Abstract
Jeju, the largest island in Korea, is the most economically important in terms of marine aquaculture. We investigated the marine viral composition adjacent to Jeju Island over four seasons in 2022 and sequenced DNA libraries extracted from samples in March, June, September, and December using Illumina HiSeq 2000. We obtained 212,402, 186,542, 235,441, and 224,513 contigs from the four-season samples, respectively. Among the identified metagenomes, bacteriophages were dominant in all the samples. Bacillus phage G was the dominant species in March and June, whereas Pelagibacter phage HTVC 008M was the dominant species in September and December. Additionally, the number of viruses that infected algal hosts was higher in December than in other seasons. Marine viruses appeared in all seasons and infected marine vertebrates such as fish. Functional analysis using MG-RAST revealed that cell wall- and capsule-related metabolism groups were activated in March and June, whereas virulence-, disease-, and defense-related metabolism groups were activated in September and December. Conclusively, this study revealed seasonal changes in marine viral communities in the sea adjacent to Jeju Island. Our data will be useful in identifying emerging marine viral pathogens and for further community studies on marine organisms.
1. Introduction
Viruses exist in significant numbers in the marine environment, outnumbering marine bacteria [1,2]. In surface ocean waters, viruses are approximately 10 times more abundant than prokaryotes [3,4], although because of their small size, they make up only approximately 5% of the marine biomass. Viruses are a major cause of phytoplankton and microbial mortality [5,6]. They play an important role in controlling key components of the microbial food chain by stimulating the release of intracellular substances from host cells through cell lysis and the circulation of dissolved organic matter [7,8].
Metagenomics involves the study of genetic material recovered from environmental samples, such as microorganisms, by directly extracting DNA from an assemblage of microorganisms [9]. Advances in metagenomics have facilitated the in-depth characterization of the molecular diversity of DNA viruses in diverse environments including marine ecosystems [10,11]. Furthermore, developments in next-generation sequencing technologies and the continued decline in sequencing costs have made metagenomics a standard approach for studying microbial ecology. Approximately 1028 viral infections occur in the sea every day [12]. This affects marine systems substantially by causing host death, promoting horizontal gene transfer, and producing dissolved organic matter via cell lysis [13]. For viral metagenomics, nucleic acids are extracted directly from environmental samples enriched in viral particles and subjected to next-generation sequencing [14] to generate numerous sequences, which are then analyzed using bioinformatics. One of the greatest challenges in marine virus metagenomics is achieving sufficient analyzable concentrations of viral particles from marine environmental samples. For the concentration and extraction process to analyze marine viruses, the flocculation, filtration, and resuspension (FFR) method using FeCl3 is widely used. This method can rapidly concentrate large amounts of marine viruses without expensive equipment [15].
Korea has faced several emerging issues such as unforeseen climate change, influx of contaminants, and unknown and known pathogenic virus-associated diseases. Emerging viral pathogens can cause significant damage to the production of several marine products including seaweed, shellfish, and fish. In this study, the distribution and abundance of marine viruses showing seasonal and temporal patterns were investigated on Jeju Island, a major aquaculture region in Korea. The results of this study enhance the understanding of marine viral biogeography patterns and provide a practical baseline for investigating potential viral pathogens that pose a risk to aquaculture in Korea. This is a leading comprehensive study that reveals not only the virus population on Jeju Island, but also the seasonal changes in virus composition.
2. Materials and Methods
2.1. Sample Collection
Samples were collected from two sites on Jeju Island, Korea: Site #1 (33°23′50″ N 126°56′42″ E) and Site #2 (33°22′80″ N 126°56′50″ E). These sites were chosen to represent local conditions and cover four different seasons spanning 22 March, 15 June, 6 September, and 18 December 2022 (Figure 1). The sampling locations were neither protected nor privately owned, and the field research did not involve endangered or protected species. From each site, 100 L of ambient seawater was collected for viral analysis in sterilized 20-L plastic bottles. We examined the basic biological characteristics of the sampled areas including temperature, salinity, pH, and dissolved oxygen (DO) (Figure 1).
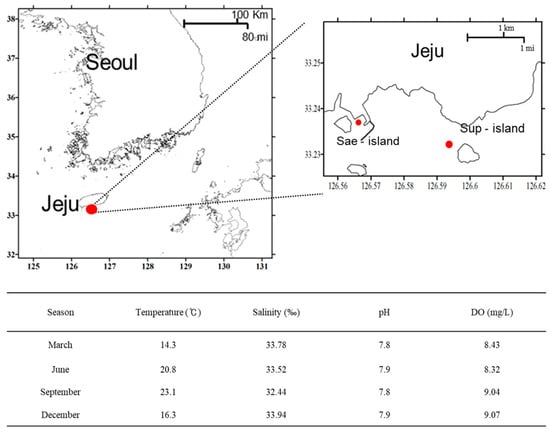
Figure 1.
Location of sample sites; Sae-island and Sup island (upper) and environmental factors at these sites (bottom) on Jeju Island, Korea. Temperature, salinity, pH, and dissolved oxygen (DO) were measured with a YSI 6600 Sonode.
2.2. Isolation of Viral Community DNA
Seawater samples were initially filtered through 3 μm (3 M paper) to remove debris and then through 0.22 μm (polycarbonate filter membrane, Millipore, Billerica, MA, USA) to remove non-analyte marine organisms including bacteria. To enrich marine viruses, we concentrated the viruses using the FeCl3-mediated agglutination method, as described in our earlier study [15]. Briefly, we added aqueous FeCl3 (6 mL) to the collected seawater (60 mL) and mixed by shaking for more than 10 min. The mixture was incubated at room temperature for more than 1 h, and the cultured mixture was filtered using a polycarbonate filter membrane (0.8 μm Millipore) (Billerica, MA, USA). The filtered filter paper samples were stored at 4 °C for subsequent experiments. Filter paper containing viral particles was cut into several pieces using sterilized scissors to extract viral DNA. Total viral DNA was extracted using a QIAeasy Plus Viral DNA/RNA Extraction Kit (Intron, Seongnam, Republic of Korea). The extracted viral DNA was used to create an NGS DNA library using a TruSeq Nano DNA Library Preparation Kit (Illumina, San Diego, CA, USA). The prepared DNA library was subjected to paired-end sequencing using an Illumina HiSeq 2000. Sequencing libraries were prepared by randomly fragmenting DNA samples followed by 5′ and 3′ adapter ligation. Additionally, tagging significantly increases the efficiency of the library preparation process by combining fragmentation and ligation reactions in a single step. The adapter-ligated fragments were amplified using PCR (Thermo Fisher Inc., Bohemia, NY, USA) and gel-purified.
2.3. Data Analysis
2.3.1. Sequence Quality Control
Fast QC and quality filtering were performed to assess sequence quality. FastQC provides a simple method for performing several quality control checks on raw sequence data from high-throughput sequencing pipelines. It provides a modular analysis to determine whether the data contained any problems. For the paired-end (PE) read pairs, sequence reads were filtered before assembly to ensure that each read had at least 90% base quality greater than or equal to Q20.
2.3.2. De Novo Sequence Data Analysis
The result was a highly accurate base-by-base sequence analysis that virtually eliminated sequence context errors, even within repeatedly analyzed sequence regions and homopolymers. Raw data were assembled using the CLC Genomics Workbench (version 12.0.3, Qiagen, Inc., Hilden, Germany). Briefly, the trim reads tool (quality limit = 0.05, ambiguous limit = 2) was used for the trimming and removal of adapter primer sequences and blasted against viral reference sequences using default parameters that matched the SILVA and NCBI viral reference 2.1.1 by BLAST with an E value of 10−3. Assembly reads were searched for functional and organism assignments using MG-RAST, which included annotation using the following databases: GenBank, Kyoto Encyclopedia of Genes and Genomes (KEGG), RefSeq, and SEED. Sequence data were submitted to the MG-RAST server under study accession no. MG-RAST (accessed on 9 December 2022, March; 4707935.3, June; 4707936.3, September; 4707939.3, December; 4707940.3), https://www.mg-rast.org/.
3. Results
3.1. Sampling Site and Environmental Characteristics
Two sampling sites were selected to represent local regions. Site #1 was selected as a port with considerable human activity, and Site #2 was chosen as a clean place. Samples from both sites were combined and analyzed over four seasons. The sampling site did not require specific permissions and was either privately owned or protected. No significant changes were observed in the salinity, pH, and DO over the four seasons (salinity; 32.14–33.64‰, pH; 7.8–7.9, DO; 8.13–9.27 mg/L). However, the water surface temperature showed clear changes depending on seasonality (14.3 °C on 16 March, 20.8 °C on 6 June, 23.1 °C on 1 September, and 16.3 °C on 14 December) (Figure 1).
3.2. Metagenome Comparisons
A pipeline for the metagenomic analysis of Jeju Island seawater was developed to identify DNA viruses. The use of the Illumina HiSeq 2000 resulted in raw sequence reads of 30,749,519 in March; 27,845,976 in June; 41,550,478 in September; and 51,644,356 in December with a read length of 100 bp. After quality filtering and trimming of the reads, 90% of the raw reads remained. Reads with an overall higher G+C content were obtained from all libraries. Metagenome libraries from the four seasons were compared by principal component analysis based on the representative hit classification against the M5nr database (the non-redundant database developed at Argonne National Laboratory containing sequences and annotations from multiple sources). The database was based on MD5 checksums of the sequences, separating the sequence data from the annotation data from multiple publicly available databases) in MG-RAST. As a result, a similar metagenome was represented between March and June, although September and December showed different locations (Figure 2).
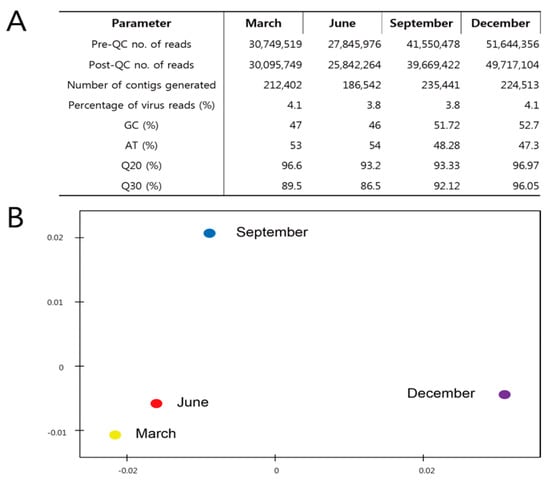
Figure 2.
Comparison of metagenome libraries. Next−generation sequencing metadata including assembly, GC content, quality score (A), and principal component analysis of the four viral metagenomes, performed using an e−value cutoff of 10−3 with the NCBI and SILVA databases implemented in the CLC genomics workbench (B).
3.3. Marine Viral Community Diversity and Composition
To identify marine viruses, we used the CLC genomics workbench program for assembled read sequences followed by a BLAST search against the NCBI database using MetaVir. Among all of the assembled contigs, DNA viruses accounted for the following distribution (ssDNA, 1.1–2.1%, dsDNA 90–94.9%) (Figure 3A). These viruses infect a wide range of hosts, including bacteria (61–67%), marine algae (3–19%), and vertebrates (0.9–5.5%) (Figure 3B). The relative abundance of the viral families indicated that, on average, more than 92% of the contigs assigned to each virome were homologous to dsDNA phages belonging to the orders Myoviridae, Podoviridae, and Siphoviridae (Caudovirales) (Figure 3C). These dsDNA phages were associated with a variety of bacterial hosts, mostly Bacillus phage G in March and June, and Pelagibacter phage HTVC008M in September and December (Table 1). The family level analysis showed that the viral community did not change significantly across the four seasons. Particularly, Myoviridae was dominant in all four seasons, followed by Siphoviridae, Podoviridae, and Phycodnaviridae. Myoviridae accounted for most distributions in September, and Podoviridae won a unit of distribution in September and December (Figure 3C). Algal viruses were present in all samples (average: 3.35%). The relative abundance of algal viruses was higher because of the significantly higher abundance of Mimiviridae and Phycodnaviridae in June. Contigs belonging to Iridoviridae (fish-hosted) were present in all seasons except June, whereas Herpesviridae were present only in December (Figure 3B,C).
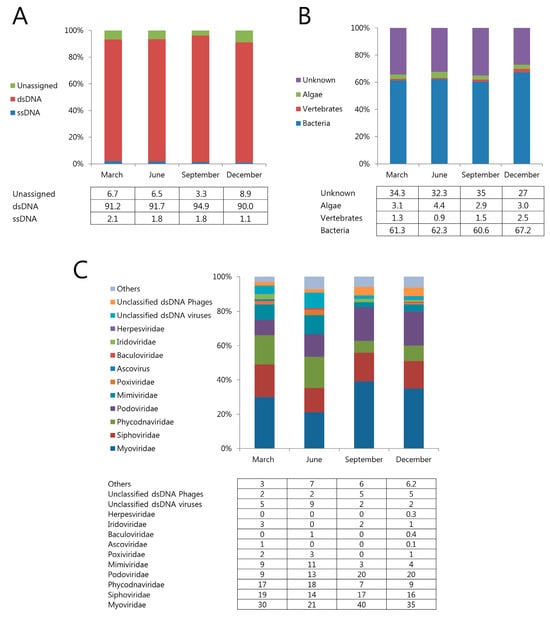
Figure 3.
Relative abundance of contigs. Type of viral genomes (A), type of virus hosts (B), and contigs assigned to viral families (C) in seasonal Jeju Island seawater viromes.

Table 1.
Top 20 marine viruses frequently identified in this study.
3.4. Seasonal Change of Marine Viruses
Seasonal changes in the dominant species of the virus communities were observed at the species level (Table 1). Bacillus phages G were most dominant in March and June, and Puniceispirllum phages HMO-2011 and Pelagibacter phages HTVC008M were dominant in September and December. It also showed seasonal changes according to the four seasons. For example, Pelagibacter phage HTVC008M and Puniceispirillum phage HMO-2011 were more common in September and December but less common in March and June. Contrastingly, Bacillus phage G was more common in March and June (Figure 4A–D). We obtained a large number of reads that matched the top 10 viruses. This makes it highly likely that the sequences obtained from the reads will cover almost the entire viral genome. All of the raw data for the top 10 viral genomes from all four seasons were aligned and identified, and as shown, most regions of the viral genome were covered by sequenced reads, and some viral genome regions were highly mapped (Table S1). Bacillus phage G, Cafeteria roenbergensis virus BV-PW1, and Pelagibacter phage HTVC008M were detected in all samples; Bacillus phage G and Cafeteria roenbergensis virus BV-PW1 were frequently found in March, and Pelagibacter phage HTVC008M was detected most frequently in September (Figure 5). Additionally, we identified viruses that host eukaryotic organisms, although not bacteriophages. Viruses infecting amoebas and vertebrates were present in all libraries. Particularly, eukaryotic large nucleocytoplasmic DNA viruses (NCLDVs, Acanthamoeba polyphaga mimivirus, Megavirus chiliensis, Pandoravirus salinus, Pandoravirus inopinatum, and Pandoravirus dulcis) were present in March and May but not in the other two seasons (Table 1).
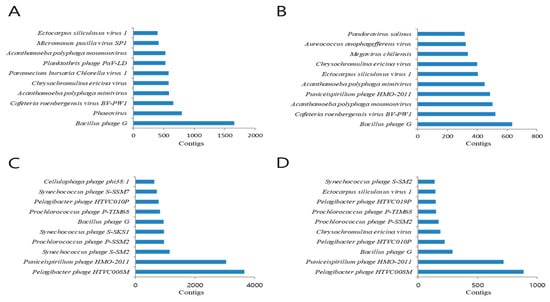
Figure 4.
Abundance of the 10 most common viral species (based on read number) in March (A), June (B), September (C), and December (D).
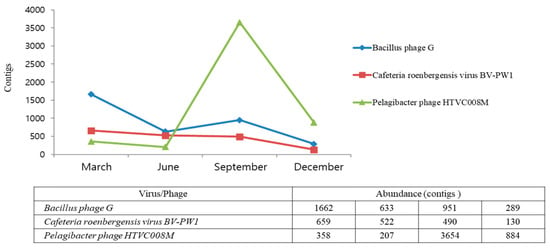
Figure 5.
Relative abundances of different viral sequences at the species level during different seasons.
3.5. Functional Classification of Identified Metagenomic Contigs
Reads from all four seasons were analyzed using the MG-RAST analysis pipeline. The reads were then subjected to functional classification. MG-RAST uses various databases for read feature annotations and includes four databases that allow for hierarchical functional annotation: KEGG Orthology (KO), COG, SEED, and eggNOG. The SEED subsystem database is manually maintained and is considered to be more accurate. Therefore, this subsystem was selected as the SEED subsystem for feature annotation.
The four reads processed by MG-RAST were compared with those of the subsystem database using a maximum E value of 10−5 and a minimum identity of 60%. Twenty-eight functional categories were assigned to the four season libraries, each subdivided into distinct subsystems. As a result, functional classification differed depending on the seasonal species composition of the dominant virus. The cell wall and capsule pathways were the largest in March and June, followed by carbohydrates, RNA metabolism, amino acids, and derivatives related to metabolic groups. September and December had the greatest matches in virulence, disease and defense pathways, carbohydrates, DNA and protein metabolism, phages, prophages, transposable elements, and plasma metabolism-related groups. Compared to those in all seasons, several metabolic pathways including membrane transport, regulation and cell signaling, cell division, and cell cycle showed very similar trends in the four seasons (Figure 6).
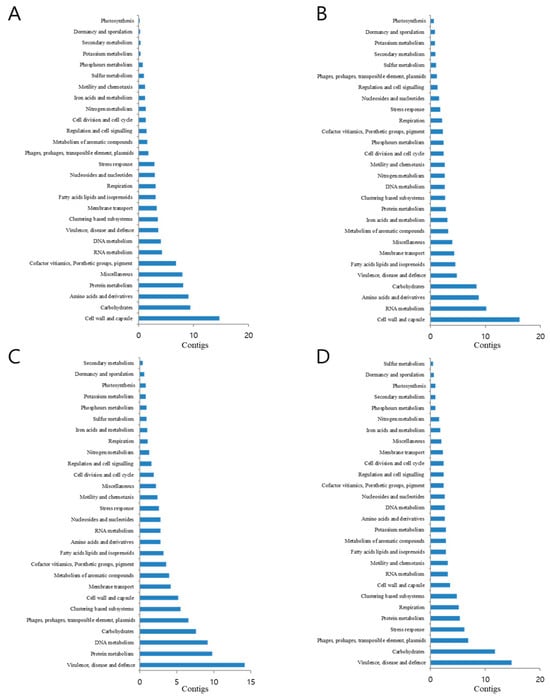
Figure 6.
Functional analysis of the four metagenomes. March (A), June (B), September (C), and December (D). Analysis was performed using the hierarchical functional annotation of the SEED subsystems on the MG-RAST web server.
4. Discussion
Viruses are the most abundant biological entities on Earth. They are considered key factors that control nutrient cycling in a variety of environments and play a particularly important role in microbial communities [16]. Virus research is important for a number of methods including oceans such as microscopy, genome size fingerprinting, and whole-genome or metagenome sequencing, which have been reviewed [17,18,19]. In this study, to explore the diversity and potential ecological role of viruses using metagenomic sequencing on Jeju Island, we analyzed and compared four metagenomes covering seasonal periods. Our results showed that most of the viruses analyzed belonged to the order Caudovirales, with the Myoviridae family having the highest distribution, followed by Sphoviridae, Podoviridae, and Phycodnaviridae. Earlier, viral abundance and diversity were investigated in various natural ecosystems in Korea including rice paddy soils, seawater, and the atmosphere near the surface [20]. The dominant species (Siphoviridae, Podoviridae, and Myoviridae) at the family level were similar to those found in our study; however, the species level was significantly different. This is thought to be influenced by seasonal changes in marine microbes and local environmental parameters (temperature, salinity, pH, and DO). Principal component analysis was used to compare the four seasonal viral metagenomes (Figure 3). This was similar to that of the metagenome of March and June, which is considered to be influenced by the DO, and shows the metagenome of September and December because of the seawater temperature.
Bacteriophages are classified based on their morphological characteristics. Although a variety of morphological phage types exist, most phages belong to one of three major families: Myoviridae, Siphoviridae, and Podoviridae [21]. Our results also showed that the most dominant order was Caudovirales (including three families: Myoviridae, Siphoviridae, and Podoviridae). The most abundant virus was Pelagibacter phage, and we identified two types (HTVC010P and HTVC008M). This result indicates that Pelagibacter ubique is also common on Jeju Island.
The food sources for these bacteria are predominantly dissolved organic carbon and nitrogen from marine ecosystems. They perform the TCA cycle via glyoxylate and various mechanisms of amino acid synthesis. They can also lead to unexpected sulfur reduction phenomena [22,23]. Pellagibacter infections caused by phages can cause a rapid decline in the host population (bacteria) and the production of abundant phage particles. These relationships play an important role in the marine ecosystem of Jeju Island.
We identified the Herpesviridae, Iridoviridae, Baculoviridae, Ascoviridae, and Poxoviridae families on Jeju Island. Herpes virus infections can cause various diseases in marine species (gastropods, mollusks, and bivalves). Particularly, ostreid herpesvirus 1(OsHV-1) is a major pathogen that adversely affects oyster growth. OsHV-1 belongs to Malacoherpes viridae (family) and Herpesvirales (order) [24,25]. Iridoviruses are large double-stranded DNA viruses that primarily infect vertebrate hosts (fish, reptiles, and molluscs) [26]. Red seabream iridovirus (RSIV) causes enormous economic losses in aquaculture. RSIV was classified into a new genus, Megalocytivirus, along with turbot reddish body iridovirus (TRBIV) [27] and infectious spleen and kidney necrosis virus (ISKNV) [28]. The Baculoviridae and Ascoviridae are double-stranded DNA viruses that primarily infect invertebrates. These viruses are arthropods; lepidopterans serve as natural hosts, especially shrimp, and poxvirus infects marine mammals. This virus can cause skin diseases, and sometimes oral nodule diseases. Poxviruses that cause disease almost always have morphological characteristics of the Parapoxvirus genus [29,30]. In this study, these five viral families were not detected in any season. However, it did appear in some seasons. We propose the possibility of infection in the marine farming industry depending on the season. Additionally, the NCLDVs identified in this study were discovered as large-scale genome size viruses called giant viruses that belonged to 10 virus families (Ascoviridae, Asfarviridae Iridoviridae, Marseilleviridae, Megaviridae, Mimiviridae, Pandoraviridae, Phycodnaviridae, Pithoviridae, and Poxviridae). NCLDVs contain genes homologous to the host and are a discovery that demonstrates the theory of viral evolution. Genes from the host can replicate and be transcribed independently [31].
The functional potential of the four metagenomes (viromes), as determined by their homology to the subsystem database, was examined for all viral scaffolds (assembly of contigs) using the MG-RAST online server. Sequence similarity searches were computed against a protein database derived from M5nr, providing a non-redundant integration of many databases; all four seasons with different metabolic pathways, cell wall, and capsule-related metabolism made up the largest group in March and June, September and December had the greatest matches in virulence, disease, and defense-related pathways. However, more research is needed to determine the dominant species (Bacillus phage G and Pelagibacter phage HTVC008M) for seasonal changes, and metabolic pathways may be relevant. The seasonal change in species composition was determined to be caused by environmental variables such as temperature and DO. Compared to those in earlier studies, the main metabolic functions were amino acids, derivatives, and carbohydrates from the soil viral metagenome, which showed significantly different metabolic functions [32].
Viral metagenomic studies of environmental samples are likely to grow rapidly due to recent significant advances in next-generation sequencing (NGS) equipment and improved viral particle concentrations and purification methods. However, not all procedures in the analytical pipeline for virus collection and bioinformatic analysis in aquatic systems have been established. In particular, water-based samples should be used carefully to concentrate the viruses. The first study on viral concentration was conducted in 1931 by Elford, who investigated viral adsorption onto collodion membranes to determine the size of virion particles [33]. Adsorption–elution methods using larger pore-size filters [34,35,36] and the pelleting of viruses with the ultracentrifugation method [37] were developed to determine the virus concentration in water-based samples. A concentration method based on chemicals (FeCl3 precipitation) was proposed, similar to that shown in our experimental method, which exhibited complete viral recovery and is widely applied for viral concentration [15].
Additionally, the lack of viral reference databases negatively affects the accurate identification of viral sequences in the analyzed virome. The reference database related to marine viruses is still insufficient to identify all species. Although the viromes analyzed in this study mostly consisted of dsDNA viruses, the BLAST results were limited to a subset of sequences that matched the reference database. Sequences of unknown marine organisms from the marine environment may represent a valuable blueprint for the discovery of new viruses as a result of advances in bioinformatics and the continued replenishment of viral sequences. Finally, additional efforts to analyze more comprehensive and in-depth genetic sequences may yield more precise results.
5. Conclusions
We used metagenomic analysis to identify the seasonal characteristics of the marine viruses distributed on Jeju Island. The results showed that the viral distribution pattern in each of the four seasons changed significantly depending on the water temperature. Our results suggest that environmental variables such as water temperature have a significant impact on the distribution of marine viruses and their hosts. Additionally, this pioneering study revealed the viral community that exists on Jeju Island, a major aquaculture area, and provides a detailed distribution of these viruses. These marine viral communities and their characterization results may be useful for research on other marine viruses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse12091480/s1, Table S1. Identification of top 10 viral genome in all seasons.
Author Contributions
Conceptualization, J.H.; Methodology, J.H. and Y.J.; Field investigation, J.H.; Software, E.G.O.; Writing-original draft preparation, J.H.; Writing-review and editing. E.G.O. and Y.J; Project administration. E.G.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project ‘Sanitary evaluation on the shellfish growing area for export and fisheries products’(R2024057) of the National Institute of Fisheries Science (NIFS), Incheon, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Welsh, J.E.; Steenhuis, P.; de Moraes, K.R.; van der Meer, J.; Thieltges, D.W.; Brussaard, C.P. Marine virus predation by non-host organisms. Sci. Rep. 2020, 10, 5221. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Parada, V.; Sintes, E.; van Aken, H.M.; Weinbauer, M.G.; Herndl, G.J. Viral abundance, decay, and diversity in the meso-and bathypelagic waters of the North Atlantic. Appl. Environ. Microbiol. 2007, 73, 4429–4438. [Google Scholar] [CrossRef]
- Mushegian, A. Are there 1031 virus particles on earth, or more, or fewer? J. Bacteriol. 2020, 202, e00052-20. [Google Scholar] [CrossRef]
- Biggs, T.E.; Huisman, J.; Brussaard, C.P. Viral lysis modifies seasonal phytoplankton dynamics and carbon flow in the Southern Ocean. ISME J. 2021, 15, 3615–3622. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Fuhrman, J.A. Effects of phytoplankton, viral communities, and warming on free-living and particle-associated marine prokaryotic community structure. Nat. Commun. 2022, 13, 7905. [Google Scholar] [CrossRef]
- Middelboe, M.; Jorgensen, N.; Kroer, N. Effects of viruses on nutrient turnover and growth efficiency of noninfected marine bacterioplankton. Appl. Environ. Microbiol. 1996, 62, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Middelboe, M.; Lyck, P.G. Regeneration of dissolved organic matter by viral lysis in marine microbial communities. Aquat. Microb. Ecol. 2002, 27, 187–194. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Sanseverino, I.; Pretto, P.; António, D.C.; Lahm, A.; Facca, C.; Loos, R.; Skejo, H.; Beghi, A.; Pandolfi, F.; Genoni, P. Metagenomics analysis to investigate the microbial communities and their functional profile during cyanobacterial blooms in Lake Varese. Microb. Ecol. 2022, 83, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M. Marine viruses: Truth or dare. Mar. Sci. 2012, 4, 425–428. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Mushegian, A.R.; Dolja, V.V.; Koonin, E.V. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 2010, 18, 11–19. [Google Scholar] [CrossRef]
- John, S.G.; Mendez, C.B.; Deng, L.; Poulos, B.; Kauffman, A.K.M.; Kern, S.; Brum, J.; Polz, M.F.; Boyle, E.A.; Sullivan, M.B. A simple and efficient method for concentration of ocean viruses by chemical flocculation. Environ. Microbiol. Rep. 2011, 3, 195–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Z.; Wu, S.; Ruan, A. Community Structure, Drivers, and Potential Functions of Different Lifestyle Viruses in Chaohu Lake. Viruses 2024, 16, 590. [Google Scholar] [CrossRef]
- Martínez Martínez, J.; Martinez-Hernandez, F.; Martinez-Garcia, M. Single-virus genomics and beyond. Nat. Rev. Microbiol. 2020, 18, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Kieft, K.; Adams, A.; Salamzade, R.; Kalan, L.; Anantharaman, K. vRhyme enables binning of viral genomes from metagenomes. Nucleic Acids Res. 2022, 50, e83. [Google Scholar] [CrossRef]
- Schulz, F.; Abergel, C.; Woyke, T. Giant virus biology and diversity in the era of genome-resolved metagenomics. Nat. Rev. Microbiol. 2022, 20, 721–736. [Google Scholar] [CrossRef]
- Kim, M.-S.; Whon, T.W.; Bae, J.-W. Comparative viral metagenomics of environmental samples from Korea. Genom. Inform. 2013, 11, 121. [Google Scholar] [CrossRef]
- Deghorain, M.; Van Melderen, L. The Staphylococci phages family: An overview. Viruses 2012, 4, 3316–3335. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, D.M.; von Meijenfeldt, F.B.; Dutilh, B.E.; Villanueva, L.; Sinninghe Damsté, J.S.; Stams, A.J.; Sánchez-Andrea, I. The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environ. Microbiol. 2021, 23, 2834–2857. [Google Scholar] [CrossRef]
- Tripp, H.J.; Kitner, J.B.; Schwalbach, M.S.; Dacey, J.W.; Wilhelm, L.J.; Giovannoni, S.J. SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 2008, 452, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Trus, B.L.; Cheng, N.; Steven, A.C.; Watson, M.S.; Cunningham, C.; Le Deuff, R.-M.; Renault, T. A novel class of herpesvirus with bivalve hosts. J. Gen. Virol. 2005, 86, 41–53. [Google Scholar] [CrossRef]
- Davison, A.J. Herpesvirus systematics. Vet. Microbiol. 2010, 143, 52–69. [Google Scholar] [CrossRef]
- Hyatt, A.; Gould, A.; Zupanovic, Z.; Cunningham, A.; Hengstberger, S.; Whittington, R.; Kattenbelt, J.; Coupar, B. Comparative studies of piscine and amphibian iridoviruses. Arch. Virol. 2000, 145, 301–331. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-Y.; Jia, K.-T.; Yang, B.; Huang, J. Complete genome sequence of a Megalocytivirus (family Iridoviridae) associated with turbot mortality in China. Virol. J. 2010, 7, 159. [Google Scholar] [CrossRef]
- He, J.; Zeng, K.; Weng, S.; Chan, S.-M. Experimental transmission, pathogenicity and physical–chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Hicks, B.D.; Worthy, G.A. Sealpox in captive grey seals (Halichoerus grypus) and their handlers. J. Wildl. Dis. 1987, 23, 1–6. [Google Scholar] [CrossRef]
- Simpson, V.; Stuart, N.; Stack, M.; Ross, H.; Head, J. Parapox infection in grey seals (Halichoerus grypus) in Cornwall. Vet. Rec. 1994, 134, 292–296. [Google Scholar] [CrossRef]
- Koonin, E.V.; Yutin, N. Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses. Intervirology 2010, 53, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Reavy, B.; Swanson, M.M.; Cock, P.J.; Dawson, L.; Freitag, T.E.; Singh, B.K.; Torrance, L.; Mushegian, A.R.; Taliansky, M. Distinct circular single-stranded DNA viruses exist in different soil types. Appl. Environ. Microbiol. 2015, 81, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Cashdollar, J.; Wymer, L. Methods for primary concentration of viruses from water samples: A review and meta-analysis of recent studies. J. Appl. Microbiol. 2013, 115, 1–11. [Google Scholar] [CrossRef]
- Borrego, J.; Morinigo, M.; Martinez-Manzanares, E.; Bosca, M.; Castro, D.; Barja, J.; Toranzo, A.E. Plasmid associated virulence properties of environmental isolates of Aeromonas hydrophila. J. Med. Microbiol. 1991, 35, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Shimasaki, A.; Ohgaki, S. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 2002, 68, 1033–1039. [Google Scholar] [CrossRef]
- Kamata, S.-I.; Suzuki, S. Concentration of marine birnavirus from seawater with a glass fiber filter precoated with bovine serum albumin. Mar. Biotechnol. 2003, 5, 157–162. [Google Scholar] [CrossRef]
- Colombet, J.; Robin, A.; Lavie, L.; Bettarel, Y.; Cauchie, H.; Sime-Ngando, T. Virioplankton ‘pegylation’: Use of PEG (polyethylene glycol) to concentrate and purify viruses in pelagic ecosystems. J. Microbiol. Methods 2007, 71, 212–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).