Radioprotection Performance Evaluation of 3D-Printed and Conventional Heat-Cured Dental Resins for Radiotherapy Prostheses
Abstract
1. Introduction
- Under EBRT and HDR conditions, the linear attenuation coefficients (μ) of 3D-printed and heat-cured resin are equal.
- The radiation attenuation of dental resins can be predicted satisfactorily as a linear function of thickness within the clinically relevant range of 6 to 14 mm.
2. Materials and Methods
2.1. Specimen Preparation
2.2. Calibration of Radiochromic Film
2.3. Phantom Assembly
2.4. Computational Method for EBT3 Film Measurement
2.5. Statistical Analysis
3. Results
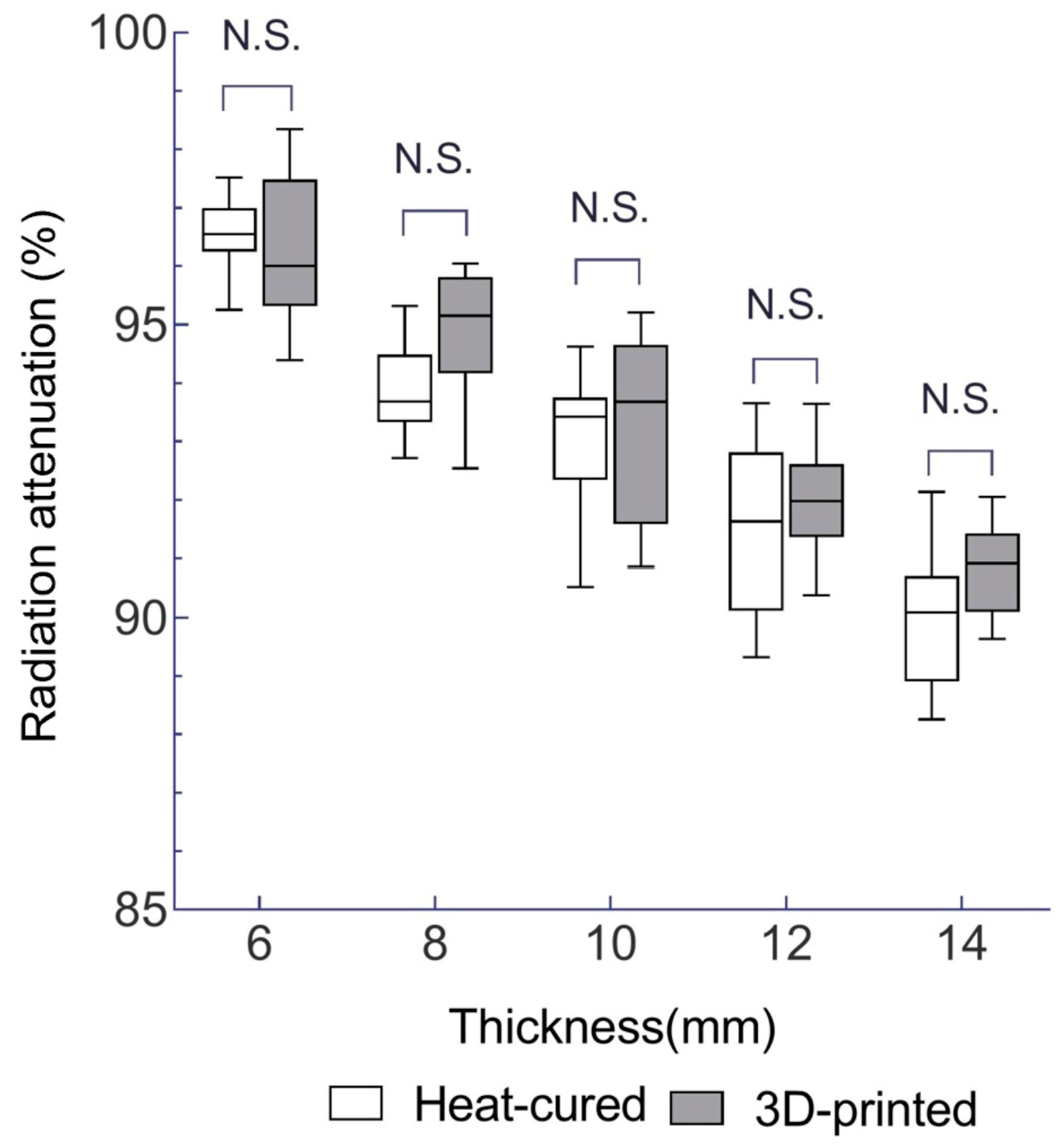
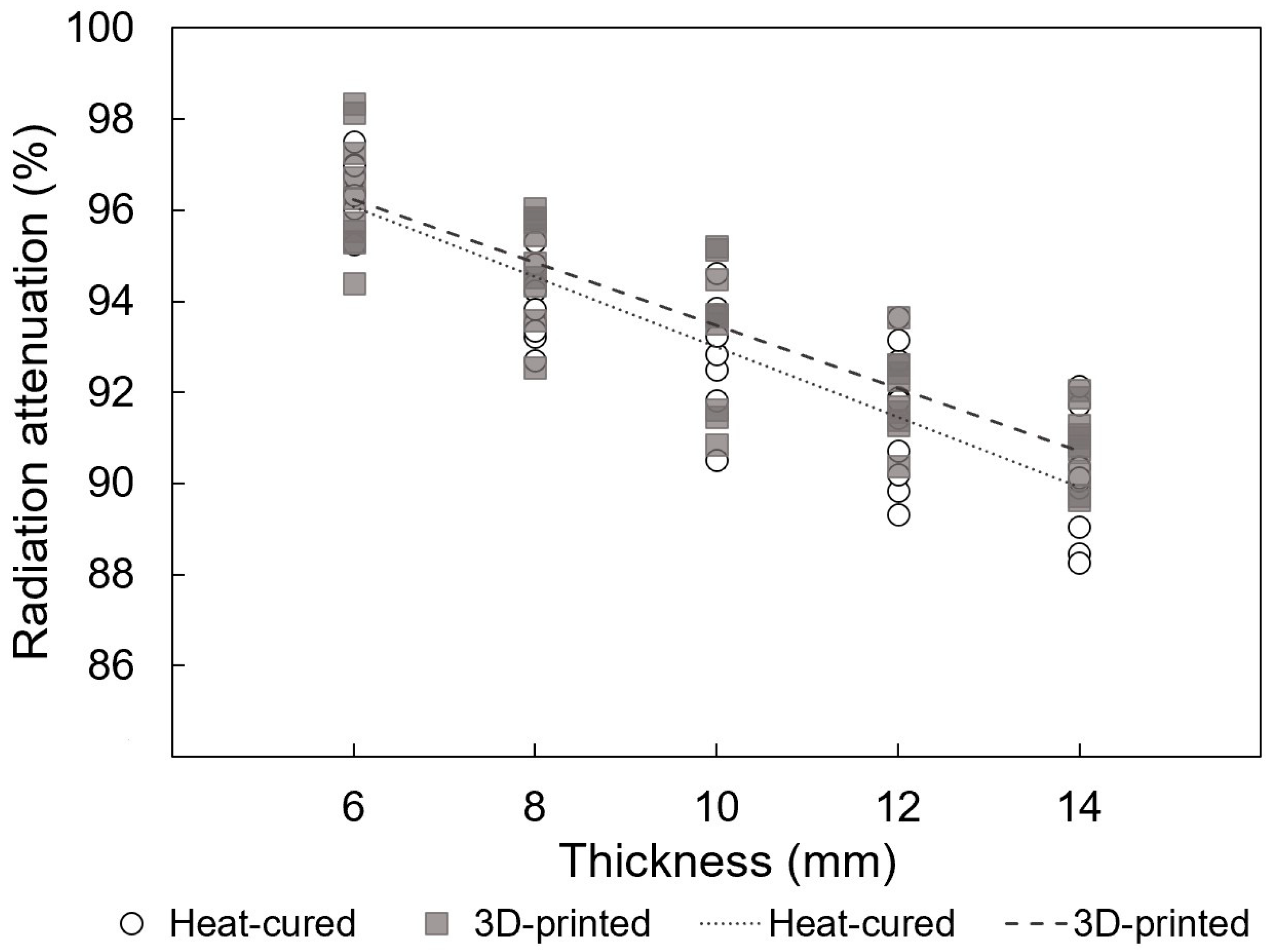
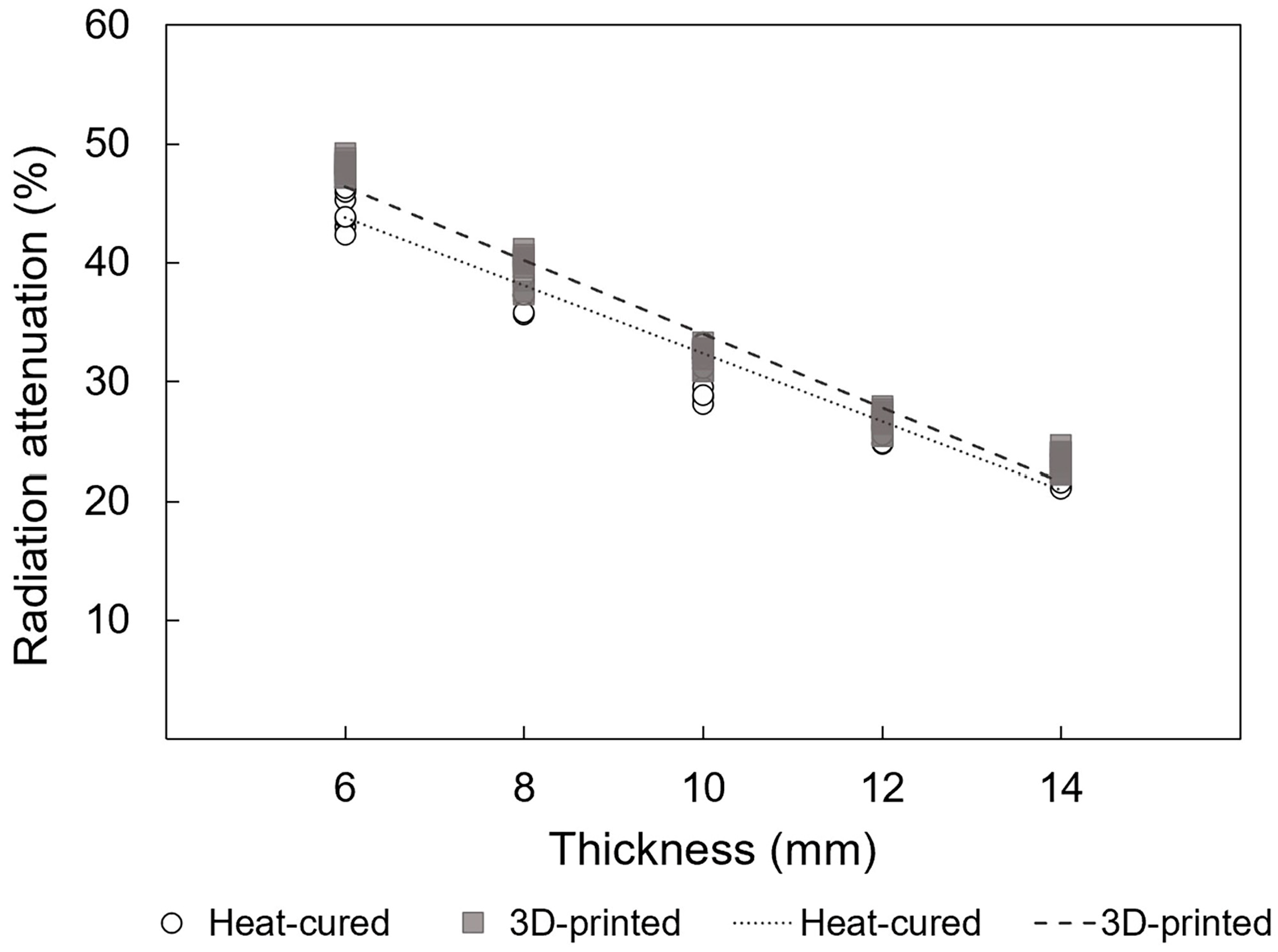
3.1. Radiation Attenuation Comparison for EBRT
3.2. Radiation Attenuation Comparison for HDR
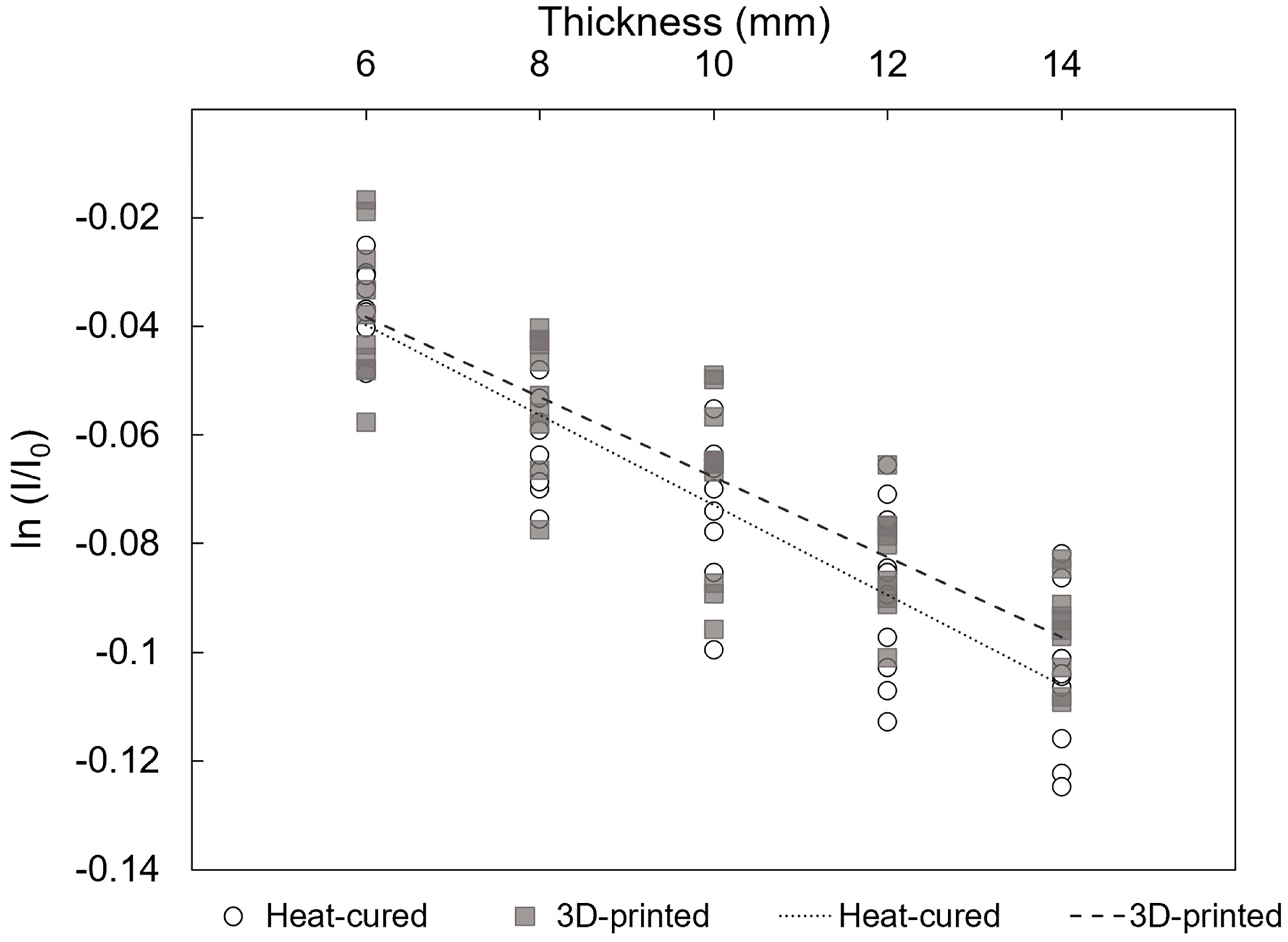
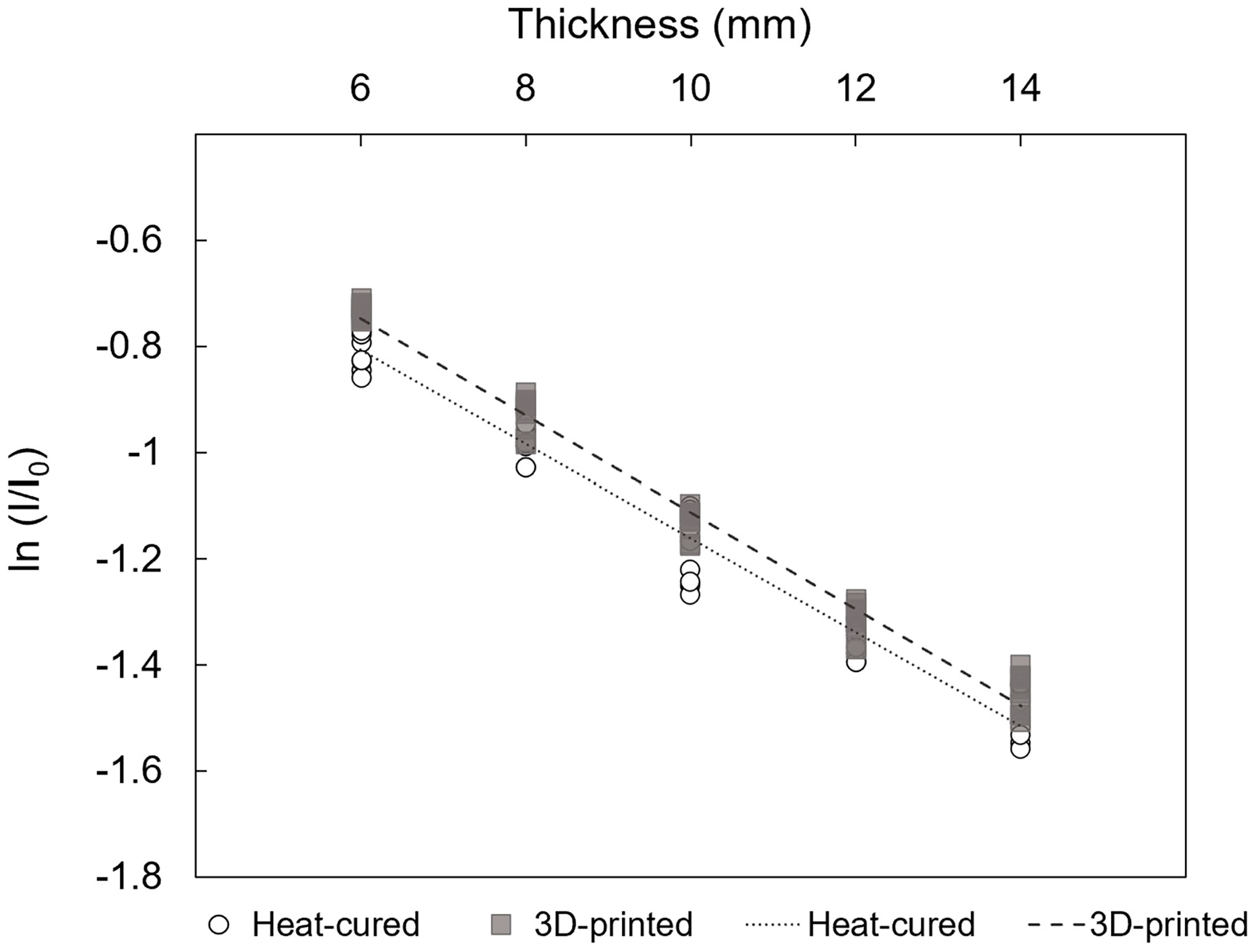
3.3. Linear Regression between the ln (I/I0) and Thickness (Δx)
3.4. Linear Regression between the (I/I0) and Thickness (Δx)
4. Discussion
5. Conclusions
- The 3D-printed resin exhibits slightly different radiation attenuation coefficients compared to heat-cured PMMA, and the 95% confidence intervals overlapped. This suggests that 3D-printed resin can be considered a viable alternative for radiotherapy prostheses.
- Within a clinically relevant thickness range (6–14 mm), the radiation attenuation rate shows a strong linear relationship with resin thickness. This indicates that linear estimation can be roughly used to predict radiation shielding effectiveness based on thickness.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Andjela, L.; Abdurahmanovich, V.M.; Vladimirovna, S.N.; Mikhailovna, G.I.; Yurievich, D.D.; Alekseevna, M.Y. A Review on Vat Photopolymerization 3D-Printing Processes for Dental Application. Dent. Mater. 2022, 38, e284–e296. [Google Scholar] [CrossRef] [PubMed]
- Formlabs. Surgical Guide Resin Product Overview; Formlabs: Somerville, MA, USA, 2024; Available online: https://dental.formlabs.com/uk/store/materials/surgical-guide-resin/ (accessed on 21 August 2024).
- Kobe, T.; Fidler, A.; Kuralt, M.; Gašpirc, B.; Gašperšič, R. Retentive Design of a Small Surgical Guide for Implant Surgery: An In-Vitro Study. J. Dent. 2023, 128, 104384. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Donaldson, C.D.; Wertheim, D.; Naini, F.B. Accuracy and Precision of Three-Dimensionally Printed Orthognathic Surgical Splints. Appl. Sci. 2024, 14, 6089. [Google Scholar] [CrossRef]
- Lee, S.K.Y.; Bigsby, A., 3rd; Morris, J.; Deisher, A.; Mundy, D.; Muller, O. Fabrication of Custom Oral Positioning Devices in Head and Neck Proton Radiotherapy. J. Prosthodont. 2023, 32, 546–550. [Google Scholar] [CrossRef]
- Zaid, M.; Bajaj, N.; Burrows, H.; Mathew, R.; Dai, A.; Wilke, C.T.; Palasi, S.; Hergenrother, R.; Chung, C.; Fuller, C.D.; et al. Creating Customized Oral Stents for Head and Neck Radiotherapy Using 3D Scanning and Printing. Radiat. Oncol. 2019, 14, 148. [Google Scholar] [CrossRef]
- Alfouzan, A.F. Radiation Therapy in Head and Neck Cancer. Saudi Med. J. 2021, 42, 247–254. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Caudell, J.J.; Torres-Roca, J.F.; Gillies, R.J.; Enderling, H.; Kim, S.; Rishi, A.; Moros, E.G.; Harrison, L.B. The Future of Personalised Radiotherapy for Head and Neck Cancer. Lancet Oncol. 2017, 18, e266–e273. [Google Scholar] [CrossRef]
- Fingeret, M.C.; Hutcheson, K.A.; Jensen, K.; Yuan, Y.; Urbauer, D.; Lewin, J.S. Associations among Speech, Eating, and Body Image Concerns for Surgical Patients with Head and Neck Cancer. Head Neck 2013, 35, 354–360. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Ten Haken, R.K.; Giaccia, A. Principles of Radiation Oncology. In Cancer: Principles and Practice of Oncology, 8th ed.; DeVita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 409–428. [Google Scholar]
- Morcos, M.; Viswanathan, A.N.; Enger, S.A. On the Impact of Absorbed Dose Specification, Tissue Heterogeneities, and Applicator Heterogeneities on Monte Carlo-Based Dosimetry of Ir-192, Se-75, and Yb-169 in Conventional and Intensity-Modulated Brachytherapy for the Treatment of Cervical Cancer. Med. Phys. 2021, 48, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Movsas, B. Management of Radiation Toxicity in Head and Neck Cancers. Semin. Radiat. Oncol. 2017, 27, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Towithelertkul, C.; Sumita, Y.I.; Haraguchi, M.; Murase, M.; Fujita, H.; Tanabe, G.; Kanazaki, A.; Yoshi, S.; Kosaka, M.; Hattori, M. A 20-Year Clinical Survey of Radiotherapy Prostheses at the Clinic for Maxillofacial Prosthetics of Tokyo Medical and Dental University Hospital. J. Oral Sci. 2023, 65, 6–9. [Google Scholar] [CrossRef]
- Santiago, A. The Role of the Dentist in Radiotherapy. J. Prosthet. Dent. 1973, 30, 196–201. [Google Scholar] [CrossRef]
- Taniguchi, H. Radiotherapy Prostheses. J. Med. Dent. Sci. 2000, 47, 12–26. [Google Scholar]
- Obinata, K.; Ohmori, K.; Tuchiya, K.; Nishioka, T.; Shirato, H.; Nakamura, M. Clinical Study of a Spacer to Help Prevent Osteoradionecrosis Resulting from Brachytherapy for Tongue Cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 246–250. [Google Scholar] [CrossRef]
- Katsura, K.; Utsunomiya, S.; Abe, E.; Sakai, H.; Kushima, N.; Tanabe, S.; Yamada, T.; Hayakawa, T.; Yamanoi, Y.; Kimura, S.; et al. A Study on a Dental Device for the Prevention of Mucosal Dose Enhancement Caused by Backscatter Radiation from Dental Alloy During External Beam Radiotherapy. J. Radiat. Res. 2016, 57, 709–713. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, F.; Yu, X.; Wu, L.; Lu, Z.; Yan, S. The Role of Radioprotective Spacers in Clinical Practice: A Review. Quant. Imaging Med. Surg. 2018, 8, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Tamamoto, M.; Fujita, M.; Yamamoto, T.; Hamada, T. Techniques for Making Spacers in Interstitial Brachytherapy for Tongue Cancer. Int. J. Prosthodont. 1996, 9, 95–98. [Google Scholar]
- Santiago, A. Fabrication of Intraoral Radiotherapy Prostheses. J. Prosthet. Dent. 1975, 34, 212–215. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Wieckiewicz, M.; Wezgowiec, J. The Influence of Polishing and Artificial Aging on BioMed Amber® Resin’s Mechanical Properties. J. Funct. Biomater. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Wezgowiec, J.; Malysa, A.; Wieckiewicz, M. Effects of Polishing and Artificial Aging on Mechanical Properties of Dental LT Clear® Resin. J. Funct. Biomater. 2023, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Knoll, G.F. Radiation Detection and Measurement, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 51. [Google Scholar]
- Spunei, M.; Malaescu, I.; Mihai, M.; Marin, C.N. Absorbing Materials with Applications in Radiotherapy and Radioprotection. Radiat. Prot. Dosim. 2014, 162, 167–170. [Google Scholar] [CrossRef]
- Paliwal, B.R.; Rommelfanger, S.; Das, R.K. Attenuation Characteristics of a New Compensator Material: Thermo-Shield for High Energy Electron and Photon Beams. Med. Phys. 1998, 25, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Towithelertkul, C.; Sumita, Y.I.; Murakami, T.; Notake, R.; Akiyama, M.; Yoshimura, R.; Wakabayashi, N. Radiation Attenuation Properties of Materials Used to Fabricate Radiotherapy Prostheses: An In Vitro Study. J. Oral Sci. 2022, 64, 274–278. [Google Scholar] [CrossRef]
- Fujita, M.; Tamamoto, M.; Hirokawa, Y.; Kashiwado, K.; Akagi, Y.; Kashimoto, K.; Wada, T. Experimental and Clinical Studies on Dose Reduction Effects of Spacers in Interstitial Brachytherapy for Carcinoma of the Mobile Tongue. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 797–803. [Google Scholar] [CrossRef]
- Saitoh, H. Outline of Standard Dosimetry of Absorbed Dose to Water in External Beam Radiotherapy. Igaku Butsuri 2013, 33, 171–178. [Google Scholar]
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Huq, M.S.; Ibbott, G.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A Revised AAPM Protocol for Brachytherapy Dose Calculations. Med. Phys. 2004, 31, 633–674. [Google Scholar] [CrossRef]
- Sorriaux, J.; Kacperek, A.; Rossomme, S.; Lee, J.A.; Bertrand, D.; Vynckier, S.; Sterpin, E. Evaluation of Gafchromic® EBT3 Films Characteristics in Therapy Photon, Electron, and Proton Beams. Phys. Med. 2013, 29, 599–606. [Google Scholar] [CrossRef]



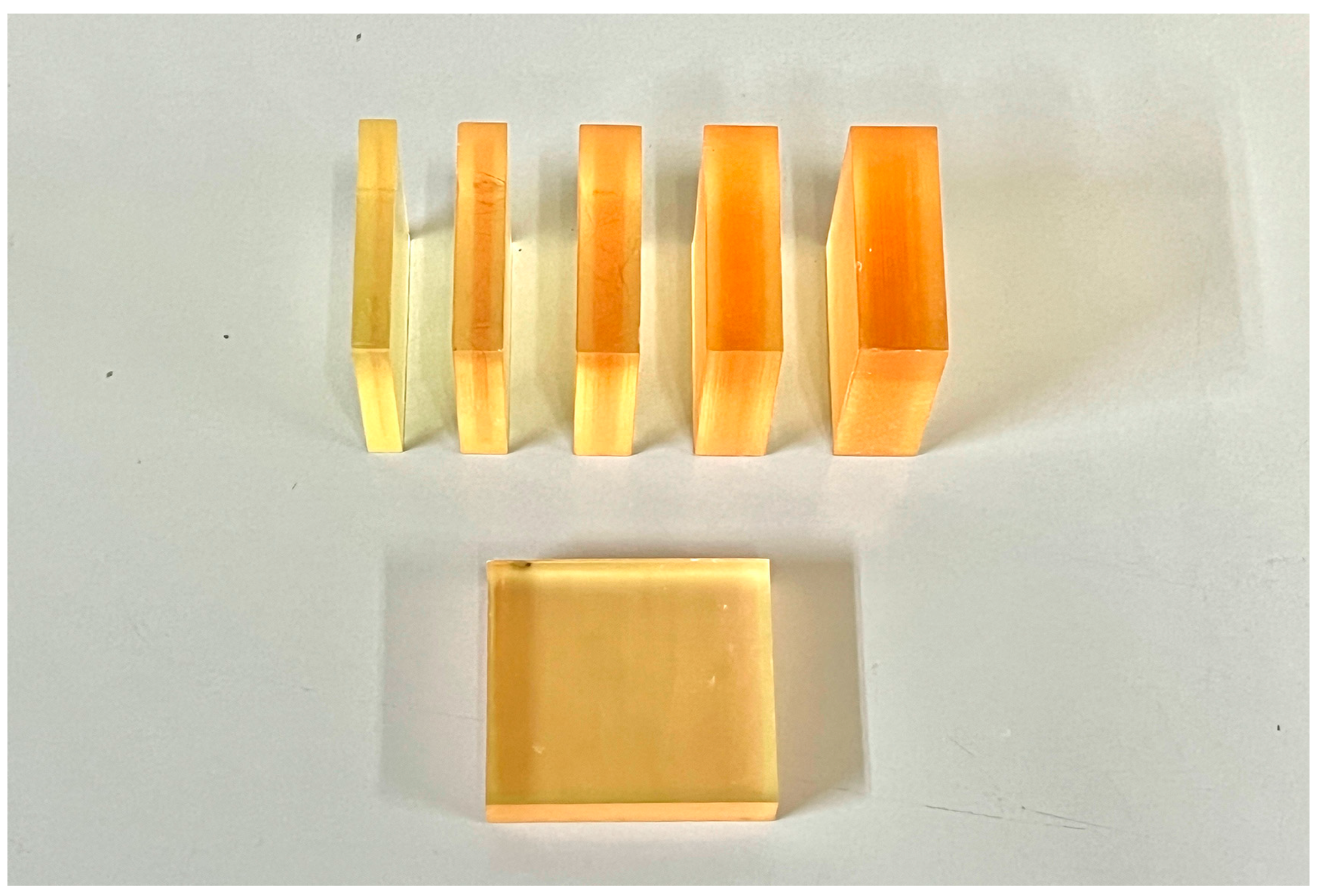
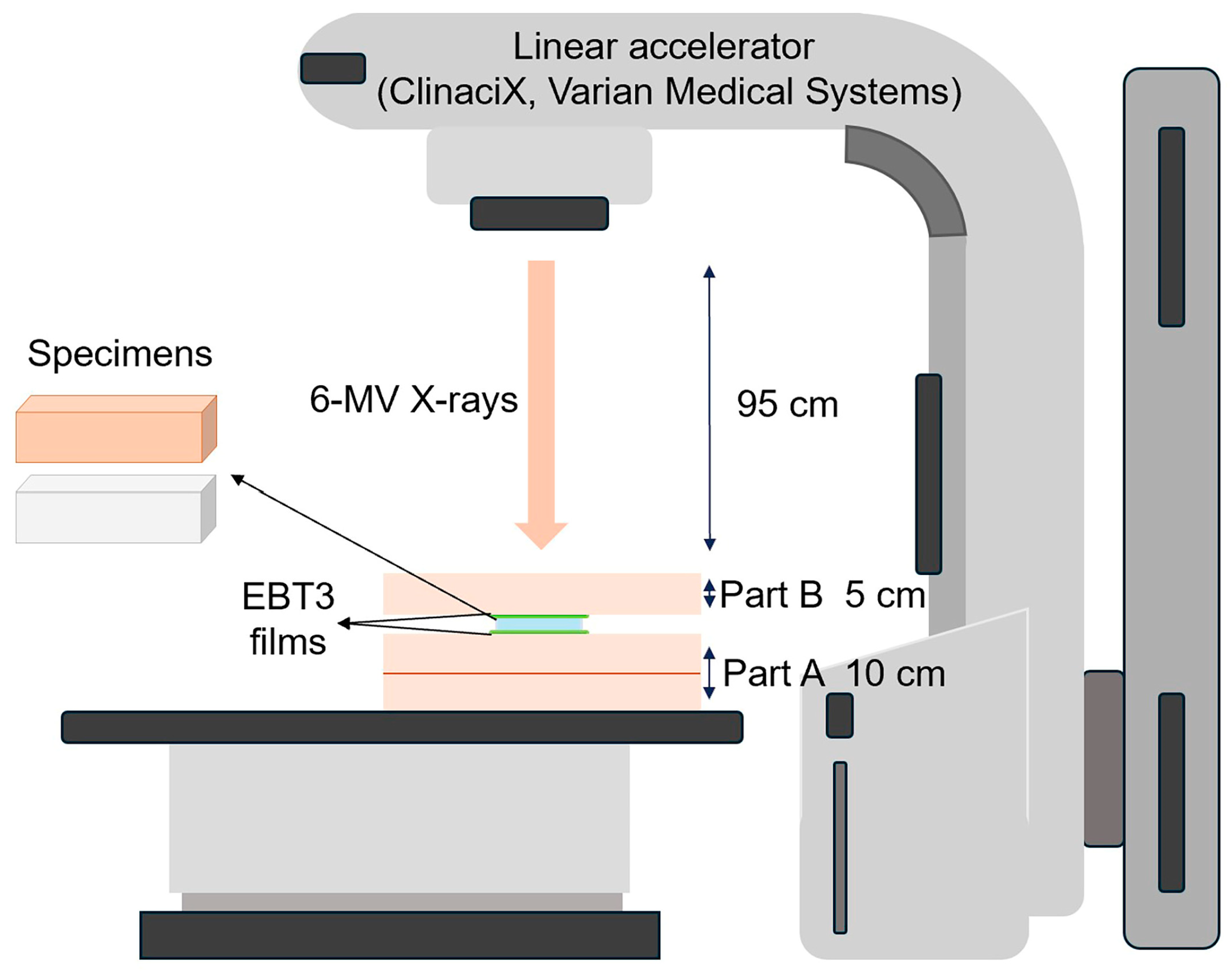
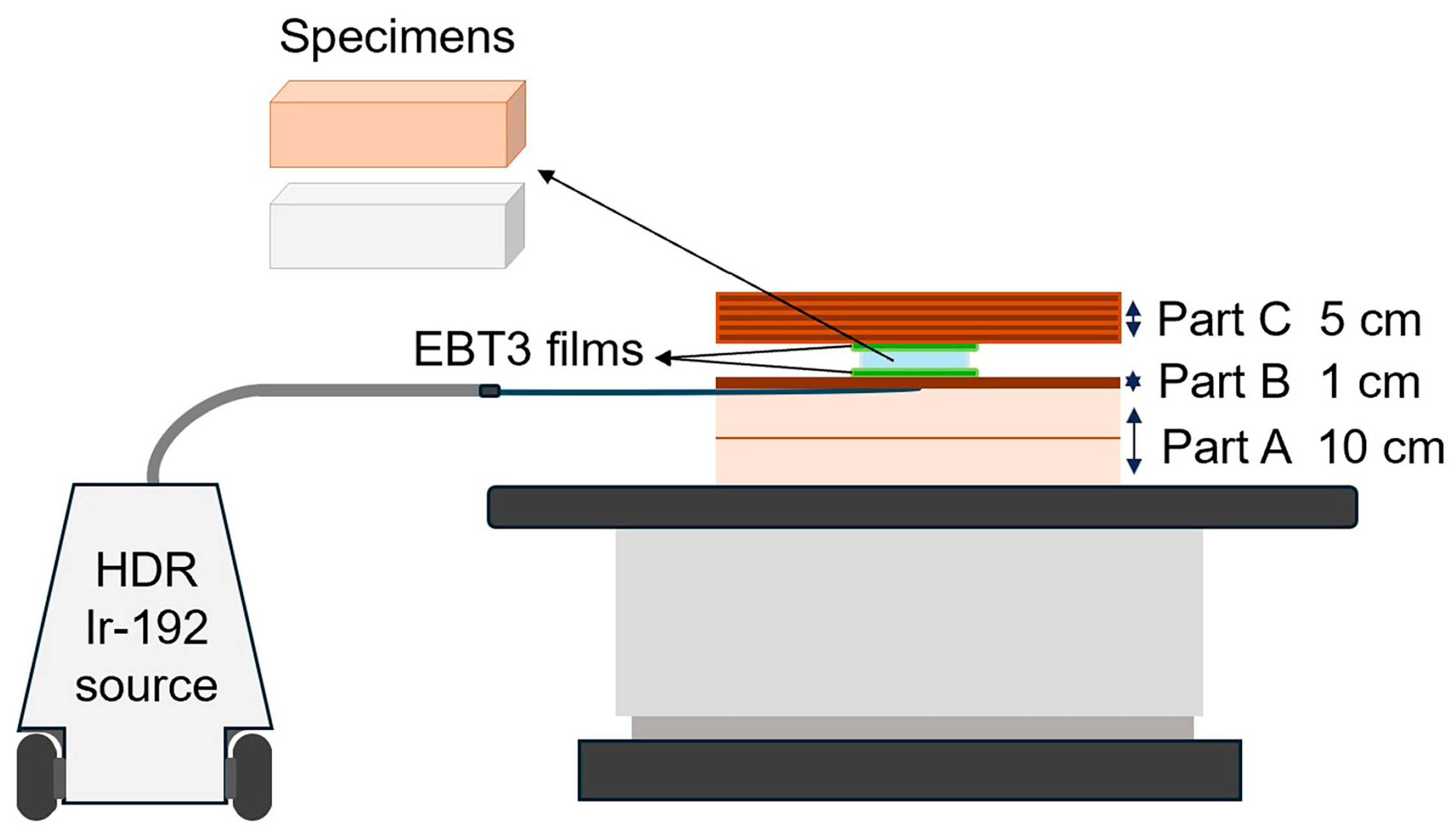

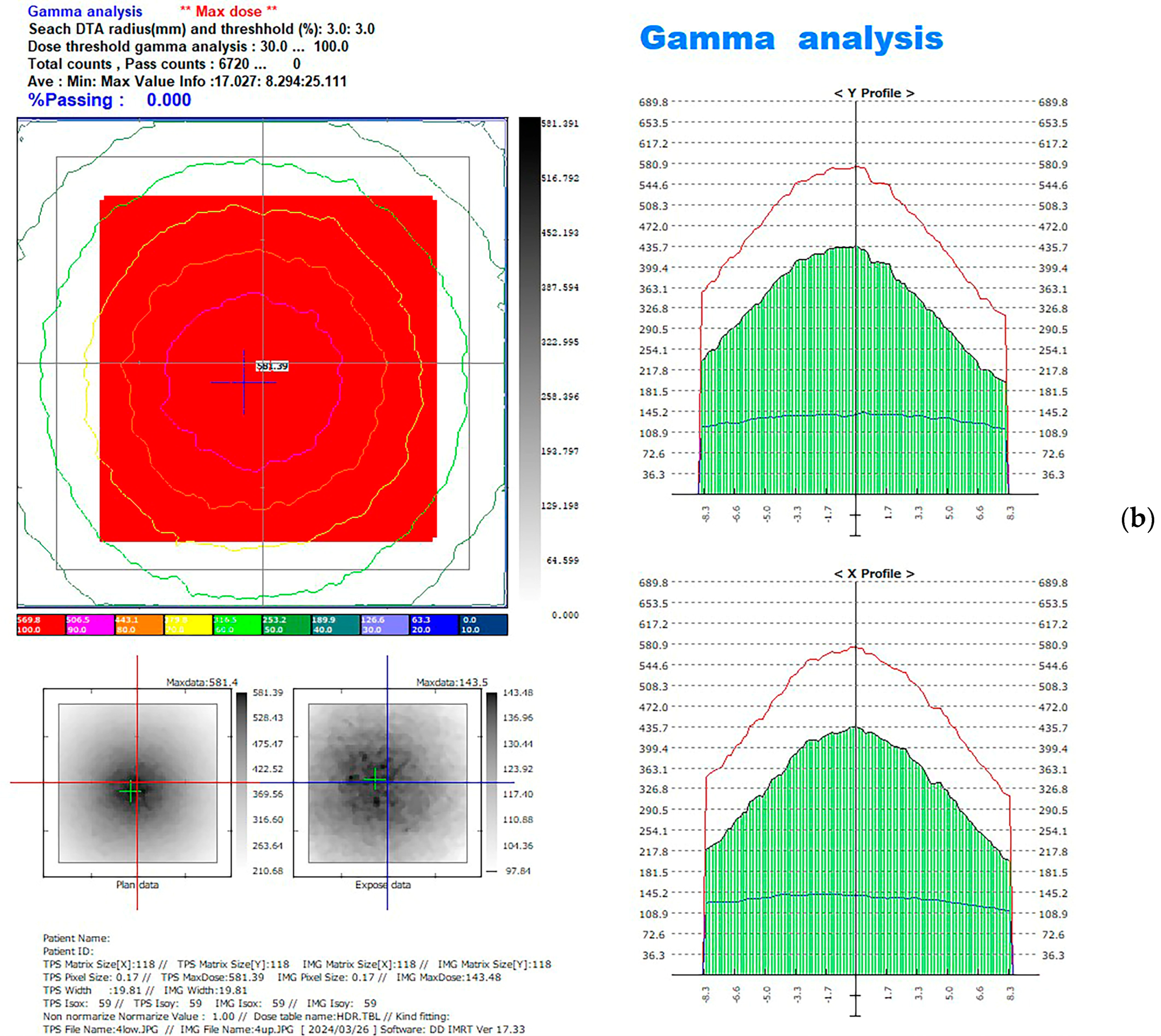

| Brand Name | Manufacturer | Processing Technique | Resin Composition | Lot | Density (g/cm3) |
|---|---|---|---|---|---|
| ACRON | GC Corp. | Heat polymerization | Powder: methacrylic acid ester polymer Liquid: methyl methacrylate | Powder: 2206021 Liquid: 2205061 | 1.20 |
| Surgical guide resin | Formlabs | Photopolymerization | Urethane dimethacrylate, methacrylate monomer (s), photoinitiator (s) | SG01220531-01 | 1.19 |
| Thickness (mm) | Radiation Attenuation (%) | |
|---|---|---|
| 3D-Printed Resin | Heat-Cured Resin | |
| 6 | 96.3 ± 1.3 | 96.5 ± 0.6 |
| 8 | 94.9 ± 1.1 | 93.9 ± 0.8 |
| 10 | 93.3 ± 1.5 | 93.0 ± 1.2 |
| 12 | 92.0 ± 0.9 | 91.5 ± 1.4 |
| 14 | 90.8 ± 0.8 | 90.0 ± 1.2 |
| Thickness (mm) | Radiation Attenuation (%) | |
|---|---|---|
| 3D-Printed Resin | Heat-Cured Resin | |
| 6 | 48.0 ± 0.7 | 45.2 ± 1.9 |
| 8 | 39.6 ± 1.3 | 37.5 ± 1.1 |
| 10 | 32.3 ± 0.8 | 30.9 ± 1.9 |
| 12 | 26.8 ± 0.9 | 25.9 ± 0.7 |
| 14 | 23.4 ± 0.9 | 22.4 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Murase, M.; Sumita, Y.I.; Notake, R.; Akiyama, M.; Yoshimura, R.; Wakabayashi, N. Radioprotection Performance Evaluation of 3D-Printed and Conventional Heat-Cured Dental Resins for Radiotherapy Prostheses. J. Funct. Biomater. 2024, 15, 282. https://doi.org/10.3390/jfb15100282
Wang J, Murase M, Sumita YI, Notake R, Akiyama M, Yoshimura R, Wakabayashi N. Radioprotection Performance Evaluation of 3D-Printed and Conventional Heat-Cured Dental Resins for Radiotherapy Prostheses. Journal of Functional Biomaterials. 2024; 15(10):282. https://doi.org/10.3390/jfb15100282
Chicago/Turabian StyleWang, Jiangyu, Mai Murase, Yuka I. Sumita, Ryoichi Notake, Masako Akiyama, Ryoichi Yoshimura, and Noriyuki Wakabayashi. 2024. "Radioprotection Performance Evaluation of 3D-Printed and Conventional Heat-Cured Dental Resins for Radiotherapy Prostheses" Journal of Functional Biomaterials 15, no. 10: 282. https://doi.org/10.3390/jfb15100282
APA StyleWang, J., Murase, M., Sumita, Y. I., Notake, R., Akiyama, M., Yoshimura, R., & Wakabayashi, N. (2024). Radioprotection Performance Evaluation of 3D-Printed and Conventional Heat-Cured Dental Resins for Radiotherapy Prostheses. Journal of Functional Biomaterials, 15(10), 282. https://doi.org/10.3390/jfb15100282







