Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Synthesis
2.3. Sample Characterization
2.4. Production of Reactive Oxygen Species
2.5. Antibacterial Activity
2.6. Antibiofilm Activity Testing
2.7. Cytotoxicity
3. Results
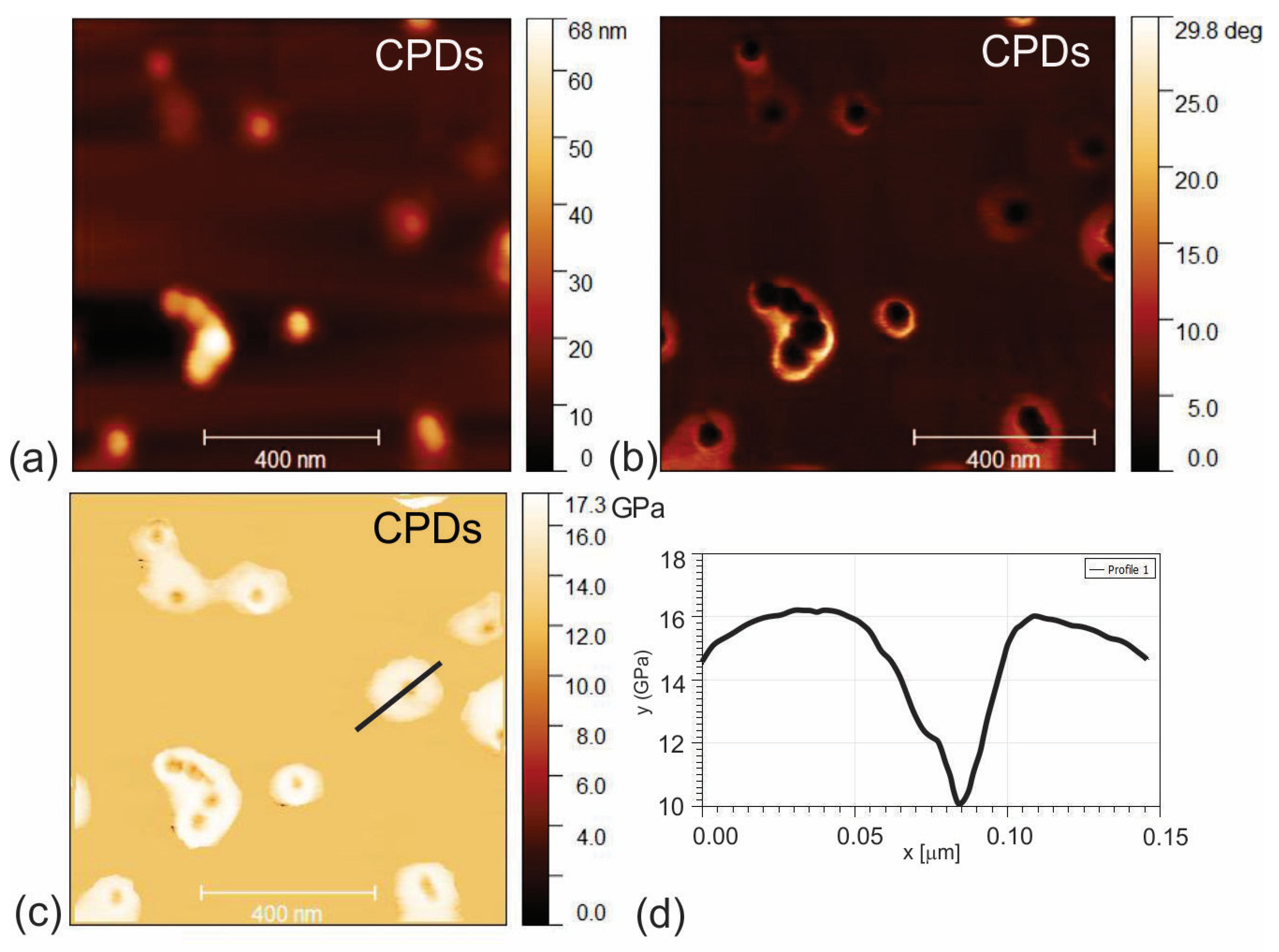
3.1. Surface Morphology of CPD, CPDs/PU and C60/PU Composite Films
3.2. Electrostatic Force Microscopy of CPDs, CPDs/PU and C60/PU
3.3. Chemical Composition
3.4. UV-Vis Spectra of CPD and C60 Nanoparticles
3.5. Production of Reactive Oxygen Species
3.5.1. Production of Singlet Oxygen
3.5.2. Production of OH Radicals
3.6. Antibacterial Activity of CPD/PU and C60/PU Polymer Composite Films
3.7. Antibiofouling Activity of CPDs/PU and C60/PU
3.8. Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm formation, and related impacts on healthcare, food Processing and packaging, industrial manufacturing, marine industries, and sanitation—A review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Percival, L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Wißmann, J.E.; Kirchhoff, L.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Persistence of pathogens on inanimate surfaces: A narrative review. Microorganisms 2021, 9, 343. [Google Scholar] [CrossRef]
- Assefa, M.; Amare, A. Biofilm-associated multi-drug resistance in hospital-acquired infections: A review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef]
- Cruz-Lopez, F.; Martinez-Melendez, A.; Garza-Gonzalez, E. How does hospital microbiota contribute to healthcare-associated infections? Microorganisms 2023, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lopez, F.; Villarreal-Treviño, L.; Morfin-Otero, R.; Martínez-Meléndez, A.; Camacho-Ortiz, A.; Rodríguez-Noriega, E.; Garza-González, E. Microbial diversity and colonization patterns of two step-down care units from a tertiary care hospital. J. Res. Med. Sci. 2021, 26, 126. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Makuła, M.; Włodarczyk-Makuła, M.; Wołejko, E.; Wydro, U.; Serra-Majem, L.; Wiater, J. Inanimate surfaces as a source of hospital infections caused by fungi, bacteria and viruses with particular emphasis on SARS-CoV-2. Int. J. Environ. Res. Public Health 2022, 19, 8121. [Google Scholar] [CrossRef]
- Kurashige, E.J.O.; Oie, S.; Furukawa, H. Contamination of environmental surfaces by methicillin-resistant Staphylococcus aureus (MRSA) in rooms of inpatients with MRSA-positive body sites. Braz. J. Microbiol. 2016, 47, 703–705. [Google Scholar] [CrossRef]
- Fazeli, H.; Akbari, R.; Moghim, S.; Narimani, T.; Arabestani, M.R.; Ghoddousi, A.R. Pseudomonas aeruginosa infections in patients, hospital means, and personnel’s specimens. J. Res. Med. Sci. 2012, 17, 332–337. [Google Scholar]
- Huang, L.; Tang, J.; Tian, G.; Tao, H.; Li, Z. Risk factors, outcomes, and predictions of extensively drug-resistant Acinetobacter baumannii nosocomial infections in patients with nervous system diseases. Infect. Drug Resist. 2023, 16, 7327–7337. [Google Scholar] [CrossRef]
- Kramer, A.; Assadian, O. Survival of Microorganisms on Inanimate Surfaces. In Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections; Borkow, G., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 7–26. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Yin, W.; Xu, S.; Wang, Y.; Zhang, Y.; Chou, S.H.; Galperin, M.Y.; He, J. Ways to control harmful biofilms: Prevention, inhibition, and eradication. Crit. Rev. Microbiol. 2021, 47, 57–78. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.; Hamblin, M.R. Photodynamic therapy for localized infections−State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.; Bäumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef]
- Kováčová, M.; Marković, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajďák, J.; Capáková, Z.; et al. Carbon quantum dots modified polyurethane nanocomposite as effective photocatalytic and antibacterial agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly efficient antibacterial polymer composites based on hydrophobic riboflavin carbon polymerized dots. Nanomaterials 2022, 12, 4070. [Google Scholar] [CrossRef]
- Savelyev, Y.; Gonchar, A.; Movchan, B.; Gornostay, A.; Vozianov, S.; Rudenko, A.; Rozhnova, R.; Travinskaya, T. Antibacterial polyurethane materials with silver and copper nanoparticles. Mater. Proc. 2017, 4, 87–94. [Google Scholar] [CrossRef]
- Miranda, C.; Castano, J.; Valdebenito-Rolack, E.; Sanhueza, F.; Toro, R.; Bello-Toledo, H.; Uarac, P.; Saez, L. Copper-polyurethane composite materials: Particle size effect on the physical-chemical and antibacterial properties. Polymers 2020, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Lim, V. Overview of antimicrobial polyurethane-based nanocomposite materials and associated signaling pathways. Eur. Polym. J. 2022, 167, 111087. [Google Scholar] [CrossRef]
- Farrokhi, Z.; Ayati, A.; Kanvisi, M.; Sillanpää, M. Recent advance in antibacterial activity of nanoparticles contained polyurethane. J. Appl. Polym. Sci. 2018, 136, 46997. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon dots as an emergent class of antimicrobial agents. Nanomaterials 2021, 11, 1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cong, H.; Yu, B.; Chen, Q. Recent advances of water-soluble fullerene derivatives in biomedical applications. Mini-Rev. Org. Chem. 2019, 16, 92–99. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. State of the art in the antibacterial and antiviral applications of carbon-based polymeric nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: Open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–218. [Google Scholar] [CrossRef]
- AM-FM Viscoelastic Mapping Mode. Available online: https://afm.oxinst.com/assets/uploads/products/asylum/documents/AM-FM-Viscoelastic-Mapping-Mode-Application-Note.pdf (accessed on 2 February 2024).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility study on quantitative measurements of singlet oxygen generation using singlet oxygen sensor green. J. Fluoresc. 2013, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.M.; Budimir, M.D.; Danko, M.; Milivojević, D.D.; Kubat, P.; Zmejkoski, D.Z.; Pavlović, V.B.; Mojsin, M.M.; Stevanović, M.J.; Todorović Marković, B.M. Structural, optical, and bioimaging characterization of carbon quantum dots solvothermally synthesized from o-phenylenediamine. Beilstein J. Nanotechnol. 2023, 14, 165–174. [Google Scholar] [CrossRef]
- ISO 22196:2007; Plastics—Measurement of Antibacterial Activity on Plastics Surfaces. ISO Central Secretariat: Vernier, Geneva, 2007. Available online: https://www.iso.org/standard/40759.html (accessed on 2 February 2024).
- Hansen, M.B.; Nielsen, S.E.; Berg, K.J. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Jaiswal, M.; Koul, V. Assessment of multicomponent hydrogel scaffolds of poly(acrylic acid-2-hydroxy ethyl methacrylate)/gelatin for tissue engineering applications. J. Biomater. Appl. 2013, 27, 848–861. [Google Scholar] [CrossRef]
- Daniel Frisbie, C. IV.C Electric Force Microscopy (EFM) and Scanning Surface Potential Microscopy (SSPM). In Encyclopedia of Physical Science and Technology; Meyers, R.A., Ed.; Elsevier: New York, NY, USA, 2001. [Google Scholar]
- Ong, H.G.; Wang, J. Study of Carbon Nanotube Based Devices Using Scanning Probe Microscope. In Physical and Chemical Properties of Carbon Nanotubes; Suzuki, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Patel, S.; Petty, C.W.; Krafcik, K.; Loyola, B.; O’Bryan, G.; Friddle, R.W. Imaging latex–carbon nanotube composites by subsurface electrostatic force microscopy. Nanotechnology 2016, 27, 415705. [Google Scholar] [CrossRef]
- Jespersen, T.S.; Nygård, J. Mapping of individual carbon nanotubes in polymer/nanotube composites using electrostatic force microscopy. Appl. Phys. Lett. 2007, 90, 183108. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Onoe, J.; Nakao, A.; Takeuchi, K. XPS study of a photopolymerized C60 film. Phys. Rev. B 1997, 55, 10051–10056. [Google Scholar] [CrossRef]
- Infrared Spectroscopy Absorption Table. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Reference_Tables/Infrared_Spectroscopy_Absorption_Table (accessed on 3 February 2024).
- Gao, Y.; Jiao, Y.; Lu, W. Carbon dots with red emission as a fluorescent and colorimetric dual-readout probe for the detection of chromium (VI) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.G.; Baragau, I.A.; Gromicova, R.; Nicolaev, A.; Thompson, S.A.J.; Rennie, A.; Power, N.P.; Sajjad, M.T. Investigating the effect of N-doping on carbon quantum dots structure, optical properties and metal ion screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Sarkar, S.; Sudolská, M.; Dubecký, M.; Reckmeier, C.J.; Rogach, A.L.; Zbořil, R.; Otyepka, M. Graphitic nitrogen doping in carbon dots causes red-shifted absorption. J. Phys. Chem. C 2016, 120, 1303–1308. [Google Scholar] [CrossRef]
- Jovanović, S.; Marković, Z.; Budimir, M.; Prekodravac, J.; Zmejkoski, D.; Kepić, D.; Bonasera, A.; Todorović Marković, B. Lights and Dots toward Therapy—Carbon-based quantum dots as new agents for photodynamic therapy. Pharmaceutics 2023, 15, 1170. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.J. Crossover between anti- and pro-oxidant activities of graphene quantum dots in the absence or presence of light. ACS Nano 2016, 10, 8690–8699. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Antonia Corsetto, P.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediators Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]
- Schurer, N.; Kohne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jost, V. Packaging related properties of commercially available biopolymers—An overview of the status quo. eXPRESS Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Philpott, D.J.; Edgeworth, J.D.; Sansonetti, P.J. The pathogenesis of Shigella flexneri infection: Lessons from in vitro and in vivo studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Stanković, N.K.; Bodik, M.; Šiffalovič, P.; Kotlar, M.; Mičušik, M.; Špitalsky, Z.; Danko, M.; Milivojević, D.D.; Kleinova, A.; Kubat, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots Langmuir−Blodgett thin films. ACS Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Li, K.; Wan, Q.; Wu, G.; Lin, Y.; Huang, T.; Wen, G. The protective role and mechanism of melanin for Aspergillus niger and Aspergillus flavus against chlorine-based disinfectants. Wat. Res. 2022, 223, 119039. [Google Scholar] [CrossRef]






| CPD | C60 | |
|---|---|---|
| Element | At% | At% |
| C 1 s | 83.1 | 90.0 |
| O 1 s | 7.3 | 10.0 |
| N 1 s | 9.6 | 0.0 |
| Characteristic Bond | Binding Energy (eV) | Relative Concentration (%) | Characteristic Bond | Binding Energy (eV) | Relative Concentration (%) |
|---|---|---|---|---|---|
| CPDs | C60 | ||||
| C 1 s peak C-C/C-H | 284.5 | 80 | C 1 s peak C-C/C-H | 284.5 | 79 |
| C 1 s peak C=O | 286.5 | 20 | C 1 s peak C-O/C-OH | 285.9 | 20 |
| O 1 s peak C=O | 531.4 | 100 | C1 s peak C-O/C-OH | 288.7 | 1 |
| N 1 s peak pyridinic | 398.7 | 45 | O 1 s peak O-H | 532.2 | 87 |
| N 1 s peak pyrrolic | 399.9 | 49 | O 1 s peak C-O/H2O | 533.3 | 13 |
| N 1 s peak C=N-C | 396.4 | 6 | - | - | - |
| Bacteria Strains | Irradiated, Incubated N (cell/cm2) | Nonirradiated, Incubated N (cell/cm2) | R | Irradiated, Incubated N (cell/cm2) | Nonirradiated, Incubated N (cell/cm2) | R |
|---|---|---|---|---|---|---|
| CPDs/PU | C60/PU | |||||
| K. pneumoniae | 0 | 0 | 5.45 | 0 | 0 | 5.45 |
| P. mirabilis | 0 | 20 × 106 | 5.52 | 0 | 0 | 5.52 |
| S. enterica | 0 | 5 × 107 | 4.81 | 0 | 2 × 107 | 4.81 |
| E. faecalis | 0 | 10 × 105 | 5.53 | 0 | 6 × 105 | 5.53 |
| E. epidermis | 0 | 0 | 4.55 | 0 | 2 × 106 | 0.11 |
| S. flexneri | 64 × 102 | 82 × 104 | 2.10 | 7.6 × 103 | 9.8 × 105 | 2.12 |
| P. aeruginosa | 0 | 70 × 105 | 4.95 | 0 | 1 × 105 | 4.95 |
| A. niger | 8.7 × 102 | 1 × 105 | 2.06 | 0 | 0 | 5.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, Z.M.; Budimir Filimonović, M.D.; Milivojević, D.D.; Kovač, J.; Todorović Marković, B.M. Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films. J. Funct. Biomater. 2024, 15, 73. https://doi.org/10.3390/jfb15030073
Marković ZM, Budimir Filimonović MD, Milivojević DD, Kovač J, Todorović Marković BM. Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films. Journal of Functional Biomaterials. 2024; 15(3):73. https://doi.org/10.3390/jfb15030073
Chicago/Turabian StyleMarković, Zoran M., Milica D. Budimir Filimonović, Dušan D. Milivojević, Janez Kovač, and Biljana M. Todorović Marković. 2024. "Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films" Journal of Functional Biomaterials 15, no. 3: 73. https://doi.org/10.3390/jfb15030073
APA StyleMarković, Z. M., Budimir Filimonović, M. D., Milivojević, D. D., Kovač, J., & Todorović Marković, B. M. (2024). Antibacterial and Antibiofouling Activities of Carbon Polymerized Dots/Polyurethane and C60/Polyurethane Composite Films. Journal of Functional Biomaterials, 15(3), 73. https://doi.org/10.3390/jfb15030073







