Enhancing the Physical, Antimicrobial, and Osteo/Odontogenic Properties of a Sol–Gel-Derived Tricalcium Silicate by Graphene Oxide for Vital Pulp Therapies
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of TCS and GO
2.1.1. Characterization of the Synthesized Materials
2.1.2. Mixing of TCS-GO Composites
2.2. Setting Time and Compressive Strength
2.3. pH Measurements
2.4. Calcium Ion Release
2.5. Antimicrobial Test
Colony Forming Units (CFUs)
2.6. Viability of DPSCs
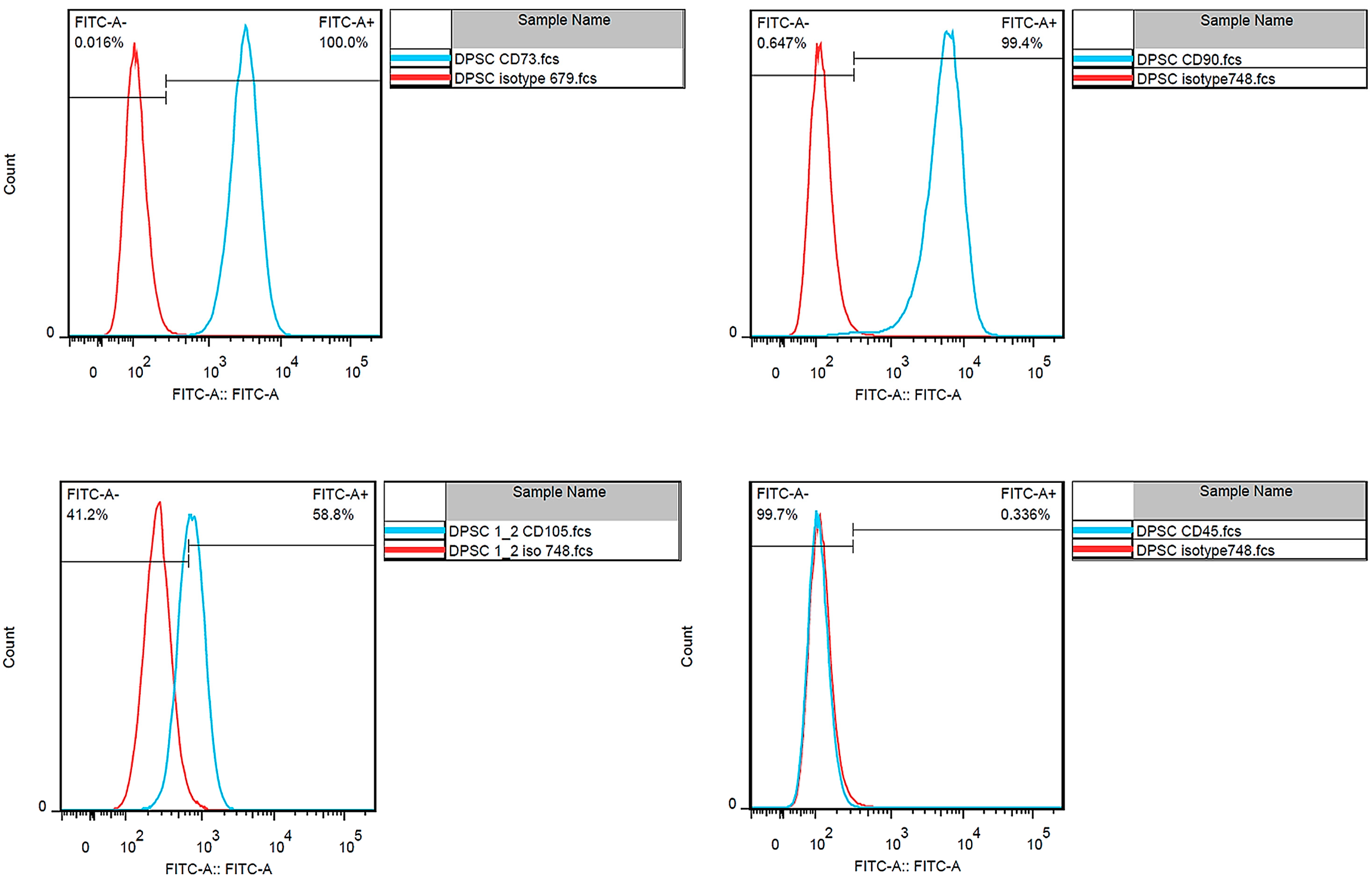
2.6.1. DPSCs’ Isolation and Characteristics
2.6.2. CCK-8 Assay
2.6.3. Live and Dead Staining Assay
2.7. In vitro Apatite Forming Ability
2.8. Quantitative Real-Time PCR (RT-qPCR)
Statistical Analysis
3. Results
3.1. Dental Pulp Stem Cell Isolation
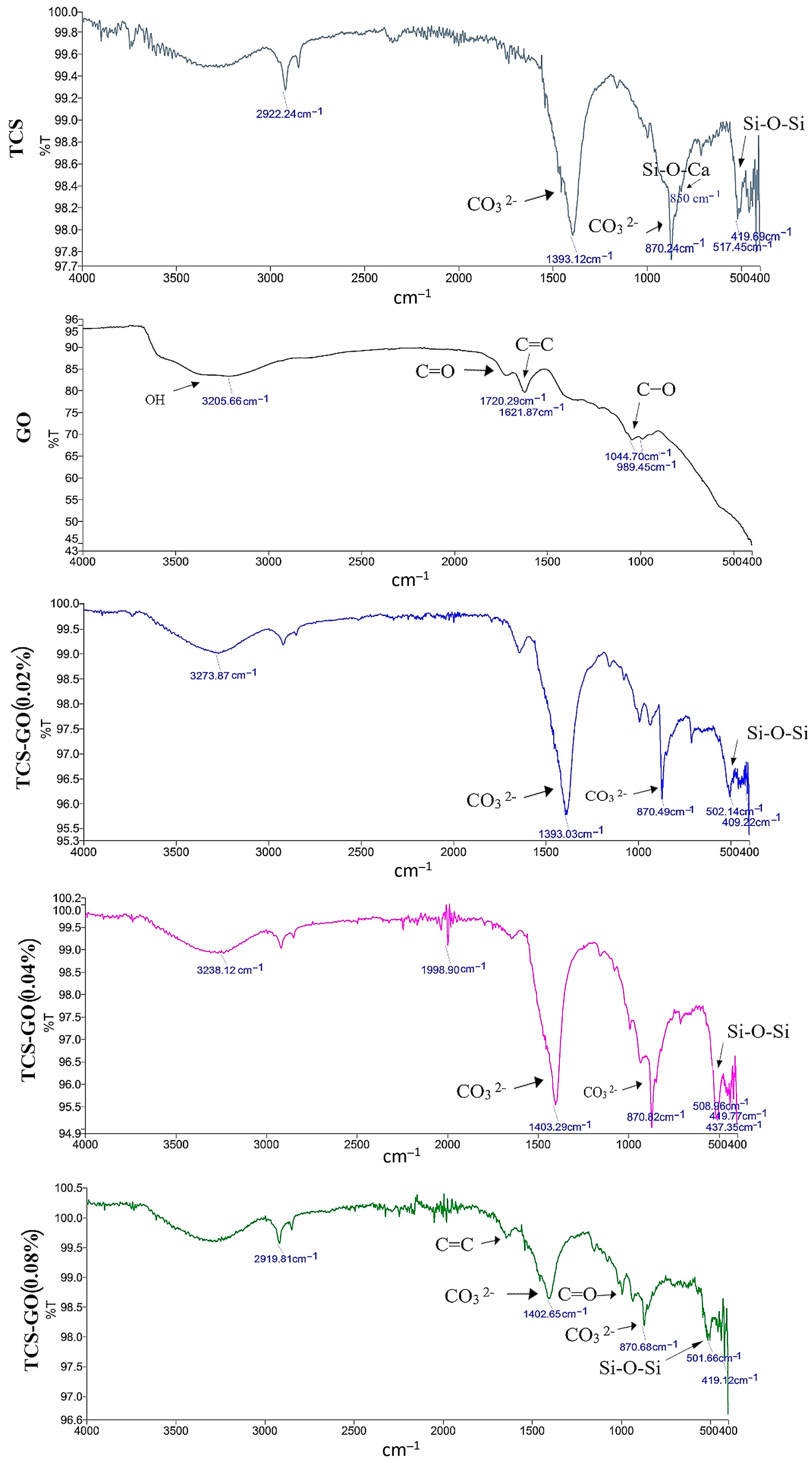
3.2. Characterization of the Prepared Materials
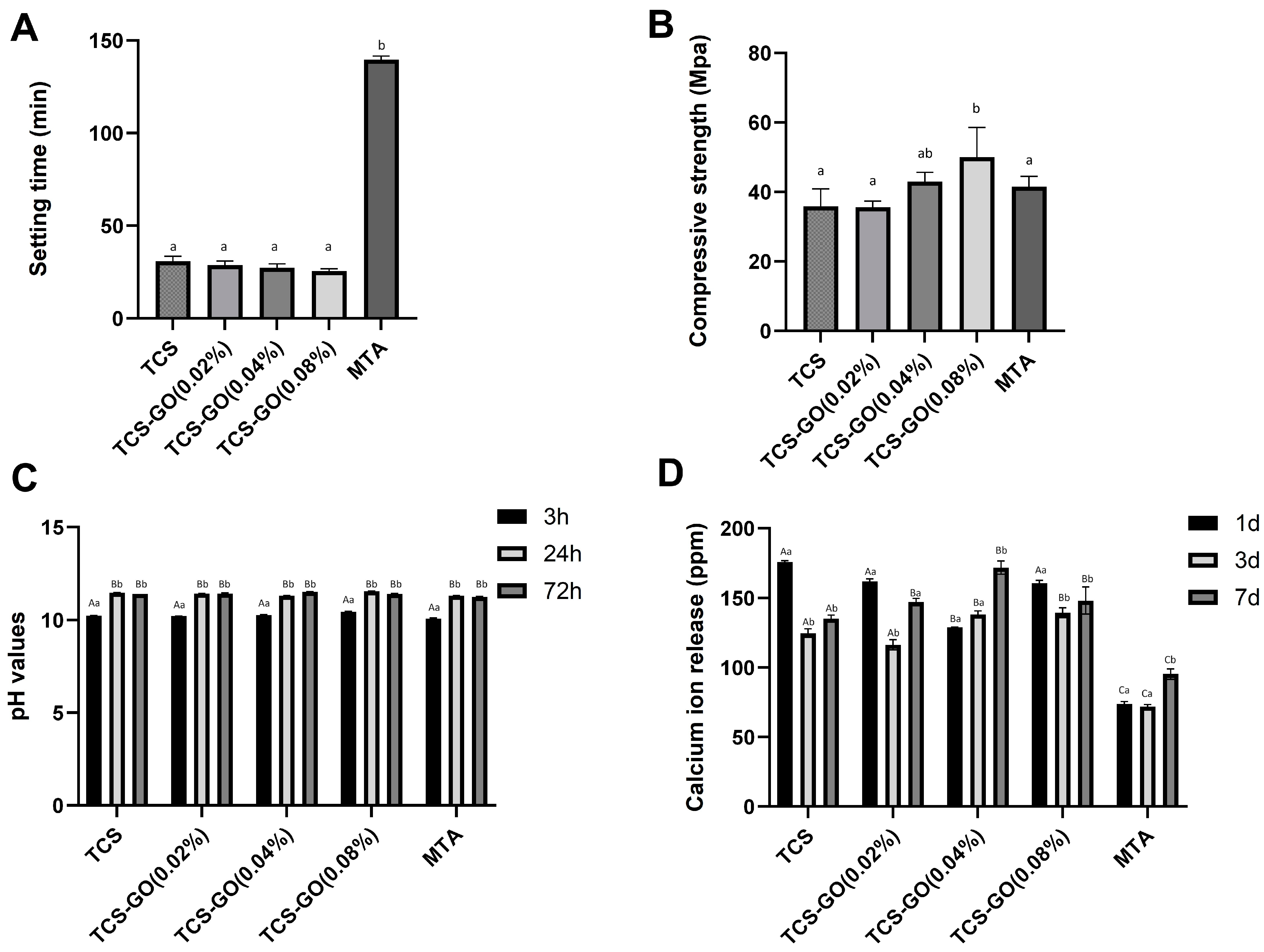
3.3. Setting Time and Compressive Strength
3.4. pH Measurements
3.5. Calcium Ion Release
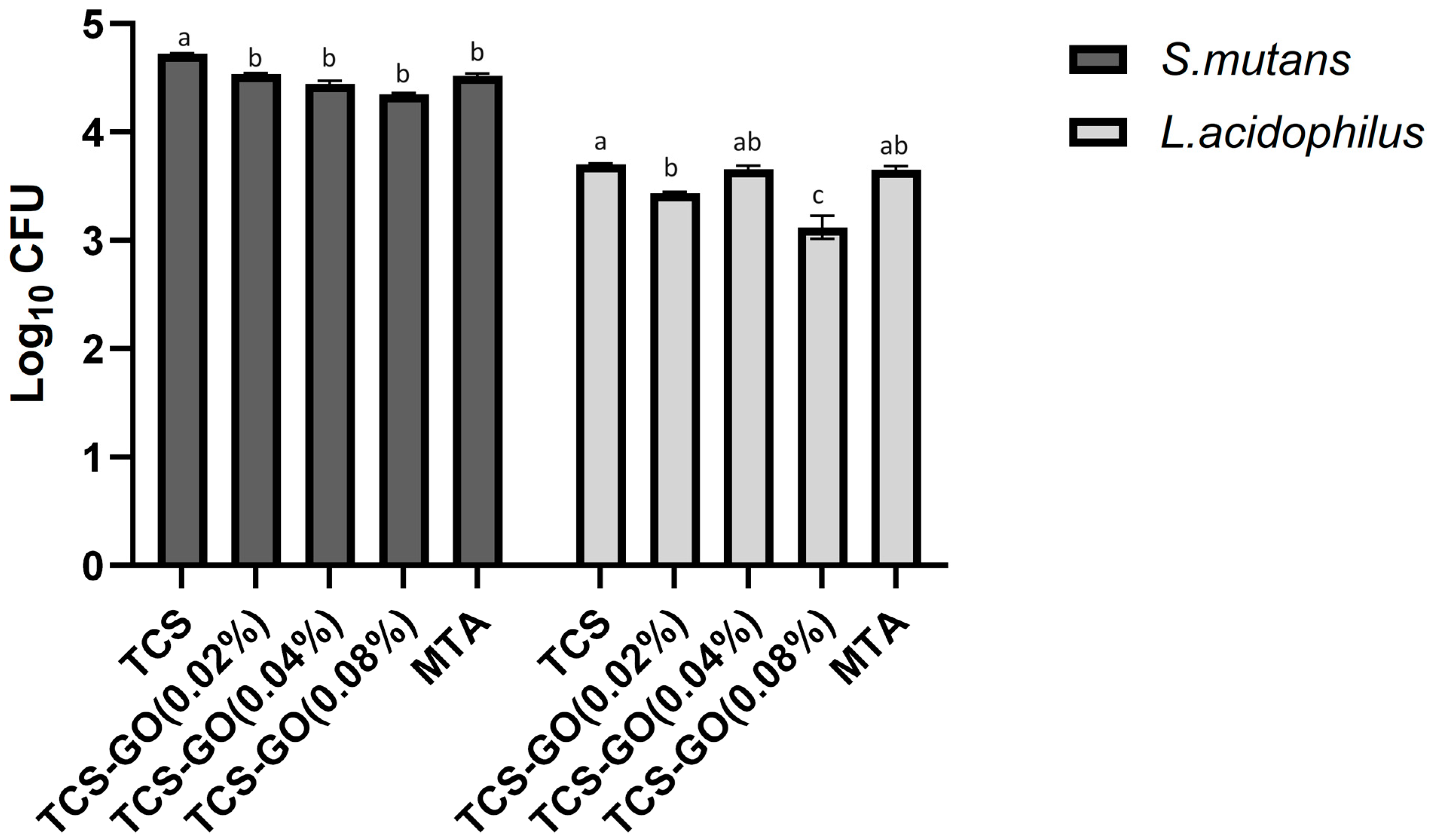
3.6. Colony Forming Units
3.7. Cell Viability (CCK-8 Assay)
Live and Dead Staining Assay
3.8. In Vitro Apatite Forming Ability
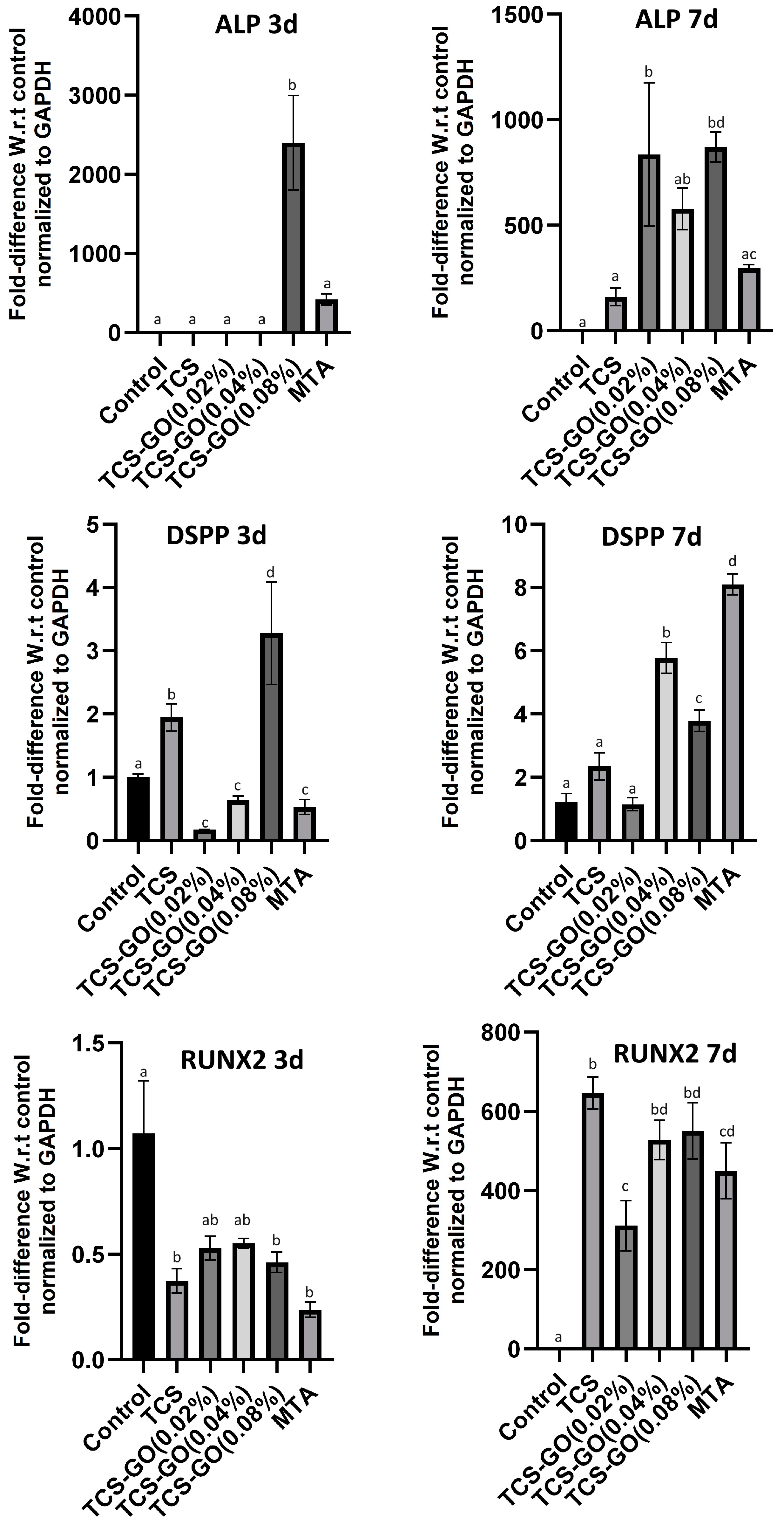
3.9. Quantitative Real-Time PCR (RT-qPCR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Tomson, P.L.; Lundy, F.T.; Cooper, P.; Clarke, M.; El Karim, I.A. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: A systematic review. J. Dent. 2019, 88, 103158. [Google Scholar] [CrossRef] [PubMed]
- Zafar, K.; Nazeer, M.R.; Ghafoor, R.; Khan, F.R. Success of pulpotomy in mature permanent teeth with irreversible pulpitis: A systematic review. J. Conserv. Dent. 2020, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Rahbaran, S.; Lewsey, J.; Gulabivala, K. Outcome of primary root canal treatment: Systematic review of the literature–Part 2. Influence of clinical factors. Int. Endod. J. 2008, 41, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.M.; Lung, C.Y.K.; Neelakantan, P.; Matinlinna, J.P. A novel, doped calcium silicate bioceramic synthesized by sol-gel method: Investigation of setting time and biological properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Love, R.; Jenkinson, H. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Atac, A.S.; Cehreli, Z.C.; Sener, B. Antibacterial activity of fifth-generation dentin bonding systems. J. Endod. 2001, 27, 730–733. [Google Scholar] [PubMed]
- No, Y.J.; Li, J.J.; Zreiqat, H. Doped calcium silicate ceramics: A new class of candidates for synthetic bone substitutes. Materials 2017, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Chen, C.-C.; Wu, I.T.; Ding, S.-J. Enhanced antibacterial activity of calcium silicate-based hybrid cements for bone repair. Mater. Sci. Eng. C 2020, 110, 110727. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Neto, A.H.C.; Rosa, V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W.H. Reinforcing effects of graphene oxide on portland cement paste. J. Mater. Civ. Eng. 2015, 27, A4014010. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Chiang, W.H.; Chen, I.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate-graphene composites. Mater. Sci. Eng. C 2017, 73, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Moghaddam, E.; Shirazi, S.F.S.; Baradaran, S.; Mehrali, M.; Latibari, S.T.; Metselaar, H.S.C.; Kadri, N.A.; Zandi, K.; Osman, N.A.A. Synthesis, mechanical properties, and in vitro biocompatibility with osteoblasts of calcium silicate–reduced graphene oxide composites. ACS Appl. Mater. Interfaces 2014, 6, 3947–3962. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jeong, S.-H.; Kim, D.-U.; Won, J.-P. Graphene oxide as an additive to enhance the strength of cementitious composites. Compos. Struct. 2020, 242, 112154. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef]
- Somaie, R.A.; El-Banna, A.; El-Korashy, D.I. Effect of incorporation of nano-graphene oxide on physicochemical, mechanical, and biological properties of tricalcium silicate cement. J. Mech. Behav. Biomed. Mater. 2023, 146, 106078. [Google Scholar] [CrossRef]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F.; Lim, C.T.; Leong, D.T.; Neto, A.H.C.; Garaj, S.; Rosa, V. Cytotoxicity survey of commercial graphene materials from worldwide. NPJ 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]
- Nizami, M.; Campéon, B.; Nishina, Y. Electrodeposition of hydroxyapatite and graphene oxide improves the bioactivity of medical grade stainless steel. Mater. Today Sustain. 2022, 19, 100193. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Nishina, Y.; Yamamoto, T.; Shinoda-Ito, Y.; Takashiba, S. Functionalized graphene oxide shields tooth dentin from decalcification. J. Dent. Res. 2020, 99, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Nizami, M.Z.I.; Campéon, B.D.L.; Satoh, A.; Nishina, Y. Graphene oxide-based multi-component antimicrobial hydrogels. Bull. Chem. Soc. Jpn. 2022, 95, 713–720. [Google Scholar] [CrossRef]
- Urban, K.; Neuhaus, J.; Donnermeyer, D.; Schäfer, E.; Dammaschke, T. Solubility and pH value of 3 different root canal sealers: A long-term investigation. J. Endod. 2018, 44, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Suchánek, J.; Visek, B.; Soukup, T.; Mohamed, S.E.-D.; Ivancakova, R.; Mokry, J.; Aboul-Ezz, E.; Omran, A. Stem cells from human exfoliated deciduous teeth-isolation, long term cultivation and phenotypical analysis. Acta Medica 2010, 53, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Carmo, S.D.; Néspoli, F.; Bachmann, L.; Miranda, C.; Castro-Raucci, L.; Oliveira, I.; Raucci-Neto, W. Influence of early mineral deposits of silicate-and aluminate-based cements on push-out bond strength to root dentine. Int. Endod. J. 2018, 51, 92–101. [Google Scholar] [CrossRef]
- Siburian, R.; Simanjuntak, C.; Supeno, M.; Lumbanraja, S.; Sihotang, H. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef]
- Rochman, R.A.; Wahyuningsih, S.; Ramelan, A.H.; Hanif, Q.A. Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012119. [Google Scholar]
- Dong, R.; Wang, L.; Zhu, J.; Liu, L.; Qian, Y. A novel SiO2–GO/acrylic resin nanocomposite: Fabrication, characterization and properties. Appl. Phys. A 2019, 125, 551. [Google Scholar] [CrossRef]
- Kaur, G.; Pickrell, G.; Sriranganathan, N.; Kumar, V.; Homa, D. Review and the state of the art: Sol-gel and melt quenched bioactive glasses for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1248–1275. [Google Scholar] [CrossRef]
- Li, P.; De Groot, K. Better bioactive ceramics through sol-gel process: Code: G2. J. Sol-Gel Sci. Technol. 1994, 2, 797–801. [Google Scholar] [CrossRef]
- Achternbosch, M.; Bräutigam, K.; Hartlieb, N.; Kupsch, C.; Richers, U.; Stemmermann, P.; Gleis, M. Heavy Metals in Cement and Concrete Resulting from the Co-Incineration of Wastes in Cement Kilns with Regard to the Legitimacy of Waste Utilization; Forschungszentrum Karlsruhe GmbH: Karlsruhe, Germany, 2003. [Google Scholar]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.-C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Lv, Y.; Cai, L.; Jiang, W.; Zhou, Y. Influence of graphene oxide on hydration characteristics of tricalcium silicate. Adv. Cem. Res. 2019, 31, 448–456. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Ouyang, D. Effect of graphene oxide on mechanical properties of cement mortar and its strengthening mechanism. Materials 2019, 12, 3753. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Peng, H.; Ge, Y.; Cai, C.; Zhang, Y.; Liu, Z. Mechanical properties and microstructure of graphene oxide cement-based composites. Constr. Build. Mater. 2019, 194, 102–109. [Google Scholar] [CrossRef]
- Song, M.; Yu, B.; Kim, S.; Hayashi, M.; Smith, C.; Sohn, S.; Kim, E.; Lim, J.; Stevenson, R.G.; Kim, R.H. Clinical and molecular perspectives of reparative dentin formation: Lessons learned from pulp-capping materials and the emerging roles of calcium. Dent. Clin. 2017, 61, 93–110. [Google Scholar]
- de Oliveira, N.G.; de Souza Araújo, P.R.; da Silveira, M.T.; Sobral, A.P.V.; Carvalho, M.V. Comparison of the biocompatibility of calcium silicate-based materials to mineral trioxide aggregate: Systematic review. Eur. J. Dent. 2018, 12, 317–326. [Google Scholar] [CrossRef]
- Bjørndal, L.; Simon, S.; Tomson, P.; Duncan, H. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shin, Y.C.; Lee, J.-J.; Bae, E.-B.; Jeon, Y.-C.; Jeong, C.-M.; Yun, M.-J.; Lee, S.-H.; Han, D.-W.; Huh, J.-B. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725. [Google Scholar] [CrossRef] [PubMed]
- Mortada, I.; Mortada, R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Neto, A.H.C.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017, 33, e13–e21. [Google Scholar] [CrossRef] [PubMed]



| Primers | |
|---|---|
| DSPP | F: ATATTGAGGGCTGGAATGGGGA R: TTTGTGGCTCCAGCATTGTCA |
| RUNX2 | F: ACTCTACCACCCCGCTGTC R: CAGAGGTGGCAGTGTCATCA |
| ALP | F: ACGTGGCTAAGAATGTCATC R: CTGGTAGGCGATGTCCTTA |
| GAPDH | F: TGGCACCCAGCACAATGAA R: CTAAGTCATAGTCCGCCTAGAAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, M.M.; Nizami, M.Z.I.; Rajasekar, V.; Basabrain, M.; Lung, C.Y.K.; Yiu, C.K.Y. Enhancing the Physical, Antimicrobial, and Osteo/Odontogenic Properties of a Sol–Gel-Derived Tricalcium Silicate by Graphene Oxide for Vital Pulp Therapies. J. Funct. Biomater. 2024, 15, 193. https://doi.org/10.3390/jfb15070193
Abdalla MM, Nizami MZI, Rajasekar V, Basabrain M, Lung CYK, Yiu CKY. Enhancing the Physical, Antimicrobial, and Osteo/Odontogenic Properties of a Sol–Gel-Derived Tricalcium Silicate by Graphene Oxide for Vital Pulp Therapies. Journal of Functional Biomaterials. 2024; 15(7):193. https://doi.org/10.3390/jfb15070193
Chicago/Turabian StyleAbdalla, Mohamed Mahmoud, Mohammed Zahedul Islam Nizami, Vidhyashree Rajasekar, Mohammed Basabrain, Christie Y. K. Lung, and Cynthia Kar Yung Yiu. 2024. "Enhancing the Physical, Antimicrobial, and Osteo/Odontogenic Properties of a Sol–Gel-Derived Tricalcium Silicate by Graphene Oxide for Vital Pulp Therapies" Journal of Functional Biomaterials 15, no. 7: 193. https://doi.org/10.3390/jfb15070193
APA StyleAbdalla, M. M., Nizami, M. Z. I., Rajasekar, V., Basabrain, M., Lung, C. Y. K., & Yiu, C. K. Y. (2024). Enhancing the Physical, Antimicrobial, and Osteo/Odontogenic Properties of a Sol–Gel-Derived Tricalcium Silicate by Graphene Oxide for Vital Pulp Therapies. Journal of Functional Biomaterials, 15(7), 193. https://doi.org/10.3390/jfb15070193








