Is There a Better Biomaterial for Dental Implants than Titanium?—A Review and Meta-Study Analysis
Abstract
:1. Introduction
2. Literature Search
3. Dental Implants
4. Dental Implant Materials
4.1. Titanium
4.2. Titanium Alloy
4.3. Zirconia
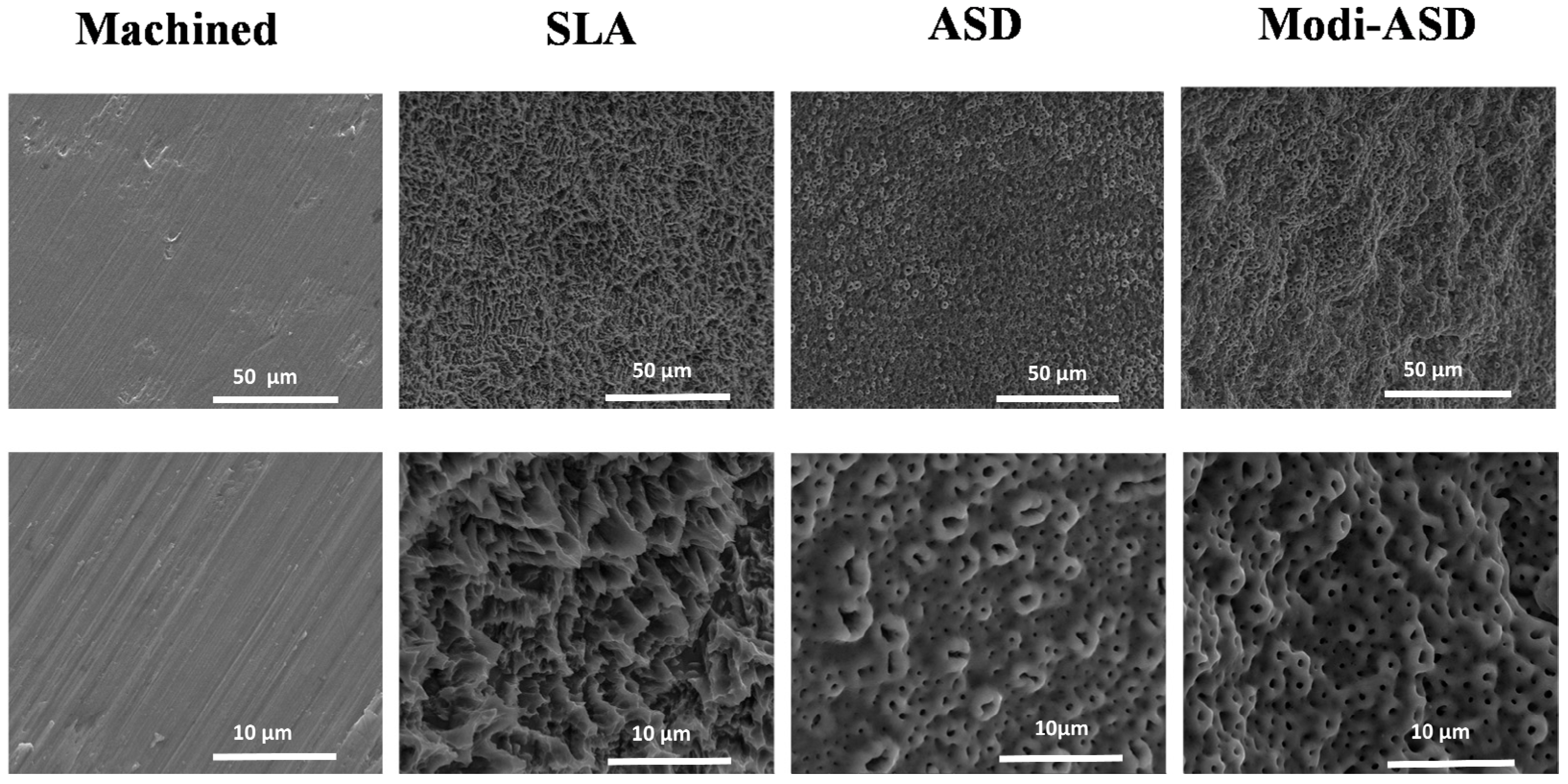
5. Implant Surface Modifications Prevent Inflammation
5.1. Improvement of Tissue Integration on Implant
5.2. Implant Surface Chemistry Alteration
6. Biomolecules
7. Meta-Study Analysis
7.1. Evaluation of Heterogeneity
7.2. On the Effect of Implant Materials on Probing Depth
7.3. Clinical Studies
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epker, B.; Frost, H. Correlation of bone resorption and formation with the physical behavior of loaded bone. J. Dent. Res. 1965, 44, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Haugen, H.J.; Aass, A.M.; Sanz, M.; Antonoglou, G.N.; Bouchard, P.; Bozic, D.; Eickholz, P.; Jepsen, K.; Jepsen, S.; et al. Peri-Implant Health and the Knowing-Doing Gap—A Digital Survey on Procedures and Therapies. Front. Dent. Med. 2021, 2. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontol. 2000 1998, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Angkaew, C.; Serichetaphongse, P.; Krisdapong, S.; Dart, M.M.; Pimkhaokham, A. Oral health-related quality of life and esthetic outcome in single anterior maxillary implants. Clin. Oral Implant. Res. 2017, 28, 1089–1096. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Hussain, B.; Karaca, E.O.; Kuru, B.E.; Gursoy, H.; Haugen, H.J.; Wohlfahrt, J.C. Treatment of residual pockets using an oscillating chitosan device versus regular curettes alone-A randomized, feasibility parallel-arm clinical trial. J. Periodontol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- Mombelli, A.; Marxer, M.; Gaberthuel, T.; Grunder, U.; Lang, N.P. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J. Clin. Periodontol. 1995, 22, 124–130. [Google Scholar] [CrossRef]
- Pontoriero, R.; Tonelli, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin. Oral Implant. Res. 1994, 5, 254–259. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.K.; Oh, J.H. Recent advances in dental implants. Maxillofac. Plast Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leventhal, G.S. Titanium, a metal for surgery. J. Bone Joint Surg. Am. 1951, 33, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Hansson, H.A.; Ivarsson, B. Interface analysis of titanium and zirconium bone implants. Biomaterials 1985, 6, 97–101. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Wintermantel, E.; Ha, S. Medizintechnik Life Science Engineering; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5. [Google Scholar]
- Steinemann, S. Titanium-the material of choice? Periodontol. 2000 1998, 17, 7. [Google Scholar] [CrossRef]
- Kasemo, B. Biocompatibility of titanium implants: Surface science aspects. J. Prosthet. Dent. 1983, 49, 832. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Material-tissue interfaces: The role of surface properties and processes. Environ. Health Perspect. 1994, 102, 41–45. [Google Scholar]
- Vora, H.D.; Shanker Rajamure, R.; Dahotre, S.N.; Ho, Y.H.; Banerjee, R.; Dahotre, N.B. Integrated experimental and theoretical approach for corrosion and wear evaluation of laser surface nitrided, Ti-6Al-4V biomaterial in physiological solution. J. Mech. Behav. Biomed. Mater. 2014, 37, 153–164. [Google Scholar] [CrossRef]
- Nicholson, J.W. The Chemistry of Medical and Dental Materials (RSC Materials Monographs); Royal Society of Chemistry: London, UK, 2002. [Google Scholar]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.; Crisan, B.; Crisan, L.; Baciut, M.; Onisor, F.; Baciut, G.; Campian, R.S.; Bran, S. Systemic drugs that influence titanium implant osseointegration. Drug Metab. Rev. 2017, 49, 92–104. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact 2009, 9, 61–71. [Google Scholar] [PubMed]
- Ellingsen, J.E.; Thomsen, P.; Lyngstadaas, S.P. Advances in dental implant materials and tissue regeneration. Periodontol. 2000 2006, 41, 136–156. [Google Scholar] [CrossRef]
- Lamolle, S.F.; Monjo, M.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Titanium implant surface modification by cathodic reduction in hydrofluoric acid: Surface characterization and in vivo performance. J. Biomed. Mater. Res. A 2009, 88, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Lamolle, S.F.; Monjo, M.; Rubert, M.; Haugen, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials 2009, 30, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Ronold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. Tensile force testing of optimized coin-shaped titanium implant attachment kinetics in the rabbit tibiae. J. Mater. Sci. Mater. Med. 2003, 14, 843–849. [Google Scholar] [CrossRef]
- Rønold, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E. Analysing the optimal value for titanium implant roughness in bone attachment using a tensile test. Biomaterials 2003, 24, 4559–4564. [Google Scholar] [CrossRef]
- Ronold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. Bone bonding assessed by tensile testing of osseointegrating coin-shaped titanium implants. J. Dent. Res. 2002, 81, A488. [Google Scholar]
- Choi, J.Y.; Lee, H.J.; Jang, J.U.; Yeo, I.S. Comparison between bioactive fluoride modified and bioinert anodically oxidized implant surfaces in early bone response using rabbit tibia model. Implant Dent. 2012, 21, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Raes, F.; Renckens, L.; Aps, J.; Cosyn, J.; De Bruyn, H. Reliability of circumferential bone level assessment around single implants in healed ridges and extraction sockets using cone beam CT. Clin. Implant Dent. Relat. Res. 2013, 15, 661–672. [Google Scholar] [CrossRef]
- Collaert, B.; Wijnen, L.; De Bruyn, H. A 2-year prospective study on immediate loading with fluoride-modified implants in the edentulous mandible. Clin. Oral Implant. Res. 2011, 22, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Saulacic, N.; Bosshardt, D.D.; Bornstein, M.M.; Berner, S.; Buser, D. Bone apposition to a titanium-zirconium alloy implant, as compared to two other titanium-containing implants. Eur. Cell Mater. 2012, 23, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Geurs, N.C.; Vassilopoulos, P.J.; Reddy, M.S. Soft tissue considerations in implant site development. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef] [Green Version]
- Forbes, W.F.; Gentleman, J.F.; Maxwell, C.J. Concerning the role of aluminum in causing dementia. Exp. Gerontol. 1995, 30, 23–32. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium and tungsten derivatives as antidiabetic agents. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef]
- Boyce, B.; Byars, J.; McWilliams, S.; Mocan, M.; Elder, H.; Boyle, I.; Junor, B. Histological and electron microprobe studies of mineralisation in aluminium-related osteomalacia. J. Clin. Pathol. 1992, 45, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, Y. Effect of friction on anodic polarization properties of metallic biomaterials. Biomaterials 2002, 23, 2071–2077. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity beta-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Mi, X.; Song, X. Martensitic transformation of Ti-18Nb (at.%) alloy with zirconium. Rare Metals 2012, 31, 227–230. [Google Scholar] [CrossRef]
- Thibon, I.; Ansel, D.; Gloriant, T. Interdiffusion in β-Ti–Zr binary alloys. J. Alloy Compd. 2009, 470, 127–133. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. Conjoint corrosion and wear in titanium alloys. Biomaterials 1999, 20, 765–772. [Google Scholar] [CrossRef]
- Ho, W.F.; Chen, W.K.; Wu, S.C.; Hsu, H.C. Structure, mechanical properties, and grindability of dental Ti-Zr alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Matsumoto, S.; Doi, H.; Yoneyama, T.; Hamanaka, H. Mechanical properties of the binary titanium-zirconium alloys and their potential for biomedical materials. J. Biomed. Mater. Res. 1995, 29, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 025005. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P. Influence of abutment material on stability of peri-implant tissues: A systematic review. Int. J. Oral Maxillofac. Implant. 2008, 23, 449–456. [Google Scholar]
- Bernhard, N.; Berner, S.; De Wild, M.; Wieland, M. The binary TiZr Alloy—A newly developed Ti alloy for use in dental implants. Forum Implantol. 2009, 5, 30–39. [Google Scholar]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin. Implant Dent. Relat. Res. 2010, 14, 538–545. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Rocha-Filho, R.C.; Biaggio, S.R.; Bocchi, N. Corrosion resistance of the Ti–50Zr at.% alloy after anodization in different acidic electrolytes. Corr. Sci. 2010, 52, 4058–4063. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Hodgson, P.D. Fabrication of novel TiZr alloy foams for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1439–1444. [Google Scholar] [CrossRef]
- Frank, M.J.; Walter, M.S.; Lyngstadaas, S.P.; Wintermantel, E.; Haugen, H.J. Hydrogen content in titanium and a titanium-zirconium alloy after acid etching. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Florit, M.; Ramis, J.M.; Xing, R.; Taxt-Lamolle, S.; Haugen, H.J.; Lyngstadaas, S.P.; Monjo, M. Differential response of human gingival fibroblasts to titanium- and titanium-zirconium-modified surfaces. J. Periodontal Res. 2014, 49, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Salou, L.; Taxt-Lamolle, S.; Reseland, J.E.; Lyngstadaas, S.P.; Haugen, H.J. Surface hydride on titanium by cathodic polarization promotes human gingival fibroblast growth. J. Biomed. Mater. Res. A 2014, 102, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Lyngstadaas, S.P.; Ellingsen, J.E.; Taxt-Lamolle, S.; Haugen, H.J. The influence of surface nanoroughness, texture and chemistry of TiZr implant abutment on oral biofilm accumulation. Clin. Oral Implant. Res. 2015, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Brägger, U.; Meijer, H.J.A.; Naert, I.; Persson, R.; Perucchi, A.; Quirynen, M.; Raghoebar, G.M.; Reichert, T.E.; Romeo, E.; et al. A Double-Blind Randomized Controlled Trial (RCT) of Titanium-13Zirconium versus Titanium Grade IV Small-Diameter Bone Level Implants in Edentulous Mandibles—Results from a 1-Year Observation Period. Clin. Implant Dent. Relat. Res. 2011, 14, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Stenlund, P.; Omar, O.; Brohede, U.; Norgren, S.; Norlindh, B.; Johansson, A.; Lausmaa, J.; Thomsen, P.; Palmquist, A. Bone response to a novel Ti-Ta-Nb-Zr alloy. Acta BioMater. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Kopova, I.; Strasky, J.; Harcuba, P.; Landa, M.; Janecek, M.; Bacakova, L. Newly developed Ti-Nb-Zr-Ta-Si-Fe biomedical beta titanium alloys with increased strength and enhanced biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 230–238. [Google Scholar] [CrossRef]
- Mat-Baharin, N.H.; Razali, M.; Mohd-Said, S.; Syarif, J.; Muchtar, A. Influence of alloying elements on cellular response and in-vitro corrosion behavior of titanium-molybdenum-chromium alloys for implant materials. J. Prosthodont. Res. 2020, 64, 490–497. [Google Scholar] [CrossRef]
- Mello, D.C.R.; de Oliveira, J.R.; Cairo, C.A.A.; Ramos, L.S.B.; Vegian, M.; de Vasconcellos, L.G.O.; de Oliveira, F.E.; de Oliveira, L.D.; de Vasconcellos, L.M.R. Titanium alloys: In vitro biological analyzes on biofilm formation, biocompatibility, cell differentiation to induce bone formation, and immunological response. J. Mater. Sci. Mater. Med. 2019, 30, 108. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials-a review. Regen. BioMater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, R.N.; Totan, A.R.; Imre, M.M.; Tancu, A.M.C.; Pantea, M.; Butucescu, M.; Farcasiu, A.T. Prosthetic Materials Used for Implant-Supported Restorations and Their Biochemical Oral Interactions: A Narrative Review. Materials 2022, 15, 1016. [Google Scholar] [CrossRef] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef]
- Rohr, N.; Bergemann, C.; Nebe, J.B.; Fischer, J. Crystal structure of zirconia affects osteoblast behavior. Dent. Mater. 2020, 36, 905–913. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hammerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Videm, K.; Lamolle, S.; Monjo, M.; Ellingsen, J.E.; Lyngstadaas, S.P.; Haugen, H.J. Hydride formation on titanium surfaces by cathodic polarization. Appl. Surf. Sci. 2008, 255, 3011–3015. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R. Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Mustafa, K.; Wroblewski, J.; Lopez, B.; Wennerberg, A.; Hultenby, K.; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implant. Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.; Steinemann, S.; Fiorellini, J.; Fox, C.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Conforto, E.; Aronsson, B.; Salito, A.; Crestou, C.; Caillard, D. Rough surfaces of titanium and titanium alloys for implants and prostheses. Mater. Sci. Eng. C Mater. Biol. Appl. 2004, 24, 611–618. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontol. 2000 2017, 73, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Rønold, H.J.; Ellingsen, J.E. Effect of micro-roughness produced by TiO2 blasting. Tensile testing of bone attachment by using coin-shaped implants. Biomaterials 2002, 23, 4211–4219. [Google Scholar] [CrossRef]
- Rønold, H.J.; Lyngstadaas, S.; Ellingsen, J.E. A study on the effect of dual blasting with TiO2 on titanium implant surfaces on functional attachment in bone. J. Biomed. Mater. Res. 2003, 67A, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.B.; Liu, Q.; De Wijn, J.; De Groot, K.; Cui, F. Preparation of bioactive microporous titanium surface by a new two-step chemical treatment. J. Mater. Sci. Mater. Med. 1998, 9, 121–128. [Google Scholar] [CrossRef]
- Wen, H.; Wolke, J.; De Wijn, J.; Liu, Q.; Cui, F.; De Groot, K. Fast precipitation of calcium phosphate layers on titanium induced by simple chemical treatments. Biomaterials 1997, 18, 1471–1478. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Bollen, C.M.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; van Steenberghe, D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]
- Aronsson, B.O.; Hjörvarsson, B.; Frauchiger, L.; Taborelli, M.; Vallotton, P.H.; Descouts, P. Hydrogen desorption from sand-blasted and acid-etched titanium surfaces after glow-discharge treatment. J. Biomed. Mater. Res. 2001, 54, 20–29. [Google Scholar] [CrossRef]
- Taborelli, M.; Jobin, M.; Francois, P.; Vaudaux, P.; Tonetti, M.; Szmukler-moncler, S.; Simpson, J.; Descouts, P. Influence of surface treatments developed for oral implants on the physical and biological properties of titanium.(I) Surface characterization. Clin. Oral Implant. Res. 1997, 8, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.E.; Videm, K.; Opsahl, L.; Rønold, H.J. Implants with Modified Surfaces for Increased Biocompatibility and Method for Production Thereof. WO/2000/038753, 6 July 2000. [Google Scholar]
- Choi, J.; Heo, S.; Koak, J.; Kim, S.; Lim, Y.; Kim, S.; Lee, J. Biological responses of anodized titanium implants under different current voltages. J. Oral Rehabil. 2006, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Heo, S.; Koak, J.; Kim, S.; Lee, J.; Kim, S.; Lim, Y. Osseointegration of anodized titanium implants under different current voltages: A rabbit study. J. Oral Rehabil. 2007, 34, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cheng, X. The preliminary study on the oxide film of pure titanium treated by anodic oxidation. Zhonghua Kou Qiang Ke Za Zhi 2001, 36, 427–430. [Google Scholar]
- Sul, Y.-T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Yang, B.; Uchida, M.; Kim, H.M.; Zhang, X.; Kokubo, T. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 2004, 25, 1003–1010. [Google Scholar] [CrossRef]
- Canullo, L.; Annunziata, M.; Pesce, P.; Tommasato, G.; Nastri, L.; Guida, L. Influence of abutment material and modifications on peri-implant soft-tissue attachment: A systematic review and meta-analysis of histological animal studies. J. Prosthet. Dent. 2021, 125, 426–436. [Google Scholar] [CrossRef]
- Effah, E.A.; Bianco, P.D.; Ducheyne, P. Crystal structure of the surface oxide layer on titanium and its changes arising from immersion. J. Biomed. Mater. Res. 1995, 29, 73–80. [Google Scholar] [CrossRef]
- Sittig, C.; Textor, M.; Spencer, N.D.; Wieland, M.; Vallotton, P.H. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J. Mater. Sci. Mater. Med. 1999, 10, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Park, K.; Choi, K.H.; Kim, S.H.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Cell adhesion and in vivo osseointegration of sandblasted/acid etched/anodized dental implants. Int. J. Mol. Sci. 2015, 16, 10324–10336. [Google Scholar] [CrossRef] [Green Version]
- Ellingsen, J.E.; Lyngstadaas, S.P. Medical Prosthetic Devices Having Improved Biocompatibility. U.S. Patent 7410502B2, 12 August 2008. [Google Scholar]
- Puleo, D.; Nanci, A. Understanding and controlling the bone–implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R. Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
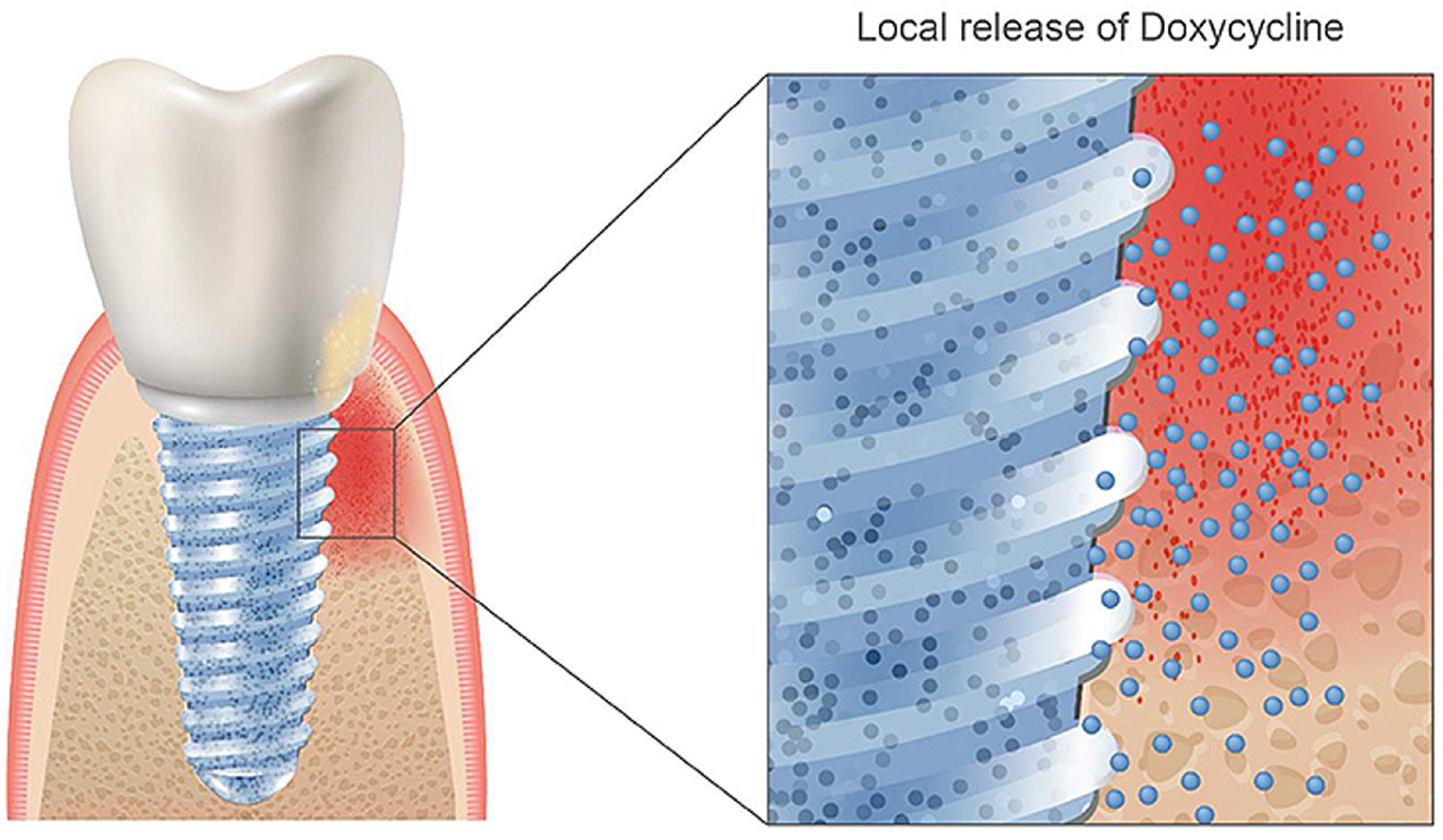
- Geissler, S.; Tiainen, H.; Haugen, H.J. Effect of cathodic polarization on coating doxycycline on titanium surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, M.S.; Frank, M.J.; Satué, M.; Monjo, M.; Rønold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Corrigendum to “Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo” [Dental 30 (2) (2014) 200–214]. Dent. Mater. 2014, 30, 463. [Google Scholar] [CrossRef]
- Rahmati, M.; Lyngstadaas, S.P.; Reseland, J.E.; Andersbakken, I.; Haugland, H.S.; Lopez-Pena, M.; Cantalapiedra, A.G.; Guzon Munoz, F.M.; Haugen, H.J. Coating doxycycline on titanium-based implants: Two in vivo studies. Bioact. Mater. 2020, 5, 787–797. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Haugen, H.J. Simvastatin-activated implant surface promotes osteoblast differentiation in vitro. J. BioMater. Appl. 2014, 28, 897–908. [Google Scholar] [CrossRef]
- Frank, M.J.; Walter, M.S.; Bucko, M.M.; Pamula, E.; Lyngstadaas, S.P.; Haugen, H.J. Polarization of modified titanium and titanium–zirconium creates nano-structures while hydride formation is modulated. Appl. Surf. Sci. 2013, 282, 7–16. [Google Scholar] [CrossRef]
- Injac, R.; Djordjevic-Milic, V.; Srdjenovic, B. Thermostability testing and degradation profiles of doxycycline in bulk, tablets, and capsules by HPLC. J. Chromatogr. Sci. 2007, 45, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Cunha, B.A.; Sibley, C.M.; Ristuccia, A.M. Doxycycline. Ther. Drug Monit. 1982, 4, 115–135. [Google Scholar] [CrossRef]
- Guerra, W.; Silva, I.R.; Azevedo, E.A.; Monteiro, A.R.S.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Silveira, J.N.; Fontes, A.P.S.; Pereira-Maia, E.C. Three new complexes of platinum (II) with doxycycline, oxytetracycline and chlortetracycline and their antimicrobial activity. J. Braz. Chem. Soc. 2006, 17, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Torres, J.; Bettini, R.; Ruggera, F.; Rueda, C.; López Ponce, M.; Lopez Cabarcos, E. Doxycycline sustained release from brushite cements for the treatment of periodontal diseases. J. Biomed. Mater. Res. A 2008, 85, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Büchter, A.; Meyer, U.; Kruse-Lösler, B.; Joos, U.; Kleinheinz, J. Sustained release of doxycycline for the treatment of peri-implantitis: Randomised controlled trial. Br. J. Oral Maxillofac. Surg. 2004, 42, 439–444. [Google Scholar] [CrossRef]
- Moutsatsos, I.K.; Turgeman, G.; Zhou, S.; Kurkalli, B.G.; Pelled, G.; Tzur, L.; Kelley, P.; Stumm, N.; Mi, S.; Müller, R. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol. Ther. 2001, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Srirangarajan, S.; Agnihotri, S.A.; Patil, S.A.; Ravindra, S.; Setty, S.B.; Aminabhavi, T.M. Development and evaluation of novel biodegradable microspheres based on poly(d,l-lactide-co-glycolide) and poly(ε-caprolactone) for controlled delivery of doxycycline in the treatment of human periodontal pocket: In vitro and in vivo studies. J. Control Release 2007, 119, 59–68. [Google Scholar] [CrossRef]
- Victor, S.P.; Kumar, T.S.S. BCP ceramic microspheres as drug delivery carriers: Synthesis, characterisation and doxycycline release. J. Mater. Sci. Mater. Med. 2008, 19, 283–290. [Google Scholar] [CrossRef]
- Ryder, M.I.; Pons, B.; Adams, D.; Beiswanger, B.; Blanco, V.; Bogle, G.; Donly, K.; Hallmon, W.; Hancock, E.B.; Hanes, P. Effects of smoking on local delivery of controlled-release doxycycline as compared to scaling and root planing. J. Clin. Periodontol. 1999, 26, 683–691. [Google Scholar] [CrossRef]
- Park, J.B. Effects of doxycycline, minocycline, and tetracycline on cell proliferation, differentiation, and protein expression in osteoprecursor cells. J. Craniofac. Surg. 2011, 22, 1839–1842. [Google Scholar] [CrossRef]
- Garrett, I.; Gutierrez, G.; Mundy, G. Statins and bone formation. Curr. Pharm. Des. 2001, 7, 715–736. [Google Scholar] [CrossRef]
- Liao, J.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [Green Version]
- Tandon, V.; Bano, G.; Khajuria, V.; Parihar, A.; Gupta, S. Pleiotropic effects of statins. Indian J. Pharmacol. 2005, 37, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Monjo, M.; Rubert, M.; Wohlfahrt, J.C.; Rønold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. In Vivo Performance Of Absorbable Collagen Sponges With Rosuvastatin In Critical-Size Cortical Bone Defects. Acta BioMater. 2009, 6, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of bone formation in vitro and in rodents by statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Rabie, A. Histologic and ultrastructural study on statin graft in rabbit skulls. Int. J. Oral Maxillofac. Surg. 2005, 63, 1515–1521. [Google Scholar] [CrossRef]
- Staal, A.; Frith, J.; French, M.; Swartz, J.; Güngör, T.; Harrity, T.; Tamasi, J.; ROGERS, M.; FEYEN, J. The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG-CoA reductase activity. J. Bone Miner. Res. 2003, 18, 88–96. [Google Scholar] [CrossRef]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G. Effects of statins on bone mineral density: A meta-analysis of clinical studies. Bone 2007, 40, 1581–1587. [Google Scholar] [CrossRef]
- Kaji, H.; Kanatani, M.; Sugimoto, T.; Chihara, K. Statins modulate the levels of osteoprotegerin/receptor activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm. Metab. Res. 2005, 37, 589–592. [Google Scholar] [CrossRef]
- Maeda, T.; Matsunuma, A.; Kurahashi, I.; Yanagawa, T.; Yoshida, H.; Horiuchi, N. Induction of Osteoblast Differentiation Indices by Statins in MC3T3-E1 Cells. J. Cell Biochem. 2004, 92, 458–471. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Yasukawa, E.; Moriyama, Y.; Ogino, Y.; Wada, H.; Atsuta, I.; Koyano, K. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 336–342. [Google Scholar] [CrossRef]
- Pagkalos, J.; Cha, J.M.; Kang, Y.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J. Bone Miner. Res. 2010, 25, 2470–2478. [Google Scholar] [CrossRef]
- Monjo, M.; Rubert, M.; Ellingsen, J.E.; Lyngstadaas, S.P. Rosuvastatin promotes osteoblast differentiation and regulates SLCO1A1 transporter gene expression in MC3T3-E1 cells. Cell Physiol. Biochem. 2010, 26, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Pardun, K.; Treccani, L.; Volkmann, E.; Li Destri, G.; Marletta, G.; Streckbein, P.; Heiss, C.; Rezwan, K. Characterization of wet powder-sprayed zirconia/calcium phosphate coating for dental implants. Clin. Implant Dent. Relat. Res. 2015, 17, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Bachle, M.; Att, W.; Chaar, S.; Altmann, B.; Renz, A.; Butz, F. Osteoblast and bone tissue response to surface modified zirconia and titanium implant materials. Dent. Mater. 2013, 29, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Sandhyarani, M.; Rameshbabu, N.; Venkateswarlu, K. Fabrication, characterization and in-vitro evaluation of nanostructured zirconia/hydroxyapatite composite film on zirconium. Surf. Coat. Technol. 2014, 238, 58–67. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Domagala, P.; Fragola, G.; Freiberger, P.; Ortiz-Vigon, A.; Rousseau, P.; Tondela, J. A Prospective Noninterventional Study to Evaluate Survival and Success of Reduced Diameter Implants Made From Titanium-Zirconium Alloy. J. Oral Implantol. 2015, 41, e118–e125. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Zarauz, C.; Strasding, M.; Sailer, I.; Zwahlen, M.; Zembic, A. A systematic review of the influence of the implant-abutment connection on the clinical outcomes of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implant. Res. 2018, 29 (Suppl. 18), 160–183. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Worsaae, N.; Schiodt, M.; Gotfredsen, K. A 3-year prospective study of implant-supported, single-tooth restorations of all-ceramic and metal-ceramic materials in patients with tooth agenesis. Clin. Oral Implant. Res. 2013, 24, 1078–1087. [Google Scholar] [CrossRef]
- Lops, D.; Bressan, E.; Chiapasco, M.; Rossi, A.; Romeo, E. Zirconia and titanium implant abutments for single-tooth implant prostheses after 5 years of function in posterior regions. Int. J. Oral Maxillofac. Implant. 2013, 28, 281–287. [Google Scholar] [CrossRef]
- Souza, J.C.; Silva, J.B.; Aladim, A.; Carvalho, O.; Nascimento, R.M.; Silva, F.S.; Martinelli, A.E.; Henriques, B. Effect of Zirconia and Alumina Fillers on the Microstructure and Mechanical Strength of Dental Glass Ionomer Cements. Open Dent. J. 2016, 10, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Payer, M.; Heschl, A.; Koller, M.; Arnetzl, G.; Lorenzoni, M.; Jakse, N. All-ceramic restoration of zirconia two-piece implants--a randomized controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 371–376. [Google Scholar] [CrossRef]
- Kumar, Y.; Jain, V.; Chauhan, S.S.; Bharate, V.; Koli, D.; Kumar, M. Influence of different forms and materials (zirconia or titanium) of abutments in peri-implant soft-tissue healing using matrix metalloproteinase-8: A randomized pilot study. J. Prosthet. Dent. 2017, 118, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zembic, A.; Bosch, A.; Jung, R.E.; Hammerle, C.H.; Sailer, I. Five-year results of a randomized controlled clinical trial comparing zirconia and titanium abutments supporting single-implant crowns in canine and posterior regions. Clin. Oral Implant. Res. 2013, 24, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Cardaropoli, G. Peri-implant hard and soft tissue integration to dental implants made of titanium and gold. Clin. Oral Implant. Res. 2007, 18, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Pontes, A.E.; Degidi, M.; Iezzi, G. Histologic studies on osseointegration: Soft tissues response to implant surfaces and components. A review. Dent. Mater. 2011, 27, 53–60. [Google Scholar] [CrossRef]
- Al-Hashedi, A.A.; Laurenti, M.; Benhamou, V.; Tamimi, F. Decontamination of titanium implants using physical methods. Clin. Oral Implant. Res. 2017, 28, 1013–1021. [Google Scholar] [CrossRef]
- Bösch, A.; Jung, R.E.; Sailer, I.; Goran, B.; Hämmerle, C.H.; Thoma, D.S. Single-tooth replacement using dental implants supporting all-ceramic and metal-based reconstructions: Results at 18 months of loading. Int. J. Periodontics Restor. Dent. 2018, 38, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Kohal, R.J.; Weng, D.; Bachle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef]
- Andersson, B.; Glauser, R.; Maglione, M.; Taylor, A. Ceramic implant abutments for short-span FPDs: A prospective 5-year multicenter study. Int. J. Prosthodont. 2003, 16, 640–646. [Google Scholar]
- Agustin-Panadero, R.; Bustamante-Hernandez, N.; Labaig-Rueda, C.; Fons-Font, A.; Fernandez-Estevan, L.; Sola-Ruiz, M.F. Influence of Biologically Oriented Preparation Technique on Peri-Implant Tissues; Prospective Randomized Clinical Trial with Three-Year Follow-Up. Part II: Soft Tissues. J. Clin. Med. 2019, 8, 2223. [Google Scholar] [CrossRef] [Green Version]
| All Implant Materials | |||
|---|---|---|---|
| Study or Subgroup | Mean (mm) | Std. Dev. (mm) | Total |
| Al-Nawas [129] | −0.20 | 0.26 | 11 |
| Altuna et al., 2016 | −0.32 | 0.72 | 14 |
| Apostu et al., 2017 | −0.08 | 0.16 | 25 |
| Balmer [68] | −0.015 | 3.65 | 20 |
| Study | Zirconia | Titanium | Weight | IV, Random at 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (mm) | SD (mm) | Total | Mean (mm) | SD (mm) | Total | |||
| De Alboroz et al. | 0.3 | 0.2 | 20 | 0.4 | 0.3 | 14 | 11.9% | −0.39 (−0.43, −0.35) |
| Hossein [131] | 0.6 | 0.07 | 11 | 0.61 | 0.12 | 15 | 12.8% | −0.03 (−0.1, −0.31) |
| Lops [132] | 0.04 | 0.01 | 15 | 0.15 | 0.11 | 21 | 11.9% | −0.20 (−0.27, −0.13) |
| Nascimento [133] | 0.1 | 0.56 | 6 | 0.45 | 0.61 | 22 | 11.0% | −0.33 (−0.61, −0.55) |
| Payer et al. [134] | 0.05 | 0.16 | 9 | 0.31 | 0.20 | 13 | 12.8% | −1.03 (−1.44, −0.62) |
| Kumar et al. [135] | 0.23 | 0.07 | 15 | 0.55 | 0.18 | 24 | 12.4% | −0.13 (−1.44, −0.62) |
| Zembic et al. [136] | 0.15 | 0.25 | 16 | 0.23 | 0.32 | 11 | 10.6% | −0.06 (−0.71, −0.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haugen, H.J.; Chen, H. Is There a Better Biomaterial for Dental Implants than Titanium?—A Review and Meta-Study Analysis. J. Funct. Biomater. 2022, 13, 46. https://doi.org/10.3390/jfb13020046
Haugen HJ, Chen H. Is There a Better Biomaterial for Dental Implants than Titanium?—A Review and Meta-Study Analysis. Journal of Functional Biomaterials. 2022; 13(2):46. https://doi.org/10.3390/jfb13020046
Chicago/Turabian StyleHaugen, Håvard J., and Hongyu Chen. 2022. "Is There a Better Biomaterial for Dental Implants than Titanium?—A Review and Meta-Study Analysis" Journal of Functional Biomaterials 13, no. 2: 46. https://doi.org/10.3390/jfb13020046
APA StyleHaugen, H. J., & Chen, H. (2022). Is There a Better Biomaterial for Dental Implants than Titanium?—A Review and Meta-Study Analysis. Journal of Functional Biomaterials, 13(2), 46. https://doi.org/10.3390/jfb13020046







