Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. Plasma Treatments
2.3. Surgical Procedures
2.4. Implant Stability Quotient (ISQ) Measurement
2.5. Micro-CT Analysis
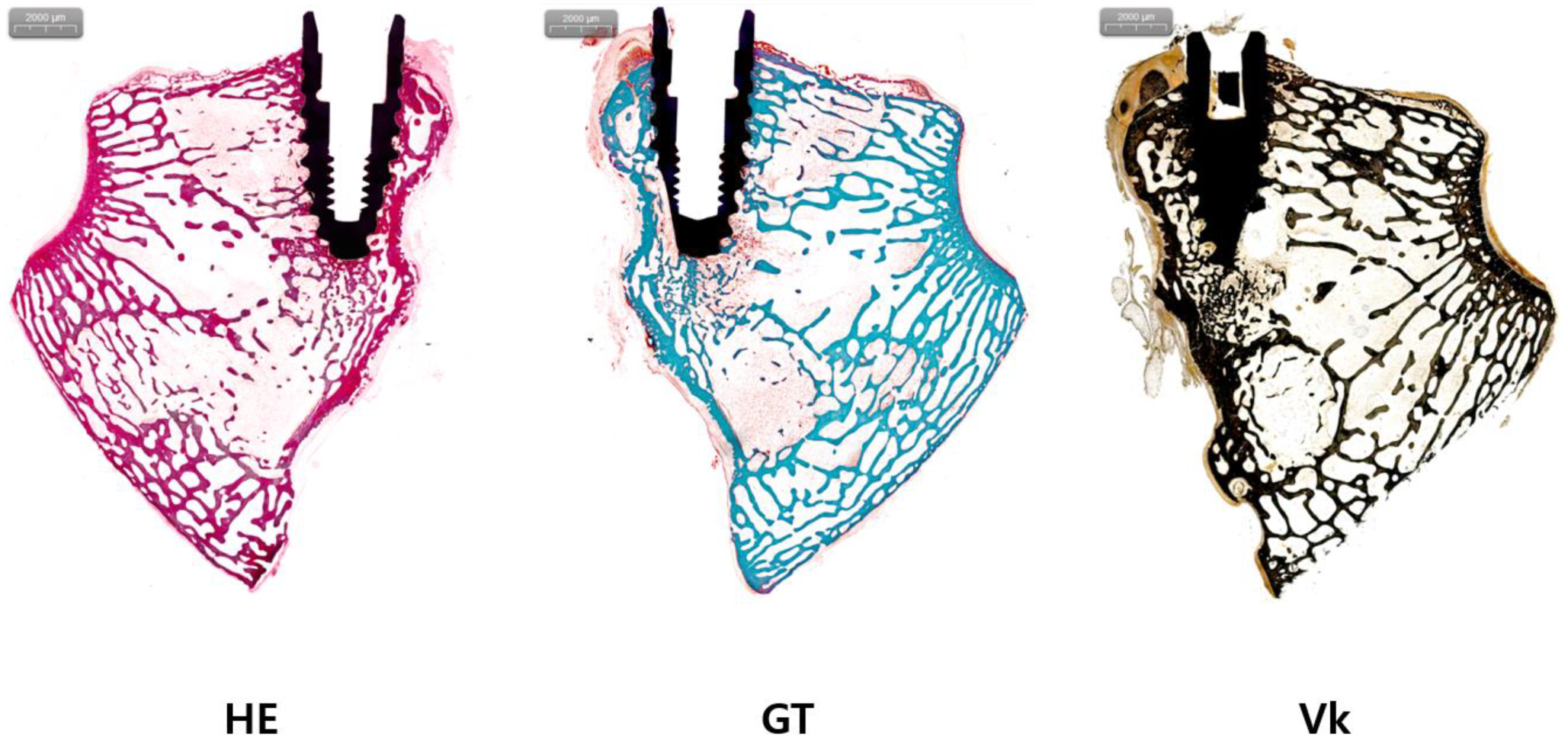
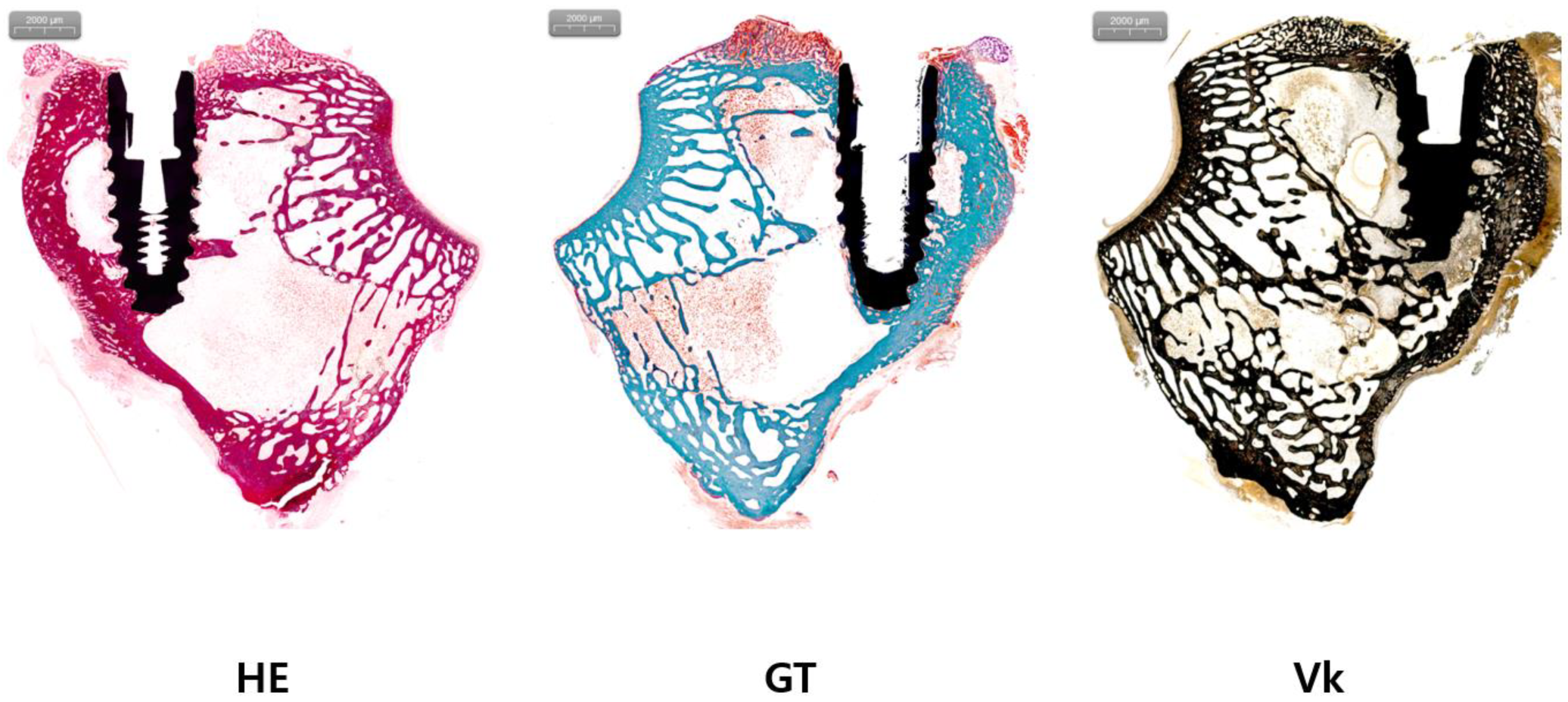
2.6. Histologic Analysis
2.7. Statistical Analysis
3. Results
3.1. Results of Micro-CT Analysis
3.2. Results of Histologic Analysis
3.3. Results of ISQ Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, P.J. Oral implant restoration for enhanced oral function. Clin. Exp. Pharmacol. Physiol. 2005, 32, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Meyer, S.; Mombelli, A.; Müller, F. Dental implants in the elderly population: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2016, 28, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surgery Suppl. 1977, 16, 1–132. [Google Scholar]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29, 106–134. [Google Scholar] [CrossRef]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 88B, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors Influencing Marginal Bone Loss around Dental Implants: A Narrative Review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jeong, W.-S.; Cha, J.-Y.; Lee, J.-H.; Lee, K.-J.; Yu, H.-S.; Choi, E.-H.; Kim, K.-M.; Hwang, C.-J. Effect of the ultraviolet light treatment and storage methods on the biological activity of a titanium implant surface. Dent. Mater. 2017, 33, 1426–1435. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.; Denzer, A.; Cochran, D.; Hoffmann, B.; Lussi, A.; Steinemann, S. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chro-mium-cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent Degradation of the Protein Adsorption Capacity of Titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Nagay, B.E.; Cordeiro, J.M.; da Cruz, N.C.; Rangel, E.C.; Ricomini-Filho, A.P.; de Avila, E.D.; Barão, V.A. UV-photofunctionalization of a biomimetic coating for dental implants application. Mater. Sci. Eng. C 2020, 110, 110657. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-S.; Sul, Y.-T.; Oh, S.-J.; Lee, H.-J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Gianfreda, F.; Antonacci, D.; Raffone, C.; Muzzi, M.; Pistilli, V.; Bollero, P. Microscopic Characterization of Bioactivate Implant Surfaces: Increasing Wettability Using Salts and Dry Technology. Materials 2021, 14, 2608. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Att, W.; Ikeda, T.; Hirota, M.; Ogawa, T. Long-Term Progressive Degradation of the Biological Capability of Titanium. Materials 2016, 9, 102. [Google Scholar] [CrossRef]
- Al Qahtani, M.S.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephan, I.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, e157–e167. [Google Scholar] [CrossRef]
- Altmann, B.; Kohal, R.-J.; Steinberg, T.; Tomakidi, P.; Bächle-Haas, M.; Wennerberg, A.; Att, W. Distinct Cell Functions of Osteoblasts on UV-Functionalized Titanium- and Zirconia-Based Implant Materials Are Modulated by Surface Topography. Tissue Eng. Part C Methods 2013, 19, 850–863. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet Photofunctionalization of Titanium Implants. Int. J. Oral Maxillofac. Implant. 2014, 29, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Yamada, M.; Ishizaki, K.; Sakurai, K. Ultraviolet-C irradiation to titanium implants increases peri-implant bone formation without impeding mineralization in a rabbit femur model. Acta Odontol. Scand. 2015, 73, 302–311. [Google Scholar] [CrossRef]
- Canullo, L.; Cassinelli, C.; Götz, W.; Tarnow, D. Plasma of Argon Accelerates Murine Fibroblast Adhesion in Early Stages of Titanium Disk Colonization. Int. J. Oral Maxillofac. Implant. 2013, 28, 957–962. [Google Scholar] [CrossRef]
- Jeon, H.J.; Jung, A.; Kim, H.J.; Seo, J.S.; Kim, J.Y.; Yum, M.S.; Gweon, B.; Lim, Y. Enhanced Osteoblast Adhesion and Proliferation on Vacuum Plasma-Treated Implant Surface. Appl. Sci. 2022, 12, 9884. [Google Scholar] [CrossRef]
- Walker, E.C.; McGregor, N.E.; Chan, A.S.M.; Sims, N.A. Measuring Bone Volume at Multiple Densities by Micro-computed Tomography. Bio Protoc. 2021, 11, e3873. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Kim, S.; Lee, B.S.; Kim, A.; Ah, C.S.; Huh, C.; Sung, G.Y.; Yun, W.S. Enhanced Protein Immobilization Efficiency on a TiO2 Surface Modified with a Hydroxyl Functional Group. Langmuir 2009, 25, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Ryu, J.-H.; Kwon, J.-S.; Kim, J.-E.; Cha, J.-Y.; Lee, K.-J.; Yu, H.-S.; Choi, E.-H.; Kim, K.-M.; Hwang, C.-J. Effect of wet storage on the bioactivity of ultraviolet light- and non-thermal atmospheric pressure plasma-treated titanium and zirconia implant surfaces. Mater. Sci. Eng. C 2019, 105, 110049. [Google Scholar] [CrossRef]
- Wennerberg, A.; Jimbo, R.; Stübinger, S.; Obrecht, M.; Dard, M.; Berner, S. Nanostructures and hydrophilicity influence osseointegration: A biomechanical study in the rabbit tibia. Clin. Oral Implant. Res. 2014, 25, 1041–1050. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments. Acta Odontol. Scand. 2014, 73, 67–75. [Google Scholar] [CrossRef]
- Dong, Y.; Long, L.; Zhang, P.; Yu, D.; Wen, Y.; Zheng, Z.; Wu, J.; Chen, W. A chair-side plasma treatment system for rapidly enhancing the surface hydrophilicity of titanium dental implants in clinical operations. J. Oral Sci. 2021, 63, 334–340. [Google Scholar] [CrossRef]
- Chou, W.-C.; Wang, R.C.-C.; Huang, C.-L.; Lee, T.-M. The effect of plasma treatment on the osseointegration of rough titanium implant: A histo-morphometric study in rabbits. J. Dent. Sci. 2018, 13, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, G.P. Plasma treatment maintains surface energy of the implant surface and enhances osse-ointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.J.; Stevens, G.C. Surface modification of polymer surfaces: Atmospheric plasma versus vacuum plasma treatments. J. Phys. D. Appl. Phys. 2001, 34, 2761–2768. [Google Scholar] [CrossRef]
- Krautwald, L.; Smeets, R.; Stolzer, C.; Rutkowski, R.; Guo, L.; Reitmeier, A.; Gosau, M.; Henningsen, A. Osseointegration of Zirconia Implants after UV-Light or Cold Atmospheric Plasma Surface Treatment In Vivo. Materials 2022, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, M.; Stoilov, L.; Enkling, N.; Stark, H.; Winter, J.; Marder, M.; Kraus, D. Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differ-entiation of Bone Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Lee, Y.-H.; Gankhuyag, B.; Chakraborty, C.; Lee, S.-S. Effect of Alumina Particles on the Osteogenic Ability of Osteoblasts. J. Funct. Biomater. 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeon, H.J.; Jung, A.; Kim, J.; Kim, J.Y.; Lee, S.H.; Kim, H.; Yeom, M.S.; Choe, W.; Gweon, B.; et al. Improvement of osseointegration efficacy of titanium implant through plasma surface treatment. Biomed. Eng. Lett. 2022, 12, 421–432. [Google Scholar] [CrossRef]
- Flörke, C.; Janning, J.; Hinrichs, C.; Behrens, E.; Liedtke, K.R.; Sen, S.; Christofzik, D.; Wiltfang, J.; Gülses, A. In-vitro assessment of the efficiency of cold atmospheric plasma on decontamination of titanium dental implants. Int. J. Implant. Dent. 2022, 8, 12. [Google Scholar] [CrossRef]

| Control | Experimental | Total | ||
|---|---|---|---|---|
| Percent bone volume in micro-CT | N | 8 | 10 | 18 |
| Mean (SD) | 52.50 (18.78) | 61.47 (8.14) | 57.48 (14.19) | |
| Median | 52.35 | 64.25 | 55.47 | |
| Min, Max | 17.78, 83.51 | 50.80, 71.51 | 17.78, 83.51 | |
| Between p-value | 0.2393 (T) | |||
| BIC (bone-to-implant contact) | N | 8 | 10 | 18 |
| Mean (SD) | 35.09 (10.92) | 31.30 (11.06) | 32.99 (10.85) | |
| Median | 35.19 | 32.21 | 32.81 | |
| Min, Max | 16.46, 54.40 | 16.60, 45.78 | 16.46, 54.40 | |
| Between p-value | 0.4781 (T) | |||
| BA (bone area ratio) | N | 8 | 10 | 18 |
| Mean (SD) | 12.31 (7.17) | 15.61 (9.98) | 14.15 (8.76) | |
| Median | 12.56 | 16.2 | 14.27 | |
| Min, Max | 2.84, 24.78 | 1.26, 31.79 | 1.26, 31.79 | |
| Between p-value | 0.4436(T) | |||
| ISQ at operation (POD#0) | N | 8 | 10 | 18 |
| Mean (SD) | 56.15 (12.51) | 60.40 (9.91) | 58.51 (11.01) | |
| Median | 56.25 | 59.5 | 58.92 | |
| Min, Max | 35.00, 74.00 | 45.00, 74.50 | 35.00, 74.50 | |
| Between p-value | 0.4318 (T) | |||
| ISQ at osseointegration (POD#1M) | N | 8 | 10 | 18 |
| Mean (SD) | 72.88 (3.18) | 70.50 (5.46) | 71.56 (4.63) | |
| Median | 72.83 | 69.75 | 71.33 | |
| Min, Max | 68.00, 77.00 | 61.83, 78.00 | 61.83, 78.00 | |
| Between p-value | 0.2928 (T) | |||
| ISQ difference (0–1 M) | N | 8 | 10 | 18 |
| Mean (SD) | 16.73 (12.98) | 10.10 (8.72) | 13.05 (11.00) | |
| Median | 19.25 | 11.83 | 12.92 | |
| Min, Max | −3.00, 33.00 | −8.67, 23.50 | −8.67, 33.00 | |
| Within p-value | 0.0082 * (P) | 0.0052 *(P) | 0.0001 * (P) | |
| Between p-value | 0.2137 (T) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahm, S.H.; Lee, S.H.; Lim, Y.; Jeon, H.J.; Yun, K.-I. Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo. J. Funct. Biomater. 2024, 15, 278. https://doi.org/10.3390/jfb15100278
Kahm SH, Lee SH, Lim Y, Jeon HJ, Yun K-I. Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo. Journal of Functional Biomaterials. 2024; 15(10):278. https://doi.org/10.3390/jfb15100278
Chicago/Turabian StyleKahm, Se Hoon, Sang Hwa Lee, Youbong Lim, Hyun Jeong Jeon, and Kyoung-In Yun. 2024. "Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo" Journal of Functional Biomaterials 15, no. 10: 278. https://doi.org/10.3390/jfb15100278
APA StyleKahm, S. H., Lee, S. H., Lim, Y., Jeon, H. J., & Yun, K.-I. (2024). Osseointegration of Dental Implants after Vacuum Plasma Surface Treatment In Vivo. Journal of Functional Biomaterials, 15(10), 278. https://doi.org/10.3390/jfb15100278







