Bioprospecting of Mangrove Filamentous Fungi for the Biodegradation of Polyethylene Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Low-Density Polyethylene Microplastics Morphological Characterization and Sterilization
2.2. Sample Collection and Isolation
2.3. Morphological Identification
2.4. Molecular Identification
2.5. Microorganisms and Culture Conditions
2.5.1. Biomass Concentration
2.5.2. Low-Density Polyethylene Microplastics Biodegradation
2.5.3. Fourier-Transform Infrared Spectroscopy
2.6. Laccase Activity Assay
3. Results
3.1. LDPEMPs Size and Morphology
3.2. Morphological and Molecular Identification
3.3. Microplastic Biodegradation
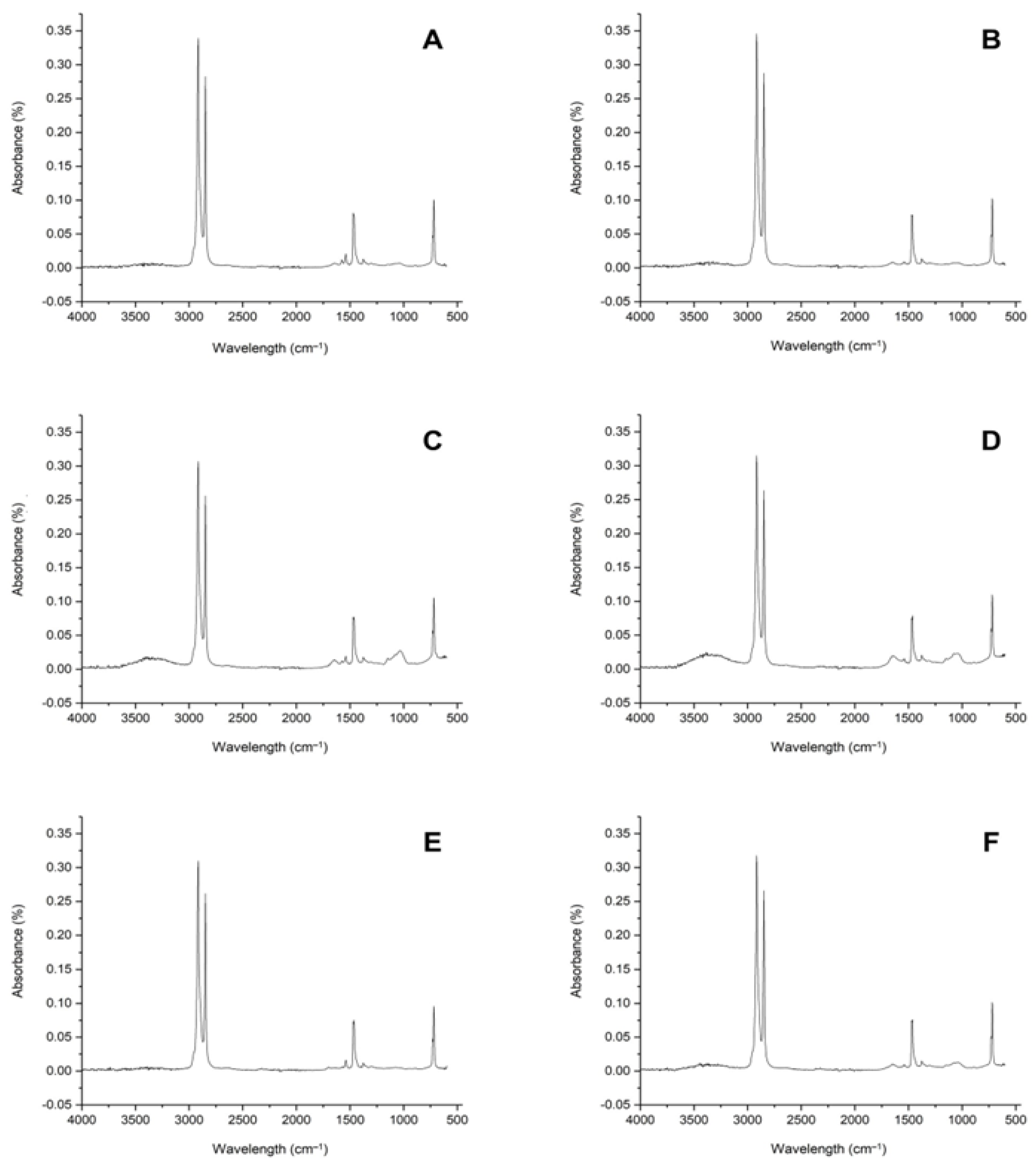
3.4. FTIR Analysis
3.5. Laccase Activity
4. Discussion
4.1. Low-Density Polyethylene Microplastics and Marine Aquatic Pollution
4.2. Fungal Isolates Identification
4.3. Microplastic Mycodegradation
4.4. Polymeric Chains Disruption
4.5. Enzymatic Activities Associated with Microplastic Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcés-Ordóñez, O.; Saldarriaga-Vélez, J.F.; Espinosa-Díaz, L.F.; Patiño, A.D.; Cusba, J.; Canals, M.; Mejía-Esquivia, K.; Fragozo-Velásquez, L.; Sáenz-Arias, S.; Córdoba-Meza, T.; et al. Microplastic pollution in water, sediments and commercial fish species from Ciénaga Grande de Santa Marta lagoon complex, Colombian Caribbean. Sci. Total Environ. 2022, 829, 154643. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, A.H.; López-Maldonado, E.A.; Alam, S.S.; López, J.R.L.; Herrera, P.F.M.; Mohamed, B.A.; Mahmoud, A.E.D.; Abutaleb, A.; Singh, L. Microplastics: Occurrences, treatment methods, regulations and foreseen environmental impacts. Environ. Res. 2022, 215, 114224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef]
- Madhumitha, C.T.; Karmegam, N.; Biruntha, M.; Arun, A.; Al Kheraif, A.A.; Kim, W.; Kumar, P. Extraction, identification, and environmental risk assessment of microplastics in commercial toothpaste. Chemosphere 2022, 296, 133976. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cárdenas, A.; Moroney, A.G.; Pelt, F.N.A.M.; O’Halloran, J.; Jansen, M.A.K. Trophic transfer of microplastics in a model freshwater microcosm; lack of a consumer avoidance response. Food Webs 2022, 31, e00228. [Google Scholar] [CrossRef]
- Rakib, M.R.J.; Sarker, A.; Ram, K.; Uddin, M.G.; Walker, T.R.; Chowdhury, T.; Uddin, J.; Khandaker, M.U.; Rahman, M.M.; Idris, A.M. Microplastic toxicity in aquatic organisms and aquatic ecosystems: A Review. Water Air Soil. Pollut. 2023, 234, 52. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef]
- Détrée, C.; Gallardo-Escárate, C. Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2018, 83, 52–60. [Google Scholar] [CrossRef]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Bacha, A.U.R.; Nabi, I.; Zaheer, M.; Jin, W.; Yang, L. Biodegradation of macro- and micro-plastics in environment: A review on mechanism, toxicity, and future perspectives. Sci. Total Environ. 2023, 858, 160108. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, S.; Mafigholami, R.; Moghimi, H.; Borghei, S.M. Feasibility study of microplastic biodegradation in effluents from South Tehran WWTP after quantitative and qualitative measurement of the particles. Appl. Water Sci. 2023, 13, 80. [Google Scholar] [CrossRef]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Singh, J.; Singh, J.; Angmo, D.; Vig, A.P. Biodegradation of different types of microplastics: Molecular mechanism and degradation efficiency. Sci. Total Environ. 2023, 877, 162912. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Yang, S.; Zheng, H.; Zheng, Y.; Jun, M.; Nagarajan, D.; Varjani, S.; Chang, J.S. Recent advances in biodegradation of emerging contaminants-microplastics (MPs): Feasibility, mechanism, and future prospects. Chemosphere 2023, 331, 138776. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, N.; Duhan, L.; Pasrija, R.; Thomas, J.; Umesh, M.; Lakkaboyana, S.K.; Andler, R.; Vangnai, A.S.; Vithanage, M.; et al. Microbial engineering strategies for synthetic microplastics clean up: A review on recent approaches. Environ. Toxicol. Pharm. 2023, 98, 104045. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Y.; Li, Y.; Lin, L.; Chen, Y.; Shen, J. Occurrence, Degradation pathways, and potential synergistic degradation mechanism of microplastics in surface water: A Review. Curr. Pollution Rep. 2023, 9, 312–326. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Duarte, K.; Costa, J.P.; Santos, P.S.M.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal enzymes involved in plastics biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- Simões, M.F.; Antunes, A.; Ottoni, C.A.; Amini, M.S.; Alam, I.; Alzubaidy, H.; Mokhtar, N.A.; Archer, J.A.; Bajic, V.B. Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the Red Sea—A metagenomic approach. Genom. Proteom. Bioinform. 2015, 13, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2021, 46, 3060–3075. [Google Scholar] [CrossRef]
- Yang, S.; Xu, W.; Zhang, K.; Hu, J.; Gao, Y.; Cui, G.; Grossart, H.-P.; Luo, Z. Fungal communities differ with microplastic types in deep sea sediment enrichments of the Eastern Pacific. Int. Biodeterior. Biodegrad. 2022, 173, 105461. [Google Scholar] [CrossRef]
- Mathur, G.; Prasad, R. Degradation of polyurethane by Aspergillus flavus (ITCC 6051) isolated from soil. Appl. Biochem. Biotechnol. 2012, 167, 1595–1602. [Google Scholar] [CrossRef]
- Simões, M.F.; Pereira, L.; Santos, C.; Lima, N. Polyphasic identification and preservation of fungal diversity: Concepts and applications. In Management of Microbial Resources in the Environment; Springer: Dordrecht, The Netherlands, 2013; pp. 91–117. [Google Scholar]
- Saif, F.A.; Yaseen, S.A.; Alameen, A.S.; Mane, S.B.; Undre, P.B. Identification and characterization of Aspergillus species of fruit rot fungi using microscopy, FT-IR, Raman and UV–Vis spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 2021. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. Genbank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. ClustalW: Improving the sensivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Altekar, G.; Dwarkadas, S.; Huelsenbeck, J.P.; Ronquist, F. Parallel Metropolis coupled Markov chain Monte Carlo for Bayesian phylogenetic inference. Bioinformatics 2004, 20, 407–415. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, v 1.4.4. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 September 2024).
- Wang, D.; Su, L.; Ruan, H.D.; Chen, J.; Lu, J.; Lee, C.H.; Jiang, S.Y. Quantitative and qualitative determination of microplastics in oyster, seawater and sediment from the coastal areas in Zhuhai, China. Mar. Pollut. Bull. 2021, 164, 112000. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef]
- Silvério, S.C.; Rodríguez, O.; Tavares, A.P.M.; Teixeira, J.A.; Macedo, E.A. Laccase recovery with aqueous two-phase systems: Enzyme partitioning and stability. J. Mol. Catal. B Enzym. 2013, 87, 37–43. [Google Scholar] [CrossRef]
- Ali, N.; Khan, M.H.; Ali, M.; Sidra; Ahmad, S.; Khan, A.; Nabi, G.; Ali, F.; Bououdina, M.; Kyzas, G.Z. Insight into microplastics in the aquatic ecosystem: Properties, sources, threats and mitigation strategies. Sci. Total Environ. 2024, 913, 169489. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Cheng, X.T.; Zeng, Y.Q. The occurrence of microplastic in aquatic environment and toxic effects for organisms. Int. J. Environ. Sci. Technol. 2023, 20, 10477–10490. [Google Scholar] [CrossRef]
- Khedre, A.M.; Ramadan, S.A.; Ashry, A.; Alaraby, M. Abundance and risk assessment of microplastics in water, sediment, and aquatic insects of the Nile River. Chemosphere 2024, 353, 141557. [Google Scholar] [CrossRef] [PubMed]
- Turra, A.; Manzano, A.B.; Dias, R.J.S.; Mahiques, M.M.; Barbosa, L.; Balthazar-Silva, D.; Moreira, F.T. Three-dimensional distribution of plastic pellets in sandy beaches: Shifting paradigms. Sci. Rep. 2014, 4, 4435. [Google Scholar] [CrossRef] [PubMed]
- Izar, G.M.; Morais, L.G.; Pereira, C.D.S.; Cesar, A.; Abessa, D.M.S.; Christofoletti, R.A. Quantitative analysis of pellets on beaches of the São Paulo coast and associated non-ingested ecotoxicological effects on marine organisms. Reg. Stud. Mar. Sci. 2019, 29, 100705. [Google Scholar] [CrossRef]
- Crew, A.; Gregory-Eavez, I.; Riciiardi, A. Distribution, abundance, and diversity of microplastics in the upper St. Lawrence River. Environ. Pollut. 2020, 260, 113994. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Reifferscheid, G. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Broccoli, A.; Bernardi, G.; Grazioli, E.; Russo, G. Chemical composition of microplastic in sediments and protected detritivores from different marine habitats (Salina Island). Mar. Pollut. Bull. 2020, 152, 110918. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Dry and wet deposition of microplastics in a semi-arid region (Shiraz, Iran). Sci. Total Environ. 2020, 786, 147358. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Sarkar, S.D.; Das, B.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci. Total Environ. 2019, 1, 133717. [Google Scholar] [CrossRef]
- Ameen, F.; Moslem, M.; Hadi, S.; Al-Sabri, A.E. Biodegradation of low density polyethylene (LDPE) by mangrove fungi from the Red Sea Coast. Prog. Rubber Plast. Recycl. Technol. 2015, 31, 125–144. [Google Scholar] [CrossRef]
- Saénz, M.; Borodulina, T.; Diaz, L.; Banchon, C. Minimal conditions to degrade low density polyethylene by Aspergillus terreus and niger. J. Ecol. Eng. 2019, 20, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sangale, M.K.; Shahnawaz, M.; Ade, A.B. Potential of fungi isolated from the dumping sites mangrove rhizosphere soil to degrade polythene. Sci. Rep. 2019, 9, 5390. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.S.; Kannan, V.R.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef]
- Ferreira-Filipe, D.A.; Oliveira, L.; Paço, A.; Fernandes, A.J.S.; Costa, F.M.; Duarte, A.C.; Rocha-Santos, T.; Silva, A.L.P. Biodegradation of e-waste microplastics by Penicillium brevicompactum. Sci. Total Environ. 2024, 935, 173334. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Boomija, R.V.; Sathiyamoorthy, T.; Mathivanan, N.; Balaji, R. Mycodegradation of low-density polyethylene by Cladosporium sphaerospermum, isolated from platisphere. Sci. Rep. 2024, 14, 8351. [Google Scholar] [CrossRef]
- Duan, Y.; Yin, Y.; Ni, Z.; Liu, J.; Gui, H.; Wu, D.; Wu, X.; Wang, L. The effective and green biodegradation of polyethylene microplastics by the screening of a strain with its degrading enzymes. Biochem. Eng. J. 2024, 210, 109429. [Google Scholar] [CrossRef]
- Khan, S.; Ali, S.A.; Ali, A.S. Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘Penicillium citrinum’ isolated from soils of plastic waste dump yard, Bhopal, India. Environ. Technol. 2022, 44, 2300–2314. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into characteristics of white rot fungus during environmental plastics adhesion and degradation mechanism of plastics. J. Hazard Mater 2023, 448, 130878. [Google Scholar] [CrossRef] [PubMed]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatya, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2018, 7, 39515. [Google Scholar] [CrossRef]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145. [Google Scholar] [CrossRef]
- Hikmah, M.; Setyaningsih, R.; Pangastuti, A. The potential of lignolytic Trichoderma isolates in LDPE (low density polyethylene) plastic biodegradation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012076. [Google Scholar] [CrossRef]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, N.; Singhal, N.; Zhuang, W.Q.; Baroutian, S. Degradation of plastic waste using stimulated and naturally occurring microbial strains. Chemosphere 2021, 263, 127975. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.T.; Rabie, G.H.; Hamed, E.A. Biodegradation of low-density polyethylene (LDPE) using the mixed culture of Aspergillus carbonarius and A. fumigates. Environ. Dev. Sustain. 2021, 23, 14556–14584. [Google Scholar] [CrossRef]
- Černoša, A.; Cortizas, A.M.; Traoré, M.; Podlogar, M.; Danevčič, T.; Gunde-Cimerman, N.; Gostinčar, C. A screening method for plastic-degrading fungi. Heliyon 2024, 10, e31130. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.B.; Song, H.A.; Lee, T.K. Accelerating the biodegradation of high-density polyethylene (HDPE) using Bjerkandera adusta TBB-03 and lignocellulose substrates. Microorganisms 2019, 7, 304. [Google Scholar] [CrossRef]
- Gómez-Méndez, L.D.; Moreno-Bayona, D.A.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Pedroza-Rodríguez, A.M.; Vargas, A.; Bogoya, J.M. Biodeterioration of plasma pretreated LDPE sheets by Pleurotus ostreatus. PLoS ONE 2018, 13, e0203786. [Google Scholar] [CrossRef]
- Restrepo-Flórez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2015, 88, 83–90. [Google Scholar] [CrossRef]

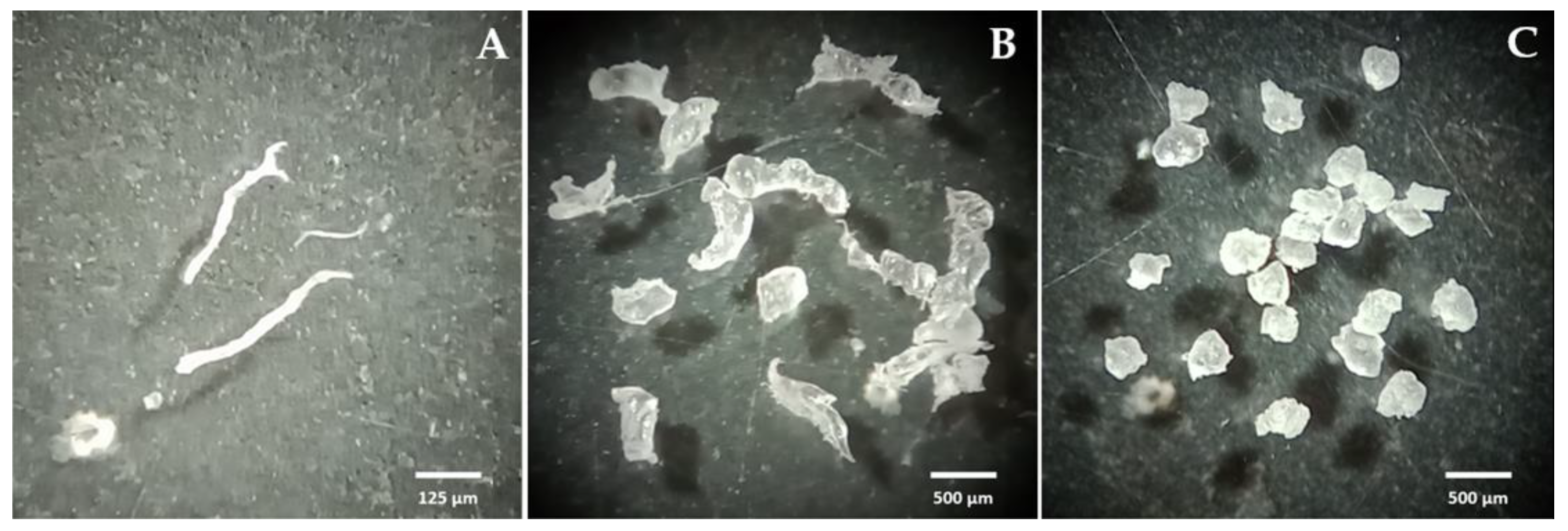
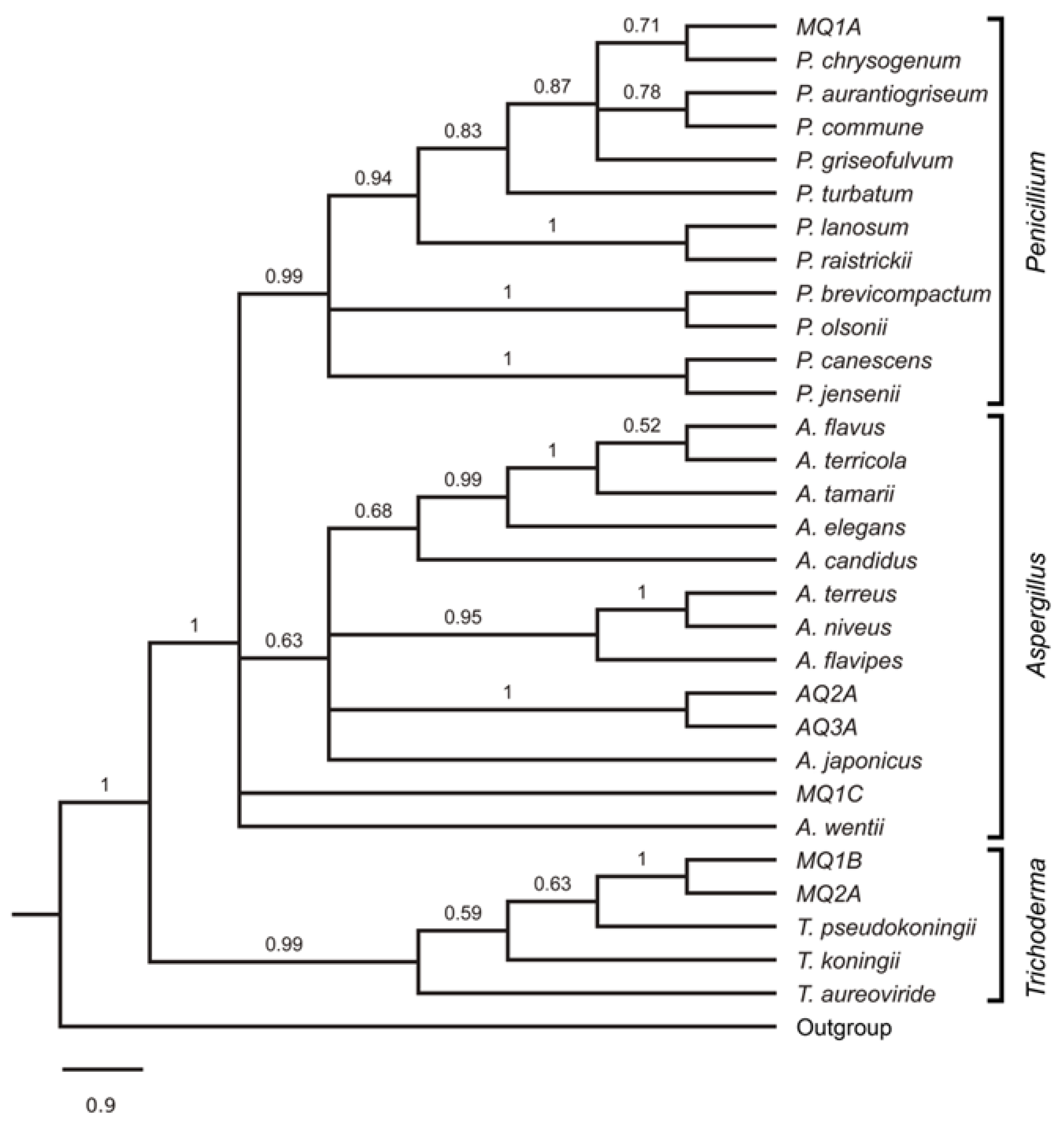
| Order | Species | GB Accession |
|---|---|---|
| Eurotiales | Aspergillus sp. AQ2A | PP980750 * |
| Aspergillus sp. AQ3A | PP980751 * | |
| Aspergillus sp. MQ1C | PP980748 * | |
| Aspergillus candidus Link | NR077149 | |
| Aspergillus elegans Gasperini | NR077196 | |
| Aspergillus flavipes (Bainer and R. Sartory), Thom and Church | NR135397 | |
| Aspergillus flavus Link | NR111041 | |
| Aspergillus japonicas Saito | NR131268 | |
| Aspergillus niveus Blochwitz | NR137476 | |
| Aspergillus tamari Kita | NR135325 | |
| Aspergillus terreus Thom | NR131276 | |
| Aspergillus terricola Marchal and É.J. Marchal | NR151785 | |
| Aspergillus wentii Wehmer | NR077152 | |
| Penicillium aff. chrysogenum MQ1A | PP980746 * | |
| Penicillium aurantiogriseum Dierckx | NT121247 | |
| Penicillium brevicompactum Dierckx | NR121299 | |
| Penicillium canescens Scopp | NR121256 | |
| Penicillium chrysogenum Thom | NR077145 | |
| Penicillium commune Thom | NR111143 | |
| Penicillium griseofulvum Dierckx | NR103692 | |
| Penicillium jensenii K.M. Zalessky | NR121297 | |
| Penicillium lanosum Westling | NR163539 | |
| Penicillium olsonii Bainier and Sartory | NR163546 | |
| Penicillium raistrickii G.Sm. | NR119493 | |
| Penicillium turbatum Westling | NR121257 | |
| Hypocreales | Trichoderma aff. pseudokoningii MQ1B | PP980747 * |
| Trichoderma aff. pseudokoningii MQ2A | PP980749 * | |
| Trichoderma aureoviride Rifai | NR144877 | |
| Trichoderma koningii Oudem | NR138456 | |
| Trichoderma pseudokoningii Rifai | NR120296 | |
| Diaporthales | Diaporthe ambigua Nitschke | NR119434 |
| Glomerellales | Colletotrichum gloesporioides (Penz.) Penz. and Sacc. | NR160754 |
| Strain Code | Macroscopic Characteristics | Macroscopic Appearance | Microscopic Characteristics | Microscopic Appearance | Species |
|---|---|---|---|---|---|
| MQ1A | Velvety white, followed by bluish-green |  |
| 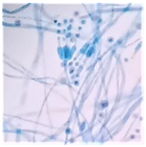 | Penicillium aff. chrysogenum |
| MQ1B | Cottony teal or dark green with concentric rings |  |
| 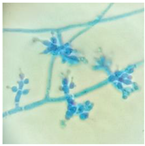 | Trichoderma aff. pseudokonigii |
| MQ1C | Furfuraceous blue-green mycelium | 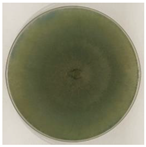 |
| 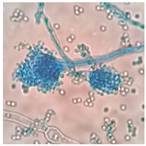 | Aspergillus sp. |
| MQ2A | Cottony teal or dark green with concentric rings | 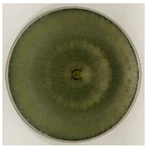 |
|  | Trichoderma aff. pseudokonigii |
| AQ2A | Velvety white mycelium with whitish to pinkish sclerotia and black vesicles | 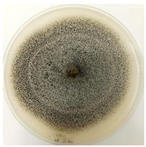 |
| 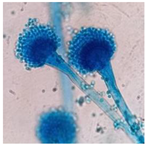 | Aspergillus sp. |
| AQ3A | Velvety white, quickly becoming powdery black | 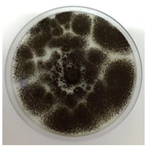 |
|  | Aspergillus sp. |
| Fungal Strain | Identified Genera/Species | Biomass | LDPEMPs | |||||
|---|---|---|---|---|---|---|---|---|
| Initial (g·mL−1) | Final (g·mL−1) | Control (g·mL−1) | Initial (g) | Final (g) | Degradation | |||
| Weight (g) | % | |||||||
| MQ1A | Penicillium aff. crisogenum | 0 | 0.0500 | 0.0465 | 0.0515 | 0.0405 | 0.0110 | 21 |
| MQ1B | Trichoderma aff. pseudokoningii. | 0 | 0.0695 | 0.0720 | 0.0505 | 0.0315 | 0.0190 | 38 |
| MQ1C | Aspergillus sp. | 0 | 0.0415 | 0.0405 | 0.0530 | 0.0315 | 0.0215 | 41 |
| MQ2A | Trichoderma aff. pseudokonongii. | 0 | 0.0360 | 0.0565 | 0.0535 | 0.0445 | 0.0090 | 17 |
| AQ2A | Aspergillus sp. | 0 | 0.0575 | 0.0395 | 0.0510 | 0.0325 | 0.0185 | 36 |
| AQ3A | Aspergillus sp. | 0 | 0.0890 | 0.0450 | 0.0520 | 0.0275 | 0.0245 | 47 |
| Fungi | Medium Carbon Source | Microplastic (µm) | Incubation Time (days) | Weight Loss (%) | References |
|---|---|---|---|---|---|
| Penicillium brevicompactum | LCM * Glucose | Printed circuit boards (PCB) 1–2 mm | 28 | 75 | [57] |
| Cladosporium sphaerospermum | LCM * (PDB **) Dextrose | LDPE *** (above 50 microns) | 7 | 15.12 | [58] |
| Aspergillus niger DL-1 | LCM * Glucose | LDPE *** film (2 × 2 cm) | 30 | 7.65 ± 0.92 | [59] |
| Penicillium citrinum | LDPE *** | LDPE *** | 90 | 47.22 ± 2.04% | [60] |
| Phanerochaete chrysosporium | Glucose | polylactic acid (PLA) polystyrene (PS) | 35 | 34.35% 19.71% | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, A.; Gama, L.; Fornari, M.; Neto, A.; de Souza, R.; Perna, R.; Castro, L.; Kovacs, S.; Simões, M.F.; Ferreira, N.; et al. Bioprospecting of Mangrove Filamentous Fungi for the Biodegradation of Polyethylene Microplastics. J. Mar. Sci. Eng. 2024, 12, 1629. https://doi.org/10.3390/jmse12091629
Aguiar A, Gama L, Fornari M, Neto A, de Souza R, Perna R, Castro L, Kovacs S, Simões MF, Ferreira N, et al. Bioprospecting of Mangrove Filamentous Fungi for the Biodegradation of Polyethylene Microplastics. Journal of Marine Science and Engineering. 2024; 12(9):1629. https://doi.org/10.3390/jmse12091629
Chicago/Turabian StyleAguiar, Arthur, Letícia Gama, Milene Fornari, Almir Neto, Rodrigo de Souza, Rafael Perna, Laura Castro, Stella Kovacs, Marta Filipa Simões, Nelson Ferreira, and et al. 2024. "Bioprospecting of Mangrove Filamentous Fungi for the Biodegradation of Polyethylene Microplastics" Journal of Marine Science and Engineering 12, no. 9: 1629. https://doi.org/10.3390/jmse12091629









