Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Dentin Discs
2.2.2. Synthesis of SrCO3, Sr0.5Ca0.5CO3, and CaCO3 Nanoparticles
2.2.3. Characterization of Nanoparticles
2.2.4. Preparation of Gels Containing Nanoparticles for Application on Dentin Discs
2.2.5. Application of Gel Containing Nanoparticles to Dentin Surface by Brushing
2.2.6. Resistance of Coating to Acid Attack
2.2.7. Chemical Characterization of Dentin Discs
2.2.8. Obliteration of Dentin Tubules
2.2.9. Cell Culture Conditions
2.2.10. Cell Viability Measurement
2.2.11. Tissue Non-Specific Alkaline Phosphatase (TNAP) Activity
2.2.12. Mineralized Nodule Formation
2.2.13. Messenger RNA (mRNA) Expression by Real-Time Polymerase Chain Reaction (RT-qPCR)
3. Results
3.1. Characterization of SrCO3, Sr0.5Ca0.5CO3, and CaCO3 Nanoparticles
3.1.1. Investigation of the Crystalline Structure of Nanoparticles by X-Ray Diffraction
3.1.2. Nanoparticle Size and Charge
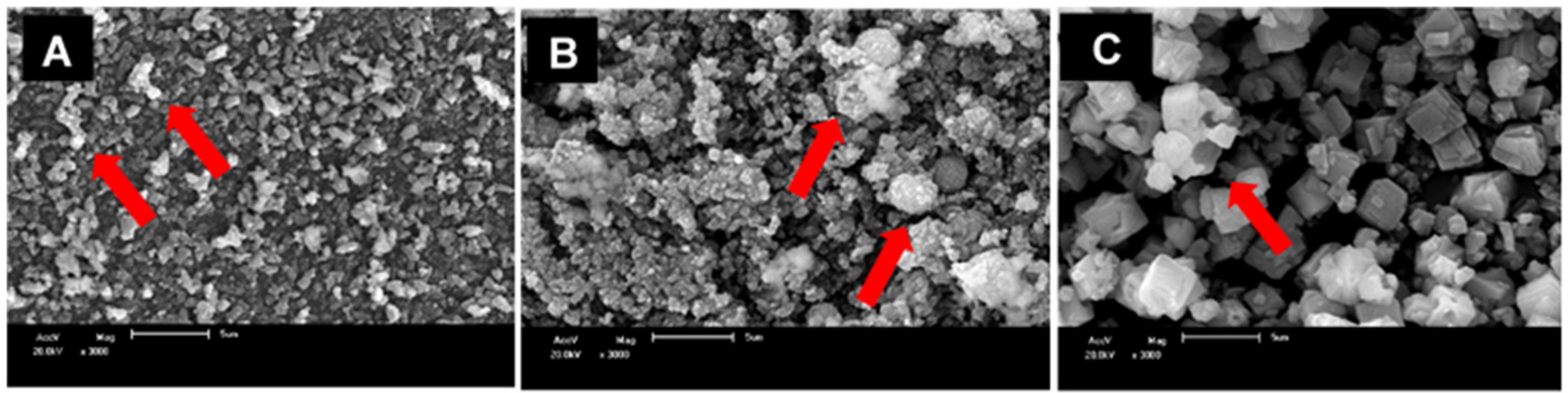
3.1.3. Nanoparticle Morphology
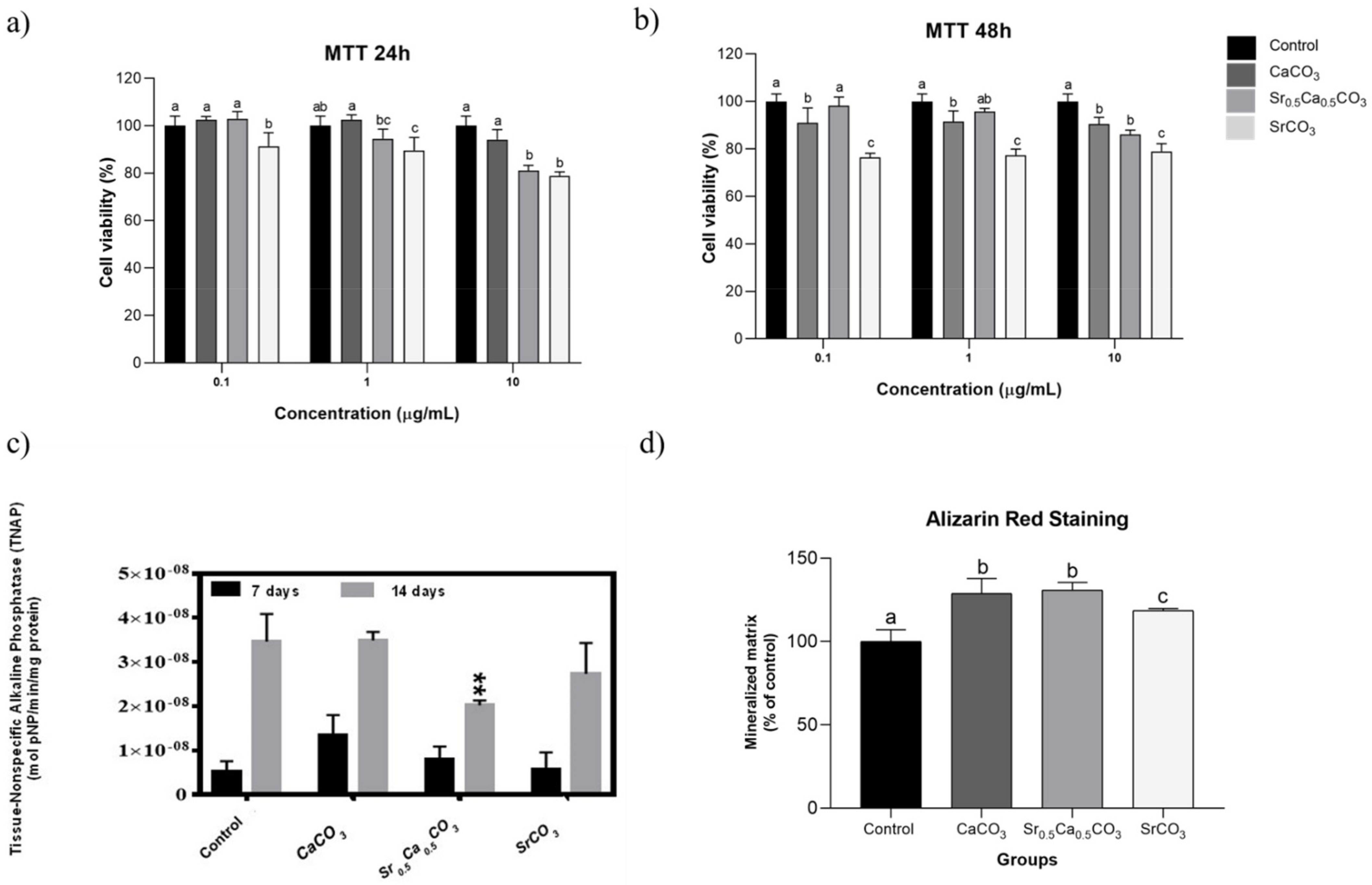
3.1.4. Effects of the Nanoparticles on the Viability of hDPSCs
3.1.5. TNAP Activity and Mineralized Nodules Formation
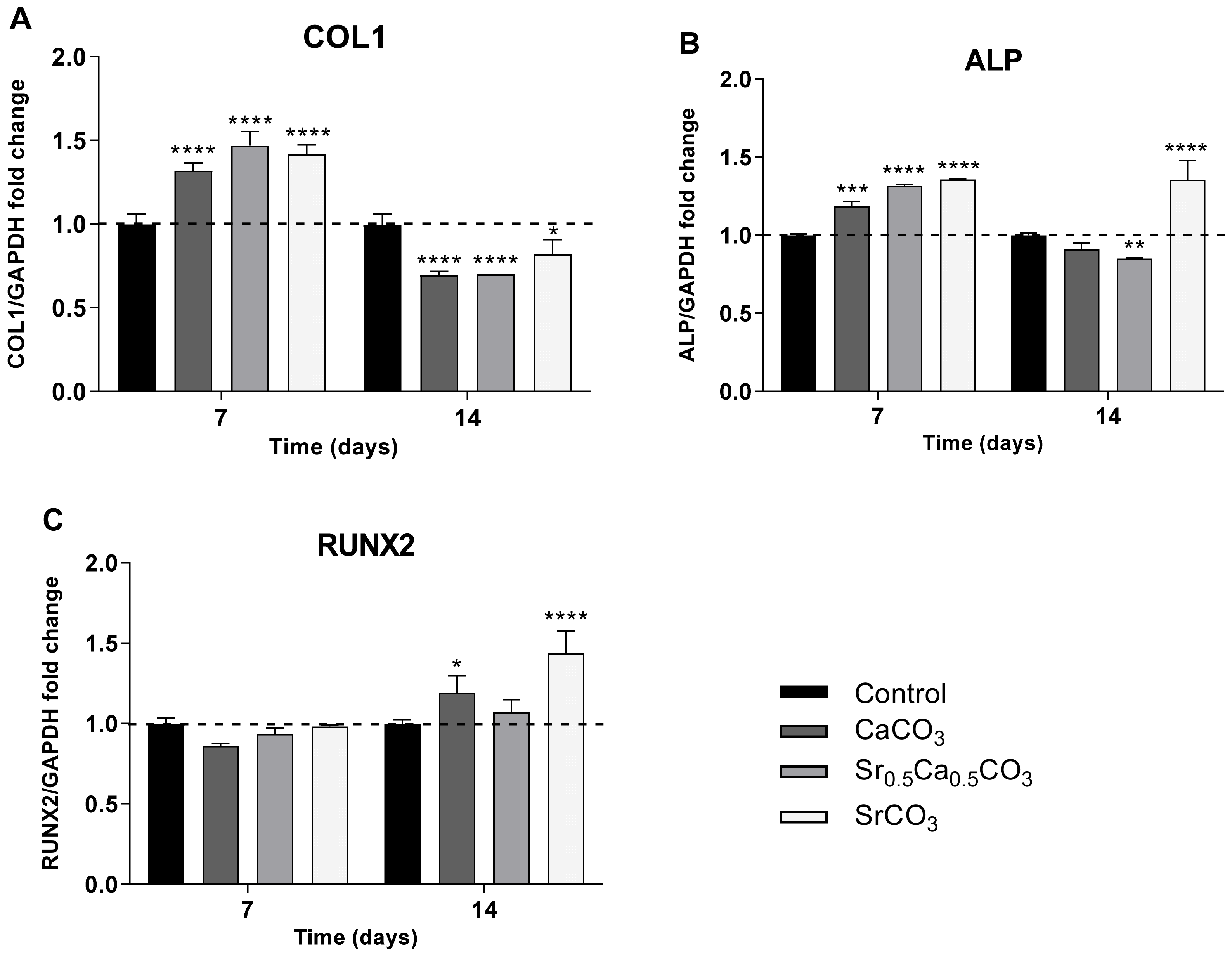
3.1.6. Expression of RUNX2, COL1, and ALP by hDPSCs
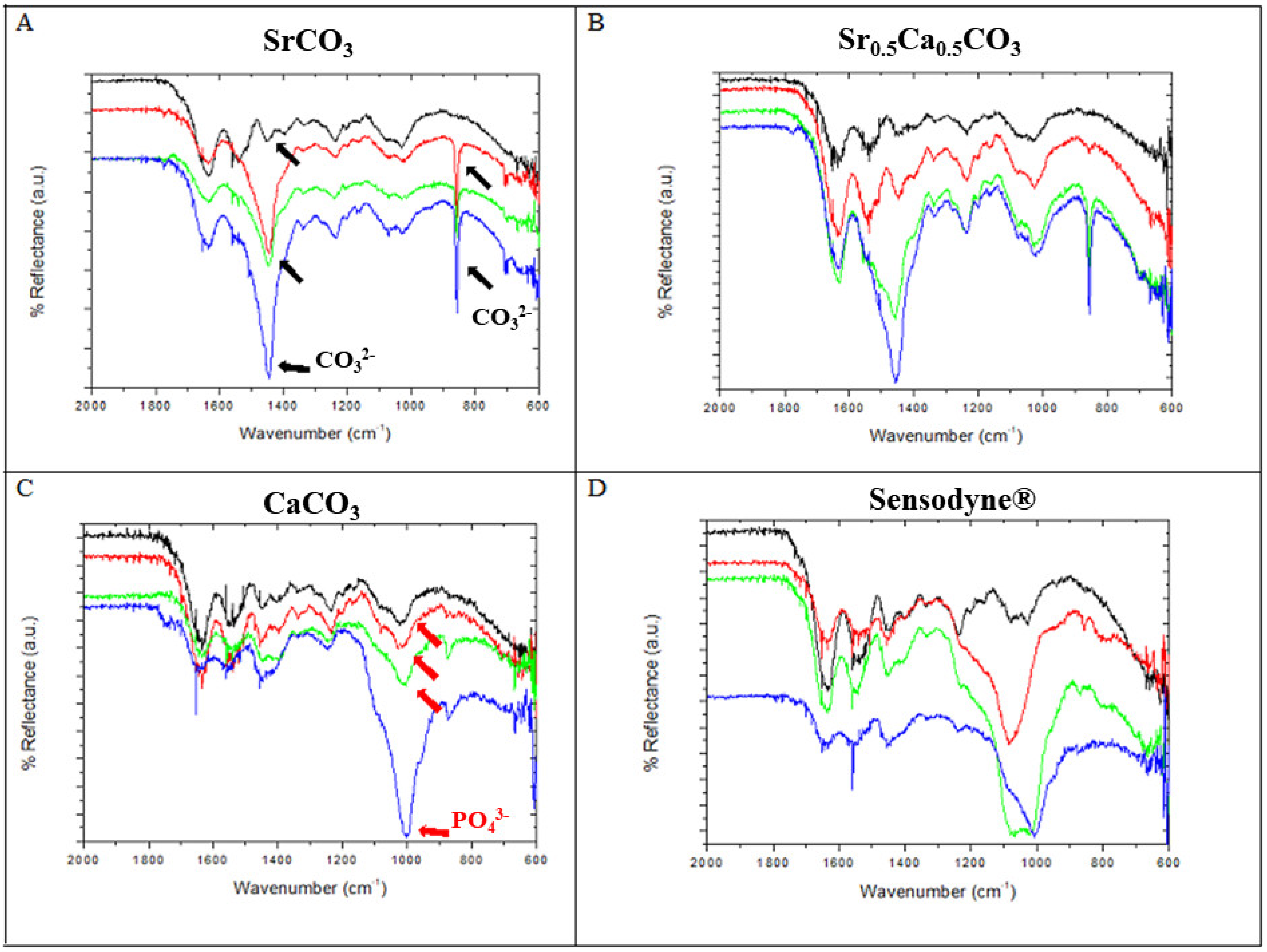
3.2. Chemical Analysis of Dentin Surface before and after Brushing with a Desensitizing Agent
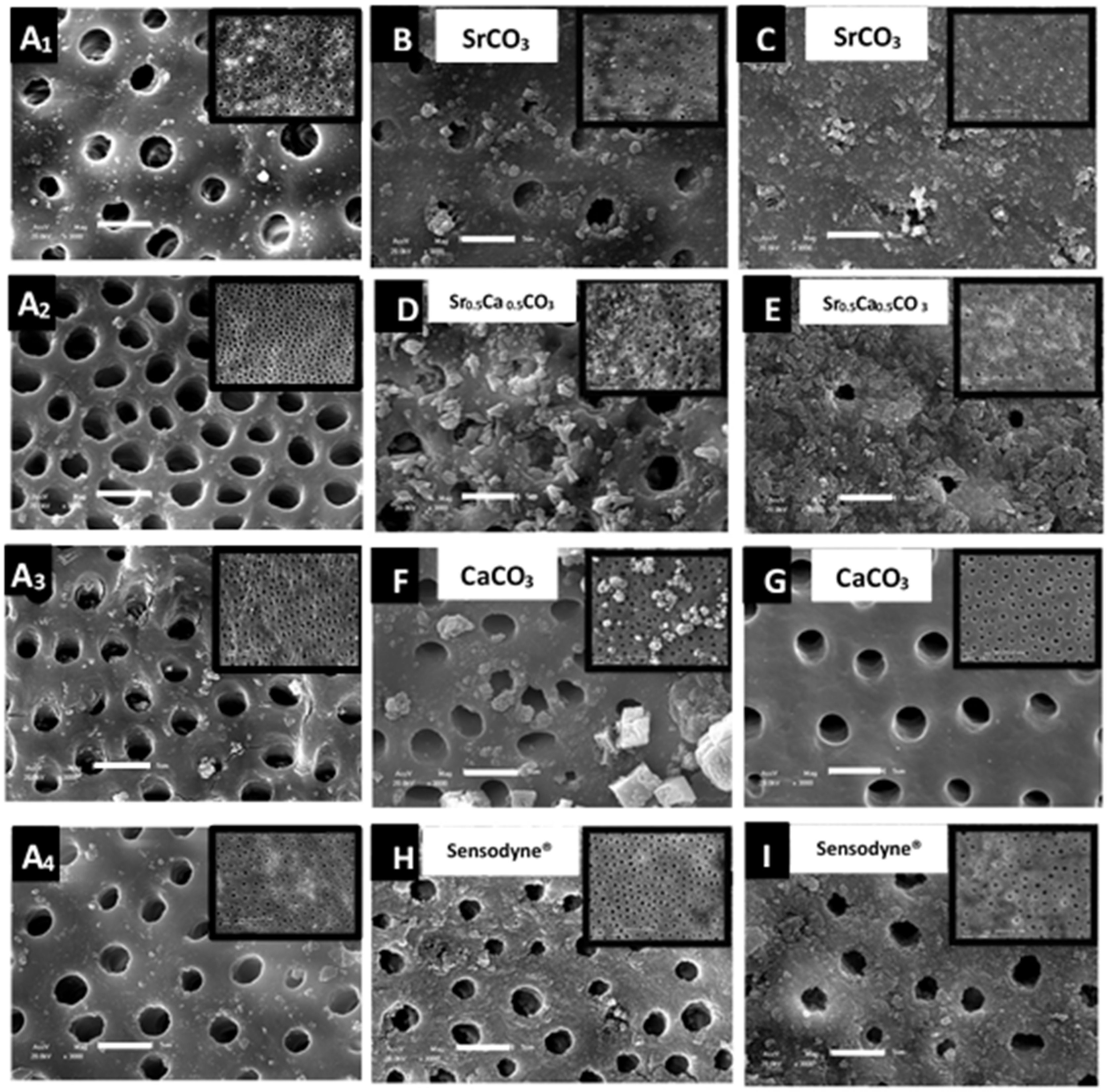
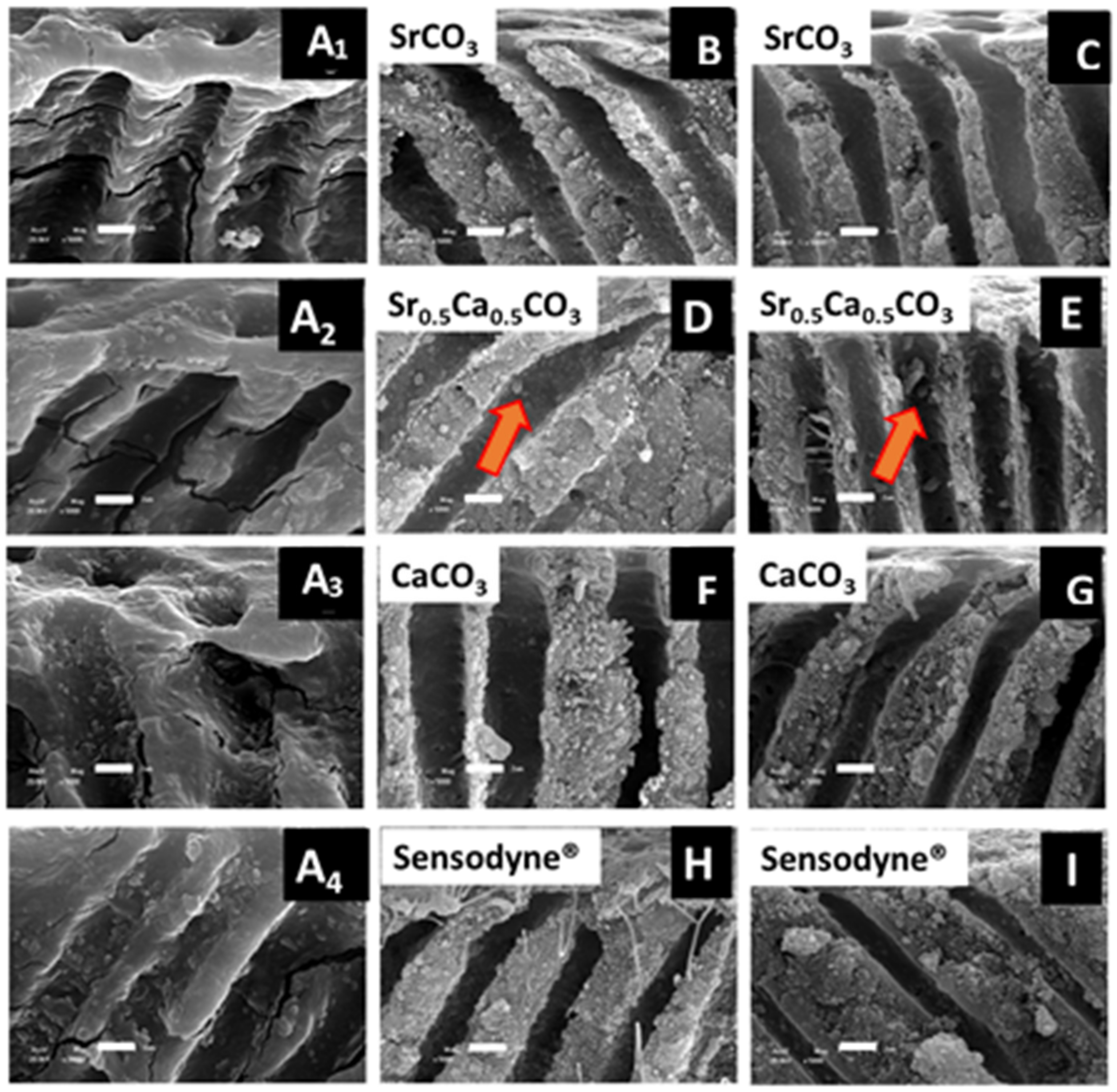
3.3. Morphological Characterization and Quantitative Analysis of Dentin Tubule Obliteration after Brushing with a Desensitizing Agent
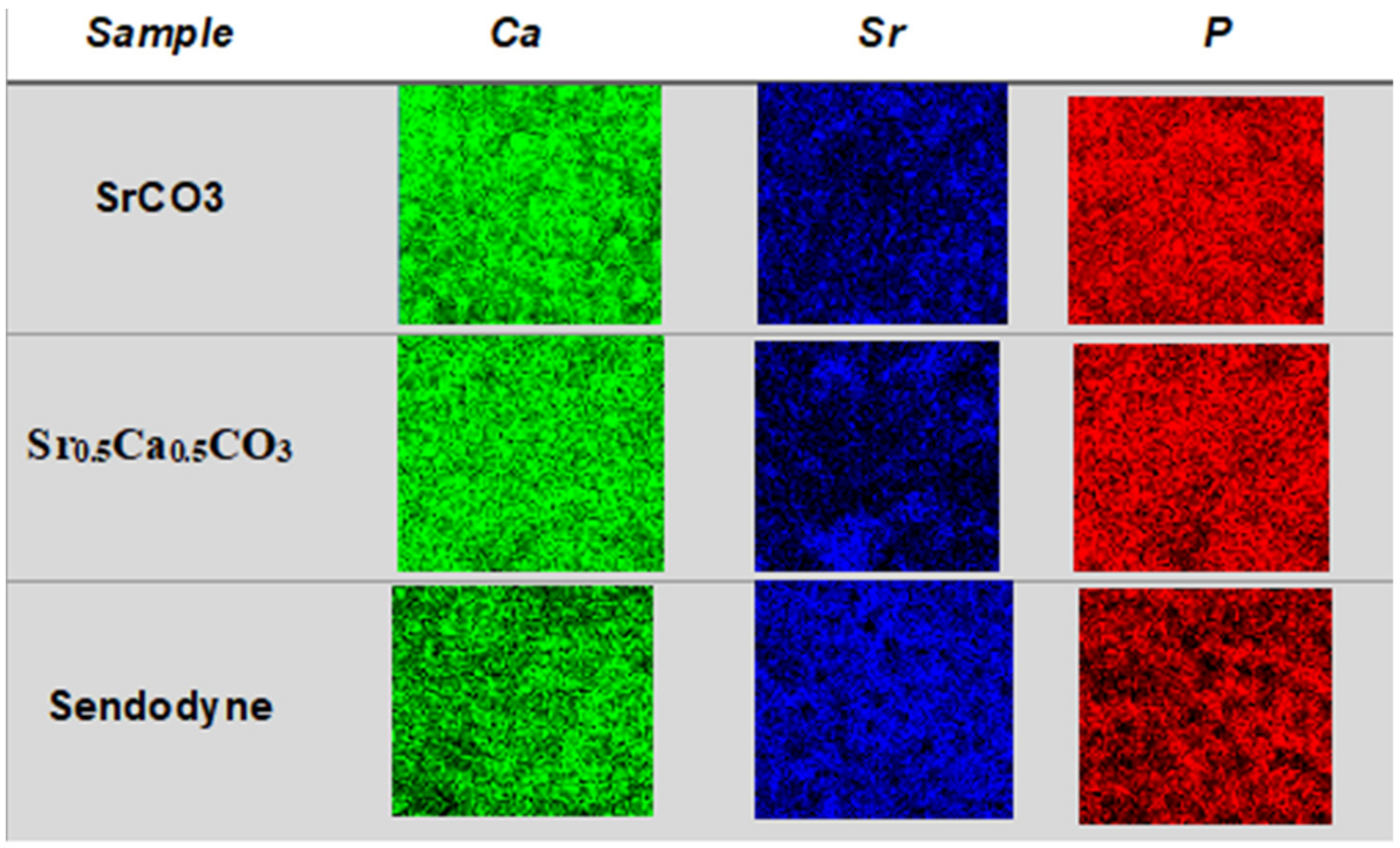
3.4. Quantification and Mapping of Ca, Sr, and P in Dentin Discs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarasena, N.; Spencer, J.; Ou, Y.; Brennan, D. Dentine hypersensitivity—Australian dentists’ perspective. Aust. Dent. J. 2010, 55, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Marshall, G.W.; Gansky, S.A.; Parkinson, C.R.; Marshall, S.J. Strontium effects on root dentin tubule occlusion and nanomechanical properties. Dent. Mater. 2016, 32, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.L.; Parolia, A.; Pau, A.; Porto, I.C.C.D.M. Comparative evaluation of the effectiveness of desensitizing agents in dentine tubule occlusion using scanning electron microscopy. Aust. Dent. J. 2015, 60, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Mason, S.; Cooke, J. Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes—A 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste—for the longer-term relief of dentine hypersensitivity. J. Dent. 2017, 60, 36–43. [Google Scholar] [CrossRef]
- Alencar, C.D.M.; de Paula, B.L.F.; Ortiz, M.I.G.; Magno, M.B.; Silva, C.M.; Maia, L.C. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: A systematic review and meta-analysis. J. Dent. 2019, 82, 11–21. [Google Scholar] [CrossRef]
- Hu, M.-L.; Zheng, G.; Zhang, Y.-D.; Yan, X.; Li, X.-C.; Lin, H. Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J. Dent. 2018, 75, 12–21. [Google Scholar] [CrossRef]
- Zeola, L.F.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- Lynch, E.; Brauer, D.S.; Karpukhina, N.; Gillam, D.G.; Hill, R.G. Multi-component bioactive glasses of varying fluoride content for treating dentin hypersensitivity. Dent. Mater. 2012, 28, 168–178. [Google Scholar] [CrossRef]
- Hughes, N.; Mason, S.; Jeffery, P.; Welton, H.; Tobin, M.; O’Shea, C.; Browne, M. A comparative clinical study investigating the efficacy of a test dentifrice containing 8% strontium acetate and 1040 ppm sodium fluoride versus a marketed control dentifrice containing 8% arginine, calcium carbonate, and 1450 ppm sodium monofluorophospha. J. Clin. Dent. 2010, 21, 49–55. [Google Scholar]
- Pinto, S.C.S.; Silveira, C.M.M.; Pochapski, M.T.; Pilatt, G.L.; Santos, F.A. Effect of desensitizing toothpastes on dentin. Braz. Oral Res. 2012, 26, 410–417. [Google Scholar] [CrossRef] [Green Version]
- Bossù, M.; Saccucci, M.; Salucci, A.; DI Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnology 2019, 17, 17. [Google Scholar] [CrossRef]
- Palazon, M.T.; Scaramucci, T.; Aranha, A.C.C.; Prates, R.A.; Lachowski, K.M.; Hanashiro, F.S.; Youssef, M.N. Immediate and Short-Term Effects of In-Office Desensitizing Treatments for Dentinal Tubule Occlusion. Photomed. Laser Surg. 2013, 31, 274–282. [Google Scholar] [CrossRef]
- Mantzourani, M.; Sharma, D. Dentine sensitivity: Past, present and future. J. Dent. 2013, 41, S3–S17. [Google Scholar] [CrossRef]
- Davies, M.; Paice, E.M.; Jones, S.B.; Leary, S.; Curtis, A.R.; West, N.X. Efficacy of desensitizing dentifrices to occlude dentinal tubules. Eur. J. Oral Sci. 2011, 119, 497–503. [Google Scholar] [CrossRef]
- Mockdeci, H.; Polonini, H.; Martins, I.; Granato, A.-P.; Raposo, N.; Chaves, M.D.G.A.M. Evaluation of ex vivo effectiveness of commercial desensitizing dentifrices. J. Clin. Exp. Dent. 2017, 9, e503–e510. [Google Scholar] [CrossRef] [Green Version]
- Olley, R.C.; Pilecki, P.; Hughes, N.; Jeffery, P.; Austin, R.S.; Moazzez, R.; Bartlett, D. An in situ study investigating dentine tubule occlusion of dentifrices following acid challenge. J. Dent. 2012, 40, 585–593. [Google Scholar] [CrossRef]
- Rajguru, S.A.; Padhye, A.M.; Gupta, H.S. Effects of two desensitizing dentifrices on dentinal tubule occlusion with citric acid challenge: Confocal laser scanning microscopy study. Indian J. Dent. Res. 2017, 28, 450. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Batitucci, R.G.; Pinheiro, M.C.; Zandim, D.L.; Spin-Neto, R.; Sampaio, J.E.C. Effect of an Acid Diet Allied to Sonic Toothbrushing on Root Dentin Permeability: An In Vitro Study. Braz. Dent. J. 2010, 21, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sa, Y.; Sauro, S.; Chen, H.; Xing, W.; Ma, X.; Jiang, T.; Wang, Y. Effect of desensitising toothpastes on dentinal tubule occlusion: A dentine permeability measurement and SEM in vitro study. J. Dent. 2010, 38, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Silvia, F.; Pashley, D.H.; Gasparotto, G.; Carlo, P. Calcium silicate coating derived from Portland cement as treatment for hypersensitive dentine. J. Dent. 2008, 36, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Mannocci, F.; Tay, F.R.; Pashley, D.H.; Cook, R.; Carpenter, G.H.; Watson, T.F. Deproteinization Effects of NaOCl on Acid-etched Dentin in Clinically-relevant vs Prolonged Periods of Application. A Confocal and Environmental Scanning Electron Microscopy Study. Oper. Dent. 2009, 34, 166–173. [Google Scholar] [CrossRef]
- Parkinson, C.; Willson, R. A comparative in vitro study investigating the occlusion and mineralization properties of commercial dentifrices in a four-day dentin model. J. Clin. Dent. 2011, 22, 68–73. [Google Scholar]
- Al-Khafaji, T.J.; Wong, F.; Fleming, P.S.; Karpukhina, N.; Hill, R. Novel fluoride and strontium-containing bioactive glasses for dental varnishes-design and bioactivity in Tris buffer solution. J. Non-Cryst. Solids 2019, 503–504, 120–130. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.-M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018, 75, 463–471. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. A comparative study on the in vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C 2018, 91, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Tsuru, K.; Nagai, H.; Fujisawa, K.; Kudoh, T.; Ohe, G.; Ishikawa, K.; Miyamoto, Y. Fabrication and evaluation of carbonate apatite-coated calcium carbonate bone substitutes for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Gjorgievska, E.S.; Nicholson, J.W.; Slipper, I.J.; Stevanovic, M.M. Remineralization of Demineralized Enamel by Toothpastes: A Scanning Electron Microscopy, Energy Dispersive X-Ray Analysis, and Three-Dimensional Stereo-Micrographic Study. Microsc. Microanal. 2013, 19, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, K. The original desensitizers: Strontium and potassium salts. J. Clin. Dent. 2009, 20, 145–151. [Google Scholar]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-Day Randomized Clinical Trial to Investigate the Desensitizing Properties of Three Dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef]
- West, N.; Newcombe, R.G.; Hughes, N.; Mason, S.; Maggio, B.; Sufi, F.; Claydon, N. A 3-day randomised clinical study investigating the efficacy of two toothpastes, designed to occlude dentine tubules, for the treatment of dentine hypersensitivity. J. Dent. 2013, 41, 187–194. [Google Scholar] [CrossRef]
- Medvecky, L.; Stulajterova, R.; Giretova, M.; Mincik, J.; Vojtko, M.; Balko, J.; Briancin, J. Effect of tetracalcium phosphate/monetite toothpaste on dentin remineralization and tubule occlusion in vitro. Dent. Mater. 2018, 34, 442–451. [Google Scholar] [CrossRef]
- Earl, J.S.; Ward, M.; Langford, R. Investigation of dentinal tubule occlusion using FIB-SEM milling and EDX. J. Clin. Dent. 2010, 21, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Jiang, T.; Liang, Y.-D.; Zhang, Z.; Wang, Y.-N. Evaluation of the osseointegration of dental implants coated with calcium carbonate: An animal study. Int. J. Oral Sci. 2017, 9, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, M.; Abdel-Fattah, A.; Soliman, Y. Radiation-induced defects in strontium carbonate rod for EPR dosimetry applications. Radiat. Phys. Chem. 2017, 131, 1–6. [Google Scholar] [CrossRef]
- Schofield, E.J.; Sarangi, R.; Mehta, A.; Jones, A.M.; Smith, A.; Mosselmans, J.F.W.; Chadwick, A.V. Strontium carbonate nanoparticles for the surface treatment of problematic sulfur and iron in waterlogged archaeological wood. J. Cult. Herit. 2015, 18, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, F.; Wang, P.; Ming, J.; Zuo, B. Preparation and characterization of silk fibroin/strontium carbonate film through rapid formation. Mater. Lett. 2017, 189, 46–49. [Google Scholar] [CrossRef]
- Seong, J.; Newcombe, R.G.; Foskett, H.L.; Davies, M.; West, N.X. A randomised controlled trial to compare the efficacy of an aluminium lactate/potassium nitrate/hydroxylapatite toothpaste with a control toothpaste for the prevention of dentine hypersensitivity. J. Dent. 2021, 108, 103619. [Google Scholar] [CrossRef]
- Huang, M.; Hill, R.G.; Rawlinson, S.C.F. Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater. 2016, 38, 201–211. [Google Scholar] [CrossRef]
- Martin-Del-Campo, M.; Rosales-Ibañez, R.; Alvarado, K.; Sampedro, J.G.; Garcia-Sepulveda, C.A.; Deb, S.; Román, J.S.; Rojo, L. Strontium folate loaded biohybrid scaffolds seeded with dental pulp stem cells induce in vivo bone regeneration in critical sized defects. Biomater. Sci. 2016, 4, 1596–1604. [Google Scholar] [CrossRef]
- Di Tinco, R.; Sergi, R.; Bertani, G.; Pisciotta, A.; Bellucci, D.; Carnevale, G.; Cannillo, V.; Bertoni, L. Effects of a Novel Bioactive Glass Composition on Biological Properties of Human Dental Pulp Stem Cells. Materials 2020, 13, 4049. [Google Scholar] [CrossRef]
- Gillam, D.G.; Tang, J.Y.; Mordan, N.J.; Newman, H.N. The effects of a novel Bioglass® dentifrice on dentine sensitivity: A scanning electron microscopy investigation. J. Oral Rehabil. 2002, 29, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Higham, S.M.; Edgar, W.M. Factors influencing the development of dental erosion in vitro: Enamel type, temperature and exposure time. J. Oral Rehabil. 1999, 26, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Osorio, E.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano, M.; Osorio, R. Improved reactive nanoparticles to treat dentin hypersensitivity. Acta Biomater. 2018, 72, 371–380. [Google Scholar] [CrossRef]
- Leguizamón, N.D.P.; Rodrigues, E.M.; de Campos, M.L.; Nogueira, A.V.B.; Viola, K.S.; Schneider, V.K.; Neo-Justino, D.M.; Tanomaru-Filho, M.; Zambuzzi, W.F.; Henrique-Silva, F.; et al. In vivo and in vitro anti-inflammatory and pro-osteogenic effects of citrus cystatin CsinCPI-2. Cytokine 2019, 123, 154760. [Google Scholar] [CrossRef]
- Nagavi-Alhoseiny, A.A.; Torshabi, M.; Rasoulianboroujeni, M.; Tayebi, L.; Tabatabaei, F.S. Effect of sodium chloride on gene expression of Streptococcus mutans and zeta potential of demineralized dentin. J. Oral Biol. Craniofacial Res. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Millán, J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Res. 2013, 93, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millán, J.L. Alkaline Phosphatases: Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006, 2, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.A.E.; Tovani, C.B.; Favarin, B.Z.; Soares, M.P.R.; Fukada, S.Y.; Ciancaglini, P.; Ramos, A.P. Synthesis of Sr–morin complex and its in vitro response: Decrease in osteoclast differentiation while sustaining osteoblast mineralization ability. J. Mater. Chem. B 2019, 7, 823–829. [Google Scholar] [CrossRef]
- Cummins, D. Dentin hypersensitivity: From diagnosis to a breakthrough therapy for everyday sensitivity relief. J. Clin. Dent. 2009, 20, 1–9. [Google Scholar] [PubMed]
- Cummins, D. Recent advances in dentin hypersensitivity: Clinically proven treatments for instant and lasting sensitivity relief. Am. J. Dent. 2010, 23, 3–13. [Google Scholar]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Tovani, C.B.; Gloter, A.; Azaïs, T.; Selmane, M.; Ramos, A.P.; Nassif, N. Formation of stable strontium-rich amorphous calcium phosphate: Possible effects on bone mineral. Acta Biomater. 2019, 92, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Zanatta, M.; da Veiga, M.; Ciancaglini, P.; Ramos, A. Lipid-mediated growth of SrCO3/CaCO3 hybrid films as bioactive coatings for Ti surfaces. Mater. Sci. Eng. C 2019, 99, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Tovani, C.B.; Oliveira, T.M.; Soares, M.P.R.; Nassif, N.; Fukada, S.Y.; Ciancaglini, P.; Gloter, A.; Ramos, A.P. Strontium Calcium Phosphate Nanotubes as Bioinspired Building Blocks for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 43422–43434. [Google Scholar] [CrossRef]
- Planchon, M.; Ferrari, R.; Guyot, F.; Gélabert, A.; Menguy, N.; Chanéac, C.; Thill, A.; Benedetti, M.F.; Spalla, O. Interaction between Escherichia coli and TiO2 nanoparticles in natural and artificial waters. Colloids Surf. B Biointerfaces 2013, 102, 158–164. [Google Scholar] [CrossRef]
- Weerkamp, A.H.; Uyen, H.M.; Busscher, H.J. Effect of Zeta Potential and Surface Energy on Bacterial Adhesion to Uncoated and Saliva-coated Human Enamel and Dentin. J. Dent. Res. 1988, 67, 1483–1487. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Cruz, M.A.E.; Trovani, C.B.; Lopes, H.B.; Beloti, M.M.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Curcumin-loaded carrageenan nanoparticles: Fabrication, characterization, and assessment of the effects on osteoblasts mineralization. Colloids Surf. B Biointerfaces 2022, 217, 112622. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Balani, D.H.; Kronenberg, H.M. Stem and progenitor cells in skeletal development. Curr. Top. Dev. Biol. 2019, 133, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Meka, S.R.K.; Jain, S.; Chatterjee, K. Strontium eluting nanofibers augment stem cell osteogenesis for bone tissue regeneration. Colloids Surf. B: Biointerfaces 2016, 146, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ilmer, M.; Karow, M.; Geissler, C.; Jochum, M.; Neth, P. Human Osteoblast–Derived Factors Induce Early Osteogenic Markers in Human Mesenchymal Stem Cells. Tissue Eng. Part A 2009, 15, 2397–2409. [Google Scholar] [CrossRef]
- De Souza, I.D.; Cruz, M.A.; de Faria, A.N.; Zancanela, D.C.; Simão, A.M.; Ciancaglini, P.; Ramos, A.P. Formation of carbonated hydroxyapatite films on metallic surfaces using dihexadecyl phosphate–LB film as template. Colloids Surf. B: Biointerfaces 2014, 118, 31–40. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, M.; Dominguez-Vidal, A.; Ayora-Cañada, M.; Molina-Díaz, A. Assessment of dentifrice adulteration with diethylene glycol by means of ATR-FTIR spectroscopy and chemometrics. Anal. Chim. Acta 2008, 620, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.B.; Kramer, K.; Chu, E.Y.; Thumbigere-Math, V.; Foster, B.L. Insights into dental mineralization from three heritable mineralization disorders. J. Struct. Biol. 2020, 212, 107597. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.C.; Cruz, M.A.; Faria, A.N.; Zancanela, D.C.; Ciancaglini, P.; Ramos, A.P. Biomimetic collagen/phospholipid coatings improve formation of hydroxyapatite nanoparticles on titanium. Mater. Sci. Eng. C 2017, 77, 102–110. [Google Scholar] [CrossRef]
- Tovani, C.B.; Oliveira, T.M.; Gloter, A.; Ramos, A.P. Sr2+-Substituted CaCO3 Nanorods: Impact on the Structure and Bioactivity. Cryst. Growth Des. 2018, 18, 2932–2940. [Google Scholar] [CrossRef]
- Frangopol, P.T.; Mocanu, A.; Almasan, V.; Garbo, C.; Balint, R.; Borodi, G.; Bratu, I.; Horovitz, O.; Tomoaia-Cotisel, M. Synthesis and structural characterization of strontium substituted hydrozyapatites. Rev. Roum. Chim. 2016, 61, 337–344. [Google Scholar]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Bertazzo, S.; Zambuzzi, W.F.; Campos, D.D.P.; Ogeda, T.L.; Ferreira, C.V.; Bertran, C.A. Hydroxyapatite surface solubility and effect on cell adhesion. Colloids Surf. B Biointerfaces 2010, 78, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Banijamali, S.; Baino, F.; Kargozar, S.; Hill, R.G. Calcium carbonate: Adored and ignored in bioactivity assessment. Acta Biomater. 2019, 91, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Chang, H.-H.; Chiang, Y.-C.; Lin, C.-H.; Lin, C.-P. Strontium ion can significantly decrease enamel demineralization and prevent the enamel surface hardness loss in acidic environment. J. Formos. Med. Assoc. 2018, 118, 39–49. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.; Darvell, B.W.; Lu, W.W.; Chang, J.; Deng, L.; Wang, D.; et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef]
- Wingender, B.; Azuma, M.; Krywka, C.; Zaslansky, P.; Boyle, J.; Deymier, A. Carbonate substitution significantly affects the structure and mechanics of carbonated apatites. Acta Biomater. 2021, 122, 377–386. [Google Scholar] [CrossRef]


| Sample | Diameter (nm) | PDI | ζ (mV) |
|---|---|---|---|
| SrCO3 | 387.0 (166) | 0.56 | −2.1 ± 3.2 |
| Sr0.5Ca0.5CO3 | 66.7 (16.7) | 0.58 | −9.1 ± 4.5 |
| CaCO3 | 590.8 (156.5) | 0.90 | −2.5 ± 4.8 |
| Sample | AA | n (%) |
|---|---|---|
| Control | − | 0% ± 0.0 |
| SrCO3nanoparticles | − | 67.7% ± 0.01 |
| + | 65.7% ± 0.08 | |
| Sr0.5Ca0.5CO3 nanoparticles | − | 93.3% ± 0.09 |
| + | 100% ± 0.0 | |
| CaCO3nanoparticles | − | 24.7% ± 0.10 |
| + | 0% ± 0.0 | |
| Sendodyne® | − | 19.5% ± 0.08 |
| + | 31.9% ± 0.15 |
| Sample | AA | Ca | Sr | P | (Ca + Sr)/P |
|---|---|---|---|---|---|
| Control | − | 63.47 | 1.00 | 35.52 | 1.81 ± 0.17 |
| SrCO3nanoparticles | − | 59.20 | 6.00 | 34.90 | 1.87 ± 0.17 |
| + | 24.90 | 26.92 | 48.18 | 1.07 ± 0.17 | |
| Sr0.5Ca0.5CO3 nanoparticles | − | 59.67 | 4.54 | 35.78 | 1.79 ± 0.17 |
| + | 43.07 | 47.03 | 9.90 | 9.10 ± 0.17 | |
| CaCO3nanoparticles | − | 59.50 | 0.90 | 39.61 | 1.52 ± 0.17 |
| + | 53.30 | 4.36 | 42.33 | 1.36 ± 0.17 | |
| Sendodyne® | − | 40.58 | 16.67 | 42.75 | 1.34 ± 0.17 |
| + | 50.49 | 5.56 | 43.95 | 1.27 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotta, T.C.; Hayann, L.; de Padua Andrade Almeida, L.; Nogueira, L.F.B.; Arnez, M.M.; Castelo, R.; Cassiano, A.F.B.; Faria, G.; Martelli-Tosi, M.; Bottini, M.; et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. J. Funct. Biomater. 2022, 13, 250. https://doi.org/10.3390/jfb13040250
Dotta TC, Hayann L, de Padua Andrade Almeida L, Nogueira LFB, Arnez MM, Castelo R, Cassiano AFB, Faria G, Martelli-Tosi M, Bottini M, et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. Journal of Functional Biomaterials. 2022; 13(4):250. https://doi.org/10.3390/jfb13040250
Chicago/Turabian StyleDotta, Tatiane Cristina, Larwsk Hayann, Leonardo de Padua Andrade Almeida, Lucas Fabrício B. Nogueira, Mayara M. Arnez, Raisa Castelo, Ana Flávia B. Cassiano, Gisele Faria, Milena Martelli-Tosi, Massimo Bottini, and et al. 2022. "Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization" Journal of Functional Biomaterials 13, no. 4: 250. https://doi.org/10.3390/jfb13040250
APA StyleDotta, T. C., Hayann, L., de Padua Andrade Almeida, L., Nogueira, L. F. B., Arnez, M. M., Castelo, R., Cassiano, A. F. B., Faria, G., Martelli-Tosi, M., Bottini, M., Ciancaglini, P., Catirse, A. B. C. E. B., & Ramos, A. P. (2022). Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. Journal of Functional Biomaterials, 13(4), 250. https://doi.org/10.3390/jfb13040250







