea Bass (Dicentrarchus labrax) Juveniles to an Acute Stress Challenge: The Impact of Partial and Total Dietary Fishmeal Replacement by an Insect Meal" Journal of Marine Science and Engineering 12, no. 5: 815. https://doi.org/10.3390/jmse12050815
APA StyleBasto, A., Peixoto, D., Machado, M., Costas, B., Murta, D., & Valente, L. M. P. (2024). Physiological Response of European Sea Bass (Dicentrarchus labrax) Juveniles to an Acute Stress Challenge: The Impact of Partial and Total Dietary Fishmeal Replacement by an Insect Meal. Journal of Marine Science and Engineering, 12(5), 815. https://doi.org/10.3390/jmse12050815








