Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering
Abstract
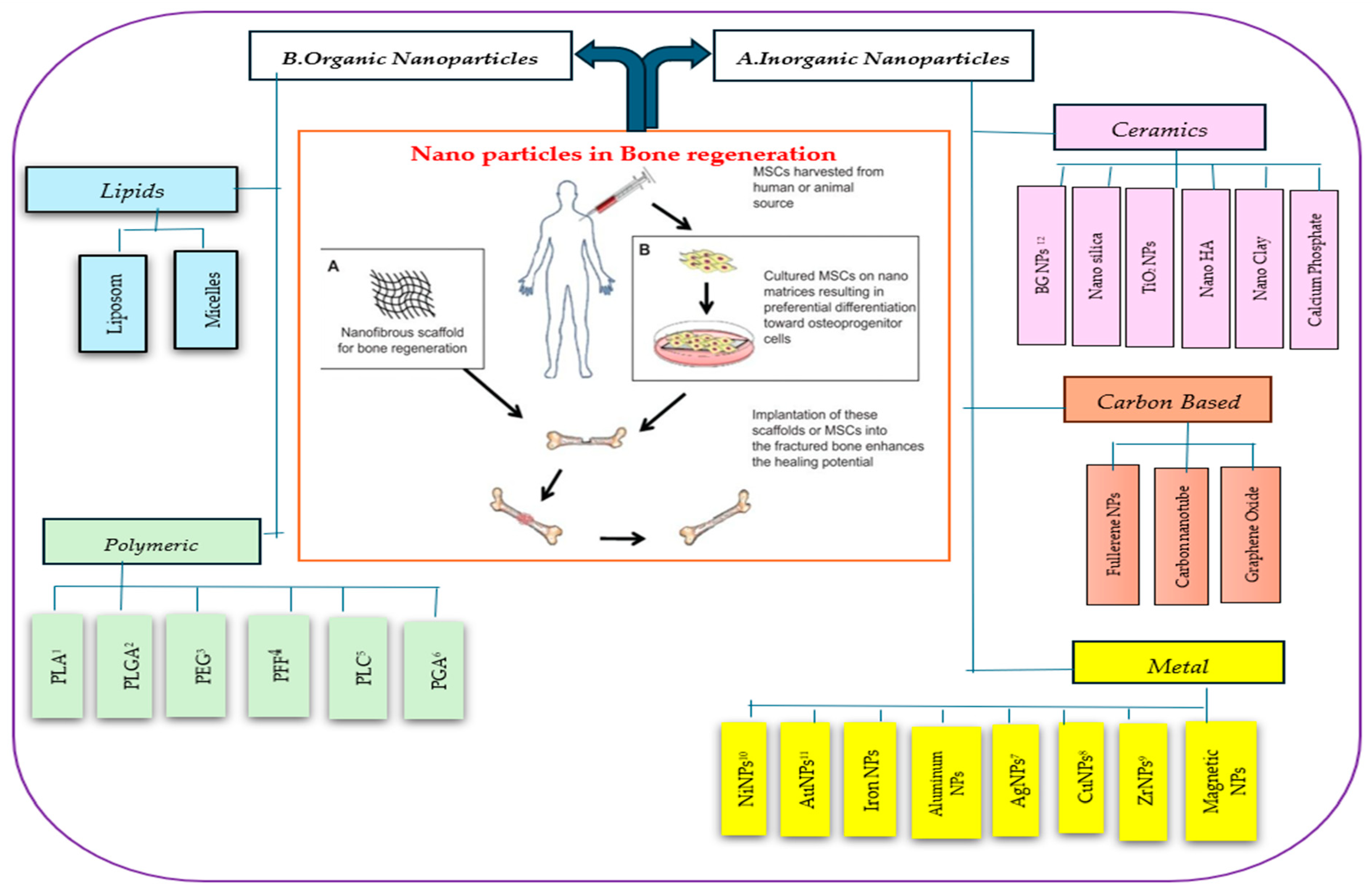
1. Introduction
2. Inorganic NPs
2.1. Ceramic NPs
2.1.1. Nanohydroxyapatite (Nano-HA) for Bone Regeneration in TE
2.1.2. Titanium Oxide Nanotubes in Bone Regeneration
2.1.3. Nanosilica for Bone Regeneration in TE
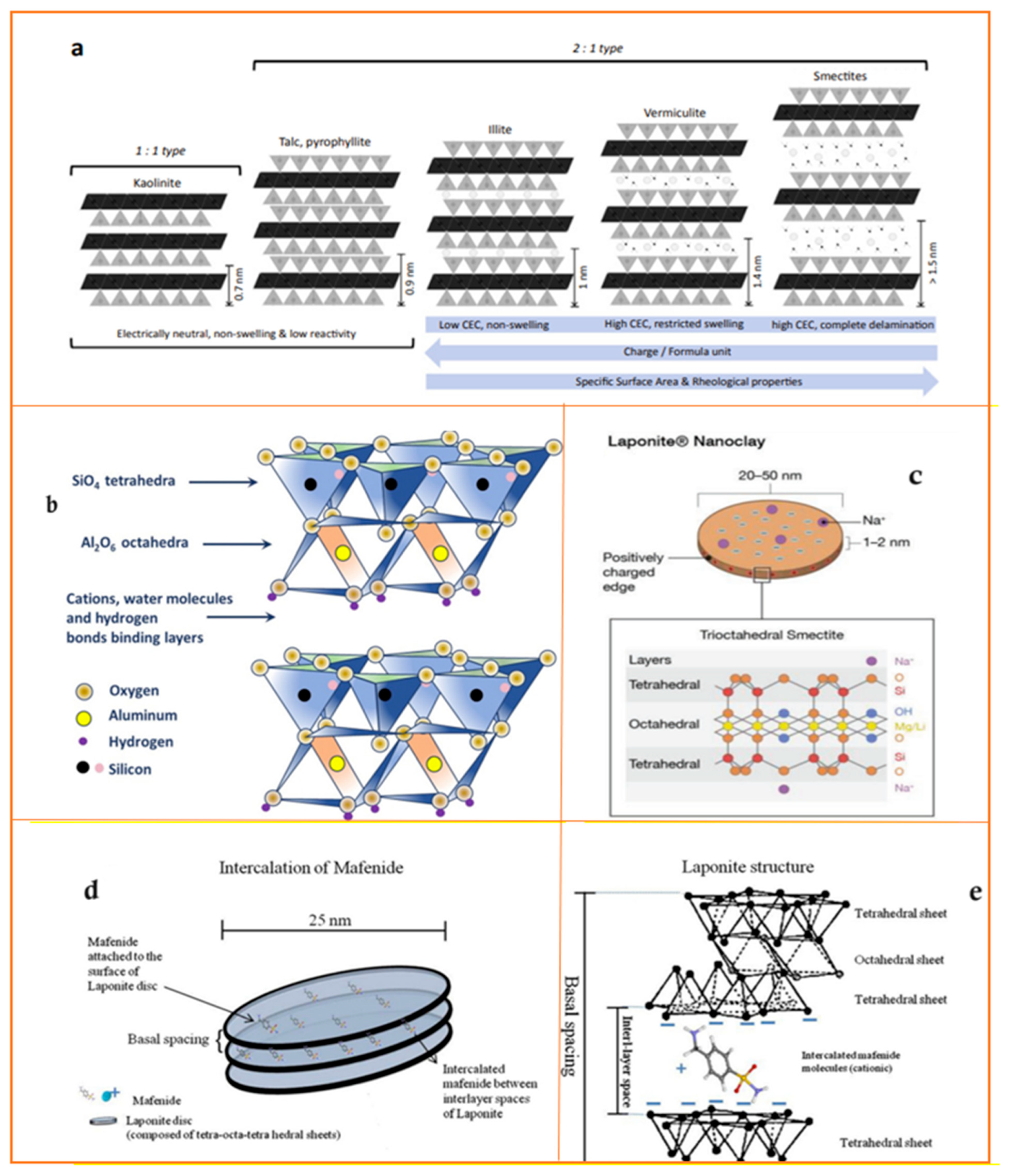
2.1.4. Nanoclay
| Structure of Clay Particles | Nanoclay | Chemical Formula/Nanoclays/ Materials Involved | Layers | Species | Animal Model | Finding Indicated | CEC 1 (meq/100 g) | Particle Size (nm) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MMT 2 | Nam(Al2.-mMgm)Si4O10(OH)2·nH2O | 2:1 | Smectites | ~80–150 | ~80–300 diameter ~1 thickness | [37,47,48] | |||
| Layered | MeGC-MMT hydrogel | Nude mice (8–12 week) | Satisfactory results only by applying the material itself | [36,49] | |||||
| PDLA - 2.5% MMT (w/w rhBMP-2) | Female mice Balb/C | Inconclusive results: 1.Comparable to control 2.Higher bone formation with rhBMP-2 | [36] | ||||||
| Layered | Kaolinite | [Si2Al2O5(OH)4·nH2O (n = 0 or 4) | 1:1 | 2 | ~50 to 600 &internal diameters ~2 to 20 | [37,50] | |||
| Layered | Halloysite | Al2Si2O5(OH)4·nH2O | 1:1 | Serpentine Kaolinite | ~10 | Nanotube diameter of ~50, lumen of ~15, length of ~1 mm | [37,40] | ||
| HNT/Ge/MA hydrogel | Sprague–Dawley rats | One regeneration improved with the presence of HNTs | [36] | ||||||
| Layered | Bentonite | (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O | 2:1 | Smectites | ~70–110 | ~100–500 diameter ~1 thickness | [40,47,48] | ||
| Layered | Laponite R | Nah(Mg3-hLih) Si4O10(OH)2.nH2O | 2:1 | Smectites | ~4–40 | ~25–30 diameter ~1 thickness | [37,40] | ||
| Laponite RD bio ceramic | Female rats and mature male pigs | 1. No obvious toxicity 2. A totally healed bone lesion | [36] | ||||||
| 1.Laponite RD functionalized TBG 2.Laponite RD + alginate gels | Nude mice | 1. Osteoconduction due to BMP-2, not clay-related 2. Gels able to localize BMP-2 to boost bone formation | [36,51] | ||||||
| PEG4K-Laponite RD scaffolds (w/wo ROB) | 12 week-old male Sprague–Dawley rats | 1. Scaffolds + ROB stimulate new bone formation 2. Better osteogenic properties for scaffolds + ROB | [36] | ||||||
| Hyaluronic-bisphosphonate hydrogel/Laponite RD/BMP-2 | MF-1 wild type mice | HABP + Lap + BMP-2 scaffolds presented synergistic effects, resulting in major bone induction in contrast to all controls | [36,52] | ||||||
| Gelatin-derived graphene/Laponite RD (GL-powder) BMP-9 | Athymic nude mice | GL-powder able to enhance BMP9-induced ectopic bone formation from MSCs in comparison with BMP-9 alone | [36,53] | ||||||
| 3D-Scaffold: poly(glycerol sebacate) (PGS)/Laponite RD | Mice | 1. From day 3 inflammatory infiltration at interfaces and at day 6 within scaffold 2. After day 21 degradation without inflammation | [36,54] | ||||||
| Layered | Illite | (K,H)Al2(Si,Al)4O10(OH)2·XH2O | 2:1 | Illite | 15 | 0.075 μm, 0.3 μm, 1.2 μm (trimodal distribution) | [37,50,55] | ||
| Layered | Rectorite | (Na,Ca)Al4(Si,Al)8O20(OH)4·2H2O | 1:1 | Rectorite | ~20–50 | ~200–300 diameter ~1–2 thickness | [37,56] | ||
| Fibrous | Palygorskite | (Mg,Al)2Si4O10(OH)·4(H2O) | Attapulgite | ~4–40 | 25~30 diameter ~1 thickness | [37,40] |
2.1.5. Zirconia
2.1.6. Bioactive Glass (BG) NPs
2.2. Metal NPs
2.2.1. Gold NPs
2.2.2. Silver (Ag) NPs
2.2.3. Iron NPs
2.2.4. Copper NPs
2.2.5. Zirconium NPs
2.2.6. Aluminum NPs
2.2.7. Nickel NPs
2.2.8. Magnetic NPs
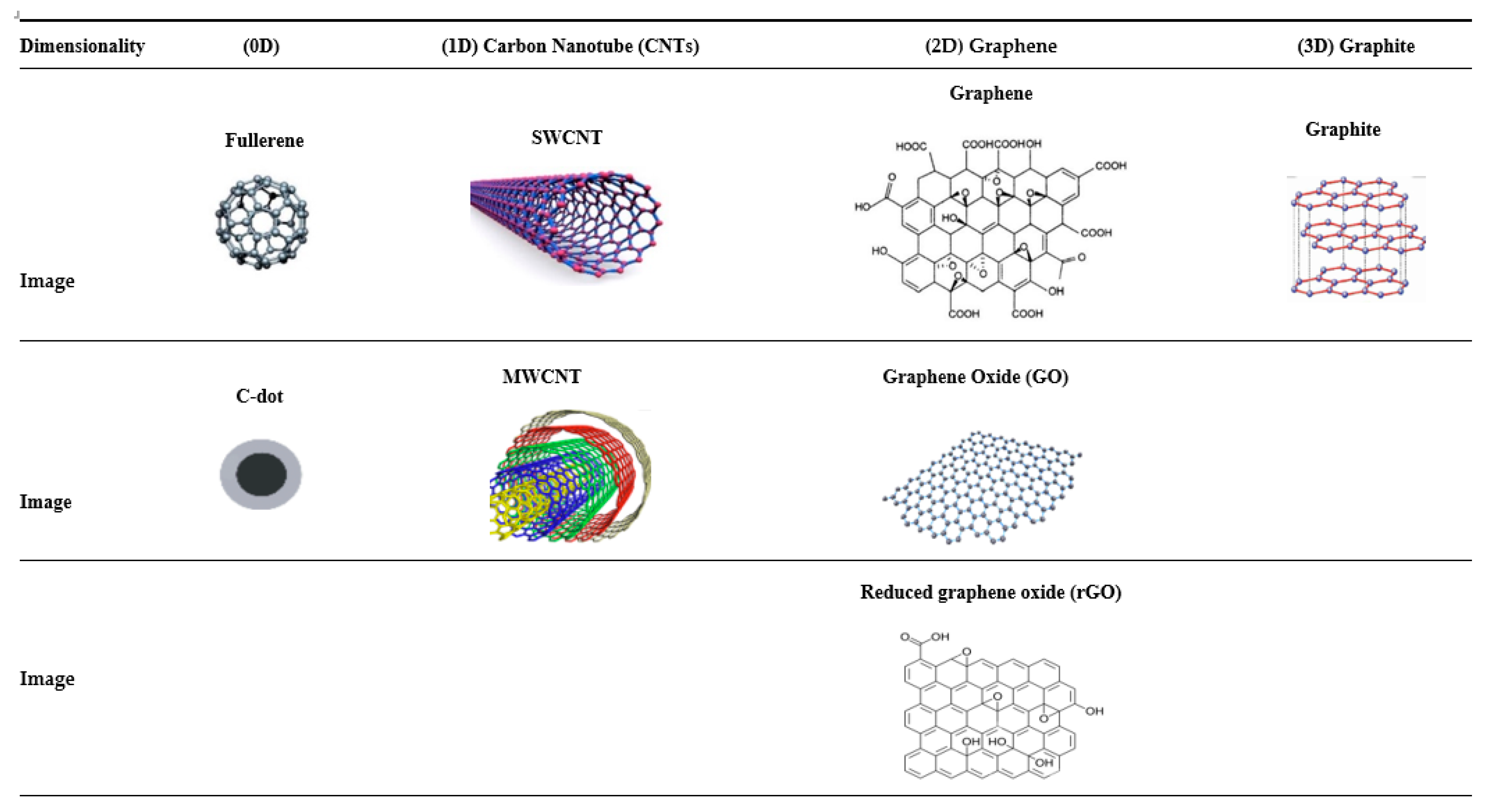
2.3. Carbon-Based NPs
2.3.1. Zero-Dimensional Carbon-Based Nanomaterials for BTE
Carbon Dots (C-Dots)
Fullerene (C60) for BTE
Nanodiamonds (NDs) for BTE
2.3.2. One-Dimensional Carbon-Based Nanomaterials for BTE
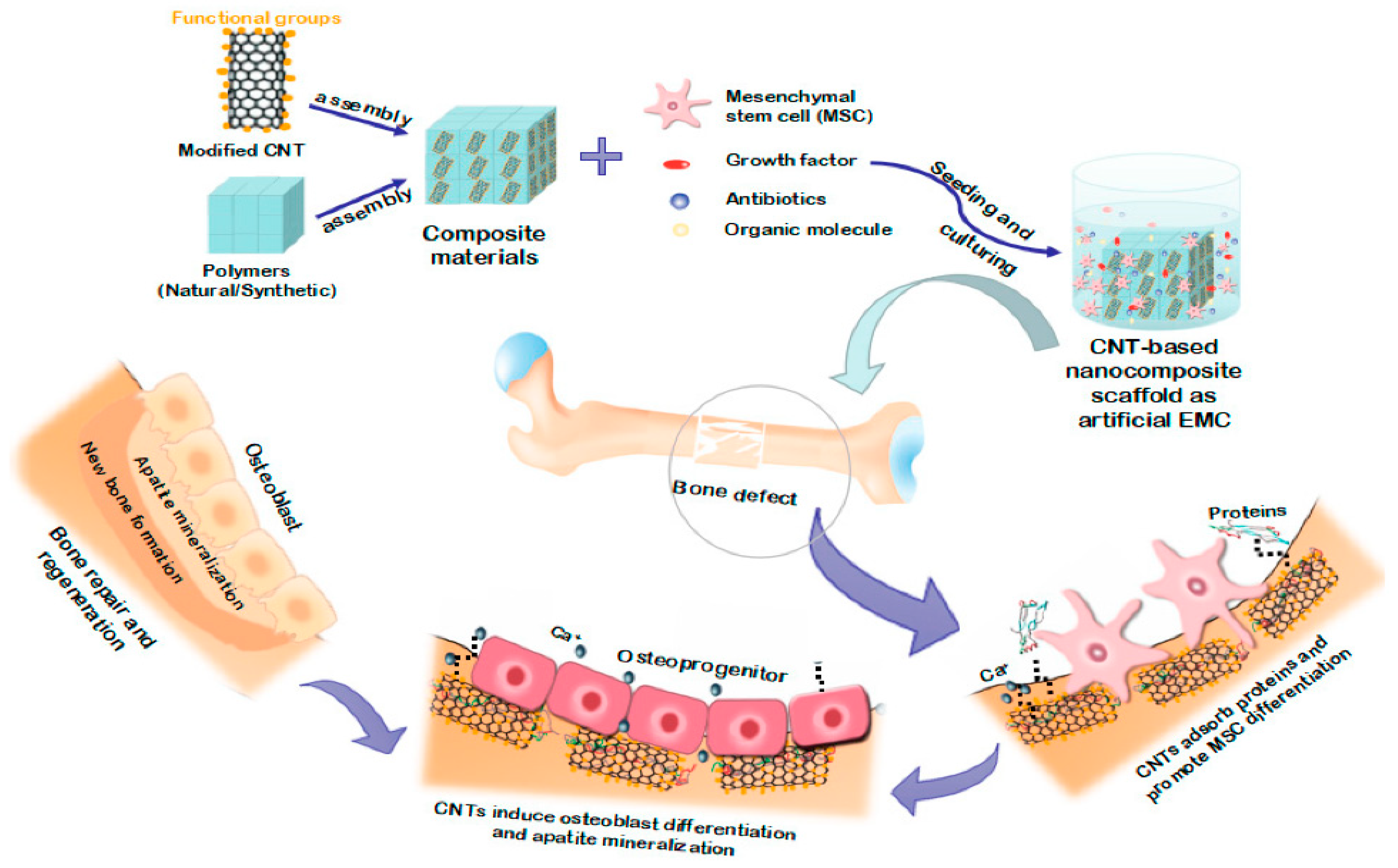
Carbon Nanotubes (CNTs)
2.3.3. Two-Dimensional Carbon-Based Nanomaterials for BTE
Graphene and Graphene Oxide (GO)
Reduced Graphene Oxide (rGO)
Three-Dimensional Carbon-Based Nanomaterials for BTE
3. Organic NPs
3.1. Synthetic Polymers
3.1.1. Poly(Lactic Acid) PLA in Bone Regeneration
3.1.2. Poly (Lactic-Co-Glycolic Acid) (PLGA)
3.1.3. Polyethylene Glycol (PEG)
3.1.4. Poly(Propylene Fumarate) (PPF)
3.1.5. Poly(ε-Caprolactone) (PCL) in Bone Regeneration
3.1.6. Poly(Glycolic Acid) (PGA)
3.2. Chitosan NPs for Bone Regeneration
4. Conclusions
Funding
Conflicts of Interest
References
- Tsuchida, S.; Nakayama, T. Recent Clinical Treatment and Basic Research on the Alveolar Bone. Biomedicines 2023, 11, 843. [Google Scholar] [CrossRef]
- Montemurro, N.; Pierozzi, E.; Inchingolo, A.M.; Pahwa, B.; De Carlo, A.; Palermo, A.; Scarola, R.; Dipalma, G.; Corsalini, M.; Inchingolo, A.D.; et al. New biograft solution, growth factors and bone regenerative approaches in neurosurgery, dentistry, and orthopedics: A review. Rev. Med. Pharmacol. Sci. 2023, 16, 7653–7664. [Google Scholar] [CrossRef]
- Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering 2023, 10, 85. [Google Scholar] [CrossRef]
- Feroz, S.; Cathro, P.; Ivanovski, S.; Muhammad, N. Biomimetic bone grafts and substitutes: A review of recent advancements and applications. Biomed. Eng. Adv. 2023, 6, 100107. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Shojaei, S.; Goodarzi, V.; Khonakdar, H.A.; Abdouss, M. Tuning properties of bio-rubbers and its nanocomposites with addition of succinic acid and ɛ-caprolactone monomers to poly (glycerol sebacic acid) as main platform for application in tissue engineering. Eur. Polym. J. 2021, 159, 110711. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, W.; Moghadas, B.K.; Baneshi, N.; Noshadi, B.; Baghaei, S.; Dehkordi, D.A. Review of recent advances in bone scaffold fabrication methods for tissue engineering for treating bone diseases and injuries. Tissue Cell 2024, 88, 102390. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Kouhjani, M.; Mohammadi, M.; Hosta-Rigau, L. Novel scaffold platforms for simultaneous induction osteogenesis and angiogenesis in bone tissue engineering: A cutting-edge approach. J. Nanobiotechnol. 2023, 21, 351. [Google Scholar] [CrossRef]
- Sagadevan, S.; Schirhagl, R.; Rahman, Z.; Bin Ismail, M.F.; Lett, J.A.; Fatimah, I.; Kaus, N.H.M.; Oh, W.-C. Recent advancements in polymer matrix nanocomposites for bone tissue engineering applications. J. Drug Deliv. Sci. Technol. 2023, 82, 104313. [Google Scholar] [CrossRef]
- Tassinari, R.; Olivi, E.; Cavallini, C.; Taglioli, V.; Zannini, C.; Marcuzzi, M.; Fedchenko, O.; Ventura, C. Mechanobiology: A landscape for reinterpreting stem cell heterogeneity and regenerative potential in diseased tissues. iScience 2023, 26, 105875. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023, 162, 157–178. [Google Scholar] [CrossRef]
- Górnicki, T.; Lambrinow, J.; Golkar-Narenji, A.; Data, K.; Domagała, D.; Niebora, J.; Farzaneh, M.; Mozdziak, P.; Zabel, M.; Antosik, P.; et al. Biomimetic Scaffolds—A Novel Approach to Three Dimensional Cell Culture Techniques for Potential Implementation in Tissue Engineering. Nanomaterials 2024, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Cai, D.; Gao, W.; He, R.; Li, Y.; Zhou, Y.; Klein, T.; Xiao, L.; Xiao, Y. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials 2023, 13, 692. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. Tissue Engineering and Regenerative Medicine. In Nanotoxicology in Nanobiomedicine; Springer International Publishing: Cham, Switzerland, 2023; pp. 125–141. [Google Scholar]
- Bhaladhare, S.; Bhattacharjee, S. Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol. 2023, 226, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Kulyar, M.F.; Farooq, U.; Ashar, A.; Mahfooz, A.; Kanwal, A.; Akhtar, M.; Asif, M.; Nawaz, S.; Li, K. Applications of functionalized nanoparticles in tissue engineering. In Antiviral and Antimicrobial Coatings Based on Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 485–513. [Google Scholar]
- Álvarez-Chimal, R.; Arenas-Alatorre, J.; Álvarez-Pérez, M.A. Nanoparticle-polymer composite scaffolds for bone tissue engineering. A review. Eur. Polym. J. 2024, 213, 113093. [Google Scholar] [CrossRef]
- Laein, S.S.; Katouzian, I.; Mozafari, M.R.; Farnudiyan-Habibi, A.; Akbarbaglu, Z.; Shadan, M.R.; Sarabandi, K. Biological and thermodynamic stabilization of lipid-based delivery systems through natural biopolymers; controlled release and molecular dynamics simulations. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–20. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Das, R.; Adhikari, M.D.; Chauhan, P.S. Future Directions in Nanomaterials Research for Biological Applications. Nanopart. Toxic. Compat. 2024, 161, 1–26. [Google Scholar]
- Grewal, N.S.; Batra, U.; Kumar, K. Polymers for Biomedical Application. In Advanced Materials for Biomedical Applications; Rajput, V.S., Bhinder, J., Eds.; Biomedical Materials for Multi-functional Applications; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Tautzenberger, A.; Kovtun, A.; Ignatius, A. Nanoparticles and their potential for application in bone. Int. J. Nanomed. 2012, 7, 4545–4557. [Google Scholar] [CrossRef]
- Sahai, N.; Ahmad, N.; Gogoi, M. Nanoparticles Based Drug Delivery for Tissue Regeneration Using Biodegradable Scaffolds: A Review. Curr. Pathobiol. Rep. 2018, 6, 219–224. [Google Scholar] [CrossRef]
- Yeo, T.; Ko, Y.G.; Kim, E.J.; Kwon, O.K.; Chung, H.Y.; Kwon, O.H. Promoting bone regeneration by 3D-printed poly (glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 2021, 94, 343–351. [Google Scholar] [CrossRef]
- Pokhrel, S. Hydroxyapatite: Preparation, properties and its biomedical applications. Adv. Chem. Eng. Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C 2017, 70, 736–744. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef]
- Abere, D.V.; Ojo, S.A.; Oyatogun, G.M.; Paredes-Epinosa, M.B.; Niluxsshun, M.C.D.; Hakami, A. Mechanical and morphological characterization of nano-hydroxyapatite (nHA) for bone regeneration: A mini review. Biomed. Eng. Adv. 2022, 4, 100056. [Google Scholar] [CrossRef]
- de Souza, W.; Gemini-Piperni, S.; Grenho, L.; Rocha, L.A.; Granjeiro, J.M.; Melo, S.A.; Fernandes, M.H.; Ribeiro, A.R. Titanium dioxide nanoparticles affect osteoblast-derived exosome cargos and impair osteogenic differentiation of human mesenchymal stem cells. Biomater. Sci. 2023, 11, 2427–2444. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Chang, Y.; Shao, Y.; Liu, Y.; Xia, R.; Tong, Z.; Zhang, J.; Zhai, Z.; Cheng, W.; Li, H. Mechanical strain promotes osteogenic differentiation of mesenchymal stem cells on TiO2 nanotubes substrate. Biochem. Biophys. Res. Commun. 2019, 511, 840–846. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Wu, K.; Chen, Z.; Wang, X.; Lin, C.; Lai, Y. Progress in TiO2nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef] [PubMed]
- Cuylear, D.L.; Elghazali, N.A.; Kapila, S.D.; Desai, T.A. Calcium Phosphate Delivery Systems for Regeneration and Biomineralization of Mineralized Tissues of the Craniofacial Complex. Mol. Pharm. 2023, 20, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2021, 29, 509–527. [Google Scholar] [CrossRef]
- Erezuma, I.; Eufrasio-Da-Silva, T.; Golafshan, N.; Deo, K.; Mishra, Y.K.; Castilho, M.; Gaharwar, A.K.; Leeuwenburgh, S.; Dolatshahi-Pirouz, A.; Orive, G. Nanoclay Reinforced Biomaterials for Mending Musculoskeletal Tissue Disorders. Adv. Healthc. Mater. 2021, 10, 2100217. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S.; Jasuja, H.; Jaswandkar, S.V.; Mohanty, S.; Katti, D.R. Nanoclays in medicine: A new frontier of an ancient medical practice. Mater. Adv. 2022, 3, 7484–7500. [Google Scholar] [CrossRef]
- Satish, S.; Tharmavaram, M.; Rawtani, D. Halloysite nanotubes as a nature’s boon for biomedical applications. Nanobiomedicine 2019, 6, 1849543519863625. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.; Avery, R.K.; Moghaddam, K.M.; Haghiniaz, R.; Yalcintas, E.P.; de Barros, N.R.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials for Drug Delivery. Adv. Healthc. Mater. 2022, 11, e2102054. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef]
- Wu, M.; Chen, F.; Wu, P.; Yang, Z.; Zhang, S.; Xiao, L.; Deng, Z.; Zhang, C.; Chen, Y.; Cai, L. Nanoclay mineral-reinforced macroporous nanocomposite scaffolds for in situ bone regeneration: In vitro and in vivo studies. Mater. Des. 2021, 205, 109734. [Google Scholar] [CrossRef]
- Avinash, A.H.; Katti, K.S.; Katti, D. Nanoclay based composite scaffolds for bone tissue engineering applications. J. Nanotechnol. Eng. Med. 2010, 1, 031013. [Google Scholar]
- Zhang, Y.; Ma, A.; Sun, L.; Chen, J.; Hong, G.; Wu, H. Nanoclay-Modified Hyaluronic Acid Microspheres for Bone Induction by Sustained rhBMP-2 Delivery. Macromol. Biosci. 2024, 24, e2300245. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Borges, J.P.; Costa, L.C.; Silva, J.C.; Soares, P.I.P. Clay-Based Nanocomposite Hydrogels for Biomedical Applications: A Review. Nanomaterials 2022, 12, 3308. [Google Scholar] [CrossRef]
- Baker, K.C.; Maerz, T.; Saad, H.; Shaheen, P.; Kannan, R.M. In vivo bone formation by and inflammatory response to resorbable polymer-nanoclay constructs. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1871–1881. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Zou, Y.; Qazvini, N.T.; Zane, K.; Sadati, M.; Wei, Q.; Liao, J.; Fan, J.; Song, D.; Liu, J.; Ma, C.; et al. Gelatin-Derived Graphene–Silicate Hybrid Materials Are Biocompatible and Synergistically Promote BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 15922–15932. [Google Scholar] [CrossRef]
- Li, P.-R.; Wei, J.-C.; Chiu, Y.-F.; Su, H.-L.; Peng, F.-C.; Lin, J.-J. Evaluation on Cytotoxicity and Genotoxicity of the Exfoliated Silicate Nanoclay. ACS Appl. Mater. Interfaces 2010, 2, 1608–1613. [Google Scholar] [CrossRef]
- Taunton, A.E.; Gunter, M.E.; Nolan, R.P.; Phillips, J.I. Characterization of minerals in pleural plaques from lung tissue of nonhuman primates. Period. Di Mineral. 2011, 80, 167–179. [Google Scholar]
- Rodrigues, L.A.d.S.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.C.; Filho, E.C.d.S.; Leite, C.M.d.S. The systems containing clays and clay minerals from modified drug release: A review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef]
- Gibbs, D.; Black, C.; Hulsart-Billstrom, G.; Shi, P.; Scarpa, E.; Oreffo, R.; Dawson, J. Bone induction at physiological doses of BMP through localization by clay nanoparticle gels. Biomaterials 2016, 99, 16–23. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Yang, X.; Shi, L.; Lanham, S.A.; Hilborn, J.; Oreffo, R.O.C.; Ossipov, D.; Dawson, J.I. Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization. Nat. Commun. 2020, 11, 1365. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 590–600. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Bannunah, A.M. Biomedical Applications of Zirconia-Based Nanomaterials: Challenges and Future Perspectives. Molecules 2023, 28, 5428. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. The silica-based formulations for drug delivery, bone treatment, and bone regeneration. ChemBioEng Rev. 2016, 3, 229–246. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.; Mo, A. Zirconia Based Dental Biomaterials: Structure, Mechanical Properties, Biocompatibility, Surface Modification, and Applications as Implant. Front. Dent. Med. 2021, 2, 689198. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 12, 1687–1714. [Google Scholar] [CrossRef]
- Wang, G.; Meng, F.; Ding, C.; Chu, P.K.; Liu, X. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010, 6, 990–1000. [Google Scholar] [CrossRef]
- Bhowmick, A.; Jana, P.; Pramanik, N.; Mitra, T.; Banerjee, S.L.; Gnanamani, A.; Das, M.; Kundu, P.P. Multifunctional zirconium oxide doped chitosan based hybrid nanocomposites as bone tissue engineering materials. Carbohydr. Polym. 2016, 151, 879–888. [Google Scholar] [CrossRef]
- Afzal, A. Implantable zirconia bioceramics for bone repair and replacement: A chronological review. Mater. Express 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Wu, G.; Li, P.; Feng, H.; Zhang, X.; Chu, P.K. Engineering and functionalization of biomaterials via surface modification. J. Mater. Chem. B 2015, 3, 2024–2042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, S.; Ning, C.; Liu, X. rBMSC and bacterial responses to isoelastic carbon fiber-reinforced poly(ether-ether-ketone) modified by zirconium implantation. J. Mater. Chem. B 2016, 4, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Lakshmi, A.M.; Murimadugula, S.; Rao, P.V.; Chitra, S.; Perumal, G.; Doble, M.; Kumari, K.; Özcan, M.; Madaboosi, N.; et al. Texturally-enhanced 55S0P and 45S10P Bioactive Glass ceramic particles: Sol-gel fabrication, nano-characterization and comprehensive Bio-evaluation for applications in Bone tissue engineering. Ceram. Int. 2024, 50, 30699–30711. [Google Scholar] [CrossRef]
- Valenzuela, F.; Covarrubias, C.; Martínez, C.; Smith, P.; Díaz-Dosque, M.; Yazdani-Pedram, M. Preparation and bioactive properties of novel bone-repair bionanocomposites based on hydroxyapatite and bioactive glass nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1672–1682. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. Part A 2009, 88, 304–313. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sathiyasree, M.; Rahim, S.B.; Renitta, R.E.; Kasipandian, K.; Krithika Shree, S.; Rajalakshmi, D.; Shobana, N.; Dhiva, S.; Abirami, S.; et al. Scaffold using chitosan, agarose, cellulose, dextran and protein for tissue engineering—A review. Polymers 2023, 15, 1525. [Google Scholar] [CrossRef]
- Woźniak-Budych, M.J.; Staszak, K.; Staszak, M. Copper and Copper-Based Nanoparticles in Medicine—Perspectives and Challenges. Molecules 2023, 28, 6687. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mishra, A.; Singh, D.; Li, C.; Srivastava, P. In-Vitro Studies on Copper Nanoparticles and Nano-hydroxyapatite Infused Biopolymeric Composite Scaffolds for Bone Bioengineering Applications. Biotechnol. Bioprocess Eng. 2023, 28, 162–180. [Google Scholar] [CrossRef]
- Qiao, M.; Tang, W.; Xu, Z.; Wu, X.; Huang, W.; Zhu, Z.; Wan, Q. Gold nanoparticles: Promising biomaterials for osteogenic/adipogenic regulation in bone repair. J. Mater. Chem. B 2023, 11, 2307–2333. [Google Scholar] [CrossRef]
- Yoshida, Y.G.; Yan, S.; Xu, H.; Yang, J. Novel metal nanomaterials to promote angiogenesis in tissue regeneration. Eng. Regen. 2023, 4, 265–276. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Fardjahromi, M.A.; Nazari, H.; Tafti, S.A.; Razmjou, A.; Mukhopadhyay, S.; Warkiani, M. Metal-organic framework-based nanomaterials for bone tissue engineering and wound healing. Mater. Today Chem. 2022, 23, 100670. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2011, 41, 2256–2282. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Coradeghini, R.; Gioria, S.; García, C.P.; Nativo, P.; Franchini, F.; Gilliland, D.; Ponti, J.; Rossi, F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013, 217, 205–216. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Q.; Liu, Q.; Li, Y.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018, 99, 450–457. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, S.Y.; Song, M.S.; Ryu, P.D.; Joo, S.-W.; Lam, A.T.N. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C 2014, 42, 70–77. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular Uptake and Cytotoxicity of Gold Nanorods: Molecular Origin of Cytotoxicity and Surface Effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and biological characterization of the mixture of poly(lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomed. 2019, 14, 483–498. [Google Scholar] [CrossRef]
- He, J.; He, F.-L.; Li, D.-W.; Liu, Y.-L.; Yin, D.-C. A novel porous Fe/Fe-W alloy scaffold with a double-layer structured skeleton: Preparation, in vitro degradability and biocompatibility. Colloids Surf. B Biointerfaces 2016, 142, 325–333. [Google Scholar] [CrossRef]
- Akturk, A.; Taygun, M.E.; Goller, G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polym. Compos. 2020, 41, 2411–2421. [Google Scholar] [CrossRef]
- Saini, R.K.; Bagri, L.P.; Bajpai, A. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces 2019, 177, 211–218. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Cui, X.; Pan, Y.; Huang, W.; Ye, S.; Luo, S.; Rahaman, M.N.; Zhang, C.; Wang, D. Evaluation of three-dimensional silver-doped borate bioactive glass scaffolds for bone repair: Biodegradability, biocompatibility, and antibacterial activity. J. Mater. Res. 2015, 30, 2722–2735. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A.; et al. Towards the Design of 3D Fiber-Deposited Poly (-caprolactone)/Iron-Doped Hydroxyapatite Nanocomposite Magnetic Scaffolds for Bone Regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Yusop, A.H.M.; Ulum, M.F.; Al Sakkaf, A.; Hartanto, D.; Nur, H. Insight into the bioabsorption of Fe-based materials and their current developments in bone applications. Biotechnol. J. 2021, 16, 2100255. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Dhivya, S.; Ajita, J.; Selvamurugan, N. Metallic Nanomaterials for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2015, 11, 1675–1700. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Muszyński, S.; Ognik, K.; Dobrowolski, P.; Kwiecień, M.; Juśkiewicz, J.; Chocyk, D.; Świetlicki, M.; Blicharski, T.; Gładyszewska, B. Comparison of the effect of dietary copper nanoparticles with copper (II) salt on bone geometric and structural parameters as well as material characteristics in a rat model. J. Trace Elem. Med. Biol. 2017, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hejazy, M.; Koohi, M.K.; Bassiri Mohamad Pour, A.; Najafi, D. Toxicity of manufactured copper nanoparticles—A review. Nanomed. Res. J. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Vodyashkin, A.; Stoinova, A.; Kezimana, P. Promising biomedical systems based on copper nanoparticles: Synthesis, Characterization, and Applications. Colloids Surf. B Biointerfaces 2024, 237, 113861. [Google Scholar] [CrossRef]
- Jaidev, L.; Kumar, S.; Chatterjee, K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf. B Biointerfaces 2017, 159, 293–302. [Google Scholar] [CrossRef]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. [Google Scholar] [CrossRef]
- Khan, M.M.; Deen, K.M.; Haider, W. Combinatorial development and assessment of a Zr-based metallic glass for prospective biomedical applications. J. Non-Cryst. Solids 2019, 523, 119544. [Google Scholar] [CrossRef]
- Maghsoudlou, M.A.; Nassireslami, E.; Saber-Samandari, S.; Khandan, A. Bone Regeneration Using Bio-Nanocomposite Tissue Reinforced with Bioactive Nanoparticles for Femoral Defect Applications in Medicine. Avicenna J. Med. Biotechnol. 2020, 12, 68–76. [Google Scholar]
- Altuna, P.; Lucas-Taulé, E.; Gargallo-Albiol, J.; Figueras-Álvarez, O.; Hernández-Alfaro, F.; Nart, J. Clinical evidence on titanium–zirconium dental implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 842–850. [Google Scholar] [CrossRef]
- Al-Khateeb, A.; Al-Hassani, E.S.; Jabur, A.R. Metallic Implant Surface Activation through Electrospinning Coating of Nanocomposite Fiber for Bone Regeneration. Int. J. Biomater. 2023, 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, F.; Morrow, B.R.; Jiang, S.; Hottel, T.L.; Garcia-Godoy, F.; Hong, L. A novel antimicrobial and remineralizing toothpaste containing CaCl2/chitosan microspheres. Am. J. Dent. 2018, 31, 149–154. [Google Scholar]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Rajendran, V.; Kannan, N. Effect of contact angle, zeta potential and particles size on the in vitro studies of Al2O3 and SiO2 nanoparticles. IET Nanobiotechnol. 2016, 9, 27–34. [Google Scholar] [CrossRef]
- Park, J.; von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef]
- Restrepo, N.; Lopera, A.; Claudia, G.; Villegas, P.; Arroyave, J. VI Latin American Congress on Biomedical Engineering CLAIB 2014, Paraná, Argentina 29, 30; 31 October 2014; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Yu, B.; Fu, S.; Kang, Z.; Zhu, M.; Ding, H.; Luo, T.; Zhu, Y.; Zhang, Y. Enhanced bone regeneration of 3D printed β-Ca2SiO4 scaffolds by aluminum ions solid solution. Ceram. Int. 2020, 46, 7783–7791. [Google Scholar] [CrossRef]
- Kokorev, O.V.; Hodorenko, V.N.; Chekalkin, T.L.; Kim, J.-S.; Kang, S.-B.; Dambaev, G.T.; Gunther, V.E. In vitro and in vivo evaluation of porous TiNi-based alloy as a scaffold for cell tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 704–709. [Google Scholar] [CrossRef]
- Priya, B.A.; Senthilguru, K.; Agarwal, T.; Narayana, S.N.G.H.; Giri, S.; Pramanik, K.; Pal, K.; Banerjee, I. Nickel doped nanohydroxyapatite: Vascular endothelial growth factor inducing biomaterial for bone tissue engineering. RSC Adv. 2015, 5, 72515–72528. [Google Scholar] [CrossRef]
- Song, F.; Jie, W.; Zhang, T.; Li, W.; Jiang, Y.; Wan, L.; Liu, W.; Li, X.; Liu, B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016, 6, 92804–92812. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P. Enriched mechanical, thermal, and blood compatibility of single stage electrospun polyurethane nickel oxide nanocomposite for cardiac tissue engineering. Polym. Compos. 2019, 40, 2381–2390. [Google Scholar] [CrossRef]
- Pardo, A.; Gómez-Florit, M.; Barbosa, S.; Taboada, P.; Domingues, R.M.A.; Gomes, M.E. Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano 2021, 15, 175–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zheng, C.; Huang, N.; Chen, X.; Zhu, X.; Zhao, Y.; Yu, Q.; Liu, J. Ru nanoparticles coated with γ-Fe2O3 promoting and monitoring the differentiation of human mesenchymal stem cells via MRI tracking. Colloids Surf. B Biointerfaces 2018, 170, 701–711. [Google Scholar] [CrossRef]
- Kim, J.-J.; Singh, R.K.; Seo, S.-J.; Kim, T.-H.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. Magnetic scaffolds of polycaprolactone with functionalized magnetite nanoparticles: Physicochemical, mechanical, and biological properties effective for bone regeneration. RSC Adv. 2014, 4, 17325–17336. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef] [PubMed]
- García, R.S.; Stafford, S.; Gun’ko, Y.K. Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications. Appl. Sci. 2018, 8, 172. [Google Scholar] [CrossRef]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Xie, M.; Gong, T.; Wang, Y.; Li, Z.; Lu, M.; Luo, Y.; Min, L.; Tu, C.; Zhang, X.; Zeng, Q.; et al. Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors. Int. J. Mol. Sci. 2024, 25, 4139. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rahmani, A.; Tahmasebi, E.; Tebyanian, H.; Yazdanian, A.; Mosaddad, S.A. Current and Advanced Nanomaterials in Dentistry as Regeneration Agents: An Update. Mini-Rev. Med. Chem. 2021, 21, 899–918. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, e1901495. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Qu, D.; Wang, X.; Bao, Y.; Sun, Z. Recent advance of carbon dots in bio-related applications. J. Phys. Mater. 2020, 3, 022003. [Google Scholar] [CrossRef]
- DuMez, R.; Miyanji, E.H.; Corado-Santiago, L.; Barrameda, B.; Zhou, Y.; Hettiarachchi, S.D.; Leblanc, R.M.; Skromne, I. In vivo characterization of carbon dots-bone interactions: Toward the development of bone-specific nanocarriers for drug delivery. Drug Deliv. 2021, 28, 1281–1289. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.L.; Leblanc, R.M. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Kumar, V.B.; Karasik, D.; Gedanken, A. Fluorescent Nanoparticles with Tissue-Dependent Affinity for Live Zebrafish Imaging. ACS Appl. Mater. Interfaces 2017, 9, 18557–18565. [Google Scholar] [CrossRef]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent advances in bioapplications of C-dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Doshi, M.; Treglown, K.; Copik, A.; Gesquiere, A.J. Composite Conjugated Polymer/Fullerene Nanoparticles as Sensitizers in Photodynamic Therapy for Cancer. BioNanoScience 2014, 4, 15–26. [Google Scholar] [CrossRef]
- Yudoh, K.; Karasawa, R.; Masuko, K.; Kato, T. Water-soluble fullerene (C60) inhibits the osteoclast differentiation and bone destruction in arthritis. Int. J. Nanomed. 2009, 4, 233–239. [Google Scholar] [CrossRef]
- Gonzalez, K.A.; Wilson, L.J.; Wu, W.; Nancollas, G.H. Synthesis and in vitro characterization of a tissue-selective fullerene: Vectoring C60 (OH) 16AMBP to mineralized bone. Bioorg. Med. Chem. 2002, 10, 1991–1997. [Google Scholar] [CrossRef]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-Aligned Fullerene Nanowhiskers as a Scaffold for Orienting Cell Growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef]
- Adel, M.; Keyhanvar, P.; Zare, I.; Tavangari, Z.; Akbarzadeh, A.; Zahmatkeshan, M. Nanodiamonds for tissue engineering and regeneration. J. Drug Deliv. Sci. Technol. 2023, 90, 105130. [Google Scholar] [CrossRef]
- Neburkova, J.; Vavra, J.; Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 43–53. [Google Scholar] [CrossRef]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef]
- Ibrahim, M.; Xue, Y.; Ostermann, M.; Sauter, A.; Steinmueller-Nethl, D.; Schweeberg, S.; Krueger, A.; Cimpan, M.R.; Mustafa, K. In vitro cytotoxicity assessment of nanodiamond particles and their osteogenic potential. J. Biomed. Mater. Res. Part A 2018, 106, 1697–1707. [Google Scholar] [CrossRef]
- Alexander, E.; Leong, K.W. Nanodiamonds in biomedical research: Therapeutic applications and beyond. PNAS Nexus 2024, 3, 198. [Google Scholar] [CrossRef]
- Bacakova, L.; Broz, A.; Liskova, J.; Stankova, L.; Potocky, S.; Kromka, A. The application of nanodiamond in biotechnology and tissue engineering. In Diamond and Carbon Composites and Nanocomposites; IntechOpen Limited: London, UK, 2016; pp. 59–88. [Google Scholar]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef]
- Mikael, P.E.; Nukavarapu, S.P. Functionalized Carbon Nanotube Composite Scaffolds for Bone Tissue Engineering: Prospects and Progress. J. Biomater. Tissue Eng. 2011, 1, 76–85. [Google Scholar] [CrossRef]
- Newman, P.; Minett, A.; Ellis-Behnke, R.; Zreiqat, H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1139–1158. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barzegari, A.; Barar, J.; Omidian, H.; Omidi, Y. Recent advances in polymeric scaffolds containing carbon nanotube and graphene oxide for cartilage and bone regeneration. Mater. Today Commun. 2021, 26, 102097. [Google Scholar] [CrossRef]
- Xu, J.L.; Khor, K.A.; Sui, J.J.; Chen, W.N. Preparation and characterization of a novel hydroxyapatite/carbon nanotubes composite and its interaction with osteoblast-like cells. Mater. Sci. Eng. C 2009, 29, 44–49. [Google Scholar] [CrossRef]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering? Tissue Eng. Part B Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef]
- Matić, A.; Sher, E.K.; Farhat, E.K.; Sher, F. Nanostructured Materials for Drug Delivery and Tissue Engineering Applications. Mol. Biotechnol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D.; et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef]
- Duan, S.; Yang, X.; Mei, F.; Tang, Y.; Li, X.; Shi, Y.; Mao, J.; Zhang, H.; Cai, Q. Enhanced osteogenic differentiation of mesenchymal stem cells on poly(l-lactide) nanofibrous scaffolds containing carbon nanomaterials. J. Biomed. Mater. Res. Part A 2015, 103, 1424–1435. [Google Scholar] [CrossRef]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L.; Dhert, W.J.; de Bruijn, J.D.; Dalmeijer, R.A.; Leenders, H.; van Blitterswijk, C.A.; Verbout, A.J. Critical size defect in the goat’s os ilium. A model to evaluate bone grafts and substitutes. Clin. Orthop. Relat. Res. 1999, 364, 231–239. [Google Scholar] [CrossRef]
- Van Der Donk, S.; Buma, P.; Aspenberg, P.; Schreurs, B.W. Similarity of bone ingrowth in rats and goats: A bone chamber study. Comp. Study 2001, 51, 336–340. [Google Scholar]
- Xu, W.; Ganz, C.; Weber, U.; Adam, M.; Holzhüter, G.; Wolter, D.; Frerich, B.; Vollmar, B.; Gerber, T. Evaluation of injectable silica-embedded nanohydroxyapatite bone substitute in a rat tibia defect model. Int. J. Nanomed. 2011, 6, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.R.N.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kim, J.; Kim, S.E.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Lee, J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen. Biomater. 2017, 4, 159–166. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, W.-B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Sabela, M.I.; Bisetty, K. Insight into the biosensing of graphene oxide: Present and future prospects. Arab. J. Chem. 2016, 9, 238–261. [Google Scholar] [CrossRef]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 4. [Google Scholar] [CrossRef]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Wan, Z.; Dong, Q.; Guo, X.; Bai, X.; Zhang, X.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. A dual-responsive polydopamine-modified hydroxybutyl chitosan hydrogel for sequential regulation of bone regeneration. Carbohydr. Polym. 2022, 297, 120027. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, C.; Park, J.; Lee, J.S.; Kang, S.; Seo, Y.D.; Jang, J.; Kim, B.S. Three-dimensional scaffolds of carbonized polyacrylonitrile for bone tissue regeneration. Angew. Chem. 2014, 126, 9367–9371. [Google Scholar] [CrossRef]
- Gupta, P.S.; Wasnik, K.; Patra, S.; Pareek, D.; Singh, M.; Maity, S.; Pandey, M.; Paik, P. A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering (BTE) Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Rawat, K.; Agarwal, S.; Tyagi, A.; Verma, A.K.; Bohidar, H.B. Aspect Ratio Dependent Cytotoxicity and Antimicrobial Properties of Nanoclay. Appl. Biochem. Biotechnol. 2014, 174, 936–944. [Google Scholar] [CrossRef]
- Tan, X.; Liu, F.; Hu, L.; Reed, A.H.; Furukawa, Y.; Zhang, G. Evaluation of the particle sizes of four clay minerals. Appl. Clay Sci. 2017, 135, 313–324. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly (D, L-lactide-co-glycolide) and its derivatives. J. Control. Release 2008, 125, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Xu, X.; Song, J. Segmental long bone regeneration guided by degradable synthetic polymeric scaffolds. Biomater. Transl. 2020, 1, 33–45. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’Heureux, N.; Fricain, J.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, N.K.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Thanh, D.T.; Trang, P.T.; Huong, H.T.; Nam, P.T.; Phuong, N.T.; Trang, N.T.; Hoang, T.; Lam, T.D.; Seo–Park, J. Fabrication of poly (lactic acid)/hydroxyapatite (PLA/HAp) porous nanocomposite for bone regeneration. Int. J. Nanotechnol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. BioMed Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; van der Walle, C.F. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J. Pharm. Sci. 2008, 97, 71–87. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Park, S.; Miller, A.L.; Terzic, A.; Lu, L. Strontium-substituted hydroxyapatite stimulates osteogenesis on poly(propylene fumarate) nanocomposite scaffolds. J. Biomed. Mater. Res. Part A 2019, 107, 631–642. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Eftekhari, H.; Jahandideh, A.; Asghari, A.; Akbarzadeh, A.; Hesaraki, S. Histopathological evaluation of polycaprolactone nanocomposite compared with tricalcium phosphate in bone healing. J. Vet. Res. 2018, 62, 385–394. [Google Scholar] [CrossRef]
- Vasireddi, R.; Basu, B. Conceptual design of three-dimensional scaffolds of powder-based materials for bone tissue engineering applications. Rapid Prototyp. J. 2015, 21, 716–724. [Google Scholar] [CrossRef]
- Abbasi, N.; Abdal-Hay, A.; Hamlet, S.; Graham, E.; Ivanovski, S. Effects of Gradient and Offset Architectures on the Mechanical and Biological Properties of 3-D Melt Electrowritten (MEW) Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 3448–3461. [Google Scholar] [CrossRef]
- Yeo, M.; Simon, C.G.; Kim, G. Effects of offset values of solid freeform fabricated PCL–β-TCP scaffolds on mechanical properties and cellular activities in bone tissue regeneration. J. Mater. Chem. 2012, 22, 21636–21646. [Google Scholar] [CrossRef]
- Castro, A.P.G.; Pires, T.; Santos, J.E.; Gouveia, B.P.; Fernandes, P.R. Permeability versus Design in TPMS Scaffolds. Materials 2019, 12, 1313. [Google Scholar] [CrossRef]
- Egan, P.F. Integrated Design Approaches for 3D Printed Tissue Scaffolds: Review and Outlook. Materials 2019, 12, 2355. [Google Scholar] [CrossRef]
- Bavariya, A.J.; Andrew Norowski, P., Jr.; Mark Anderson, K.; Adatrow, P.C.; Garcia-Godoy, F.; Stein, S.H.; Bumgardner, J.D. Evaluation of biocompatibility and degradation of chitosan nanofiber membrane crosslinked with genipin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1084–1092. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Liu, Z.; Geng, H.; Lu, X.; Zhang, X.; Li, H.; Gao, C.; Zhang, X.; Gao, P. Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin-loaded chitosan nanoparticles. Int. J. Nanomed. 2017, 12, 8855–8866. [Google Scholar] [CrossRef]

| Nanoparticle Shapes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gold Np | Nanocluster | Nanoshell | Nanobranch | Nanotriangle | Nanocube | Nanohexagon | Nanopentagon | Nanorods | Nanosphere | Nanocage | Nanostars |
| Silver NP | Nanospheres | Nanoshell | Nanorice | Nanotriangle | Nanocube | Truncated Octahedron | Nanostar | Nanorods | Nanodisk | Nanowires | |
| Au NPs | Nanospheres | Nanoshell | Nanoflower | Nanorod | Nanocube | Nanocage | |||||
| Nano silica | Capsule | Rice | Cube | Rhombus | |||||||
 |  |  |  | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farjaminejad, S.; Farjaminejad, R.; Garcia-Godoy, F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. J. Funct. Biomater. 2024, 15, 241. https://doi.org/10.3390/jfb15090241
Farjaminejad S, Farjaminejad R, Garcia-Godoy F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. Journal of Functional Biomaterials. 2024; 15(9):241. https://doi.org/10.3390/jfb15090241
Chicago/Turabian StyleFarjaminejad, Samira, Rosana Farjaminejad, and Franklin Garcia-Godoy. 2024. "Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering" Journal of Functional Biomaterials 15, no. 9: 241. https://doi.org/10.3390/jfb15090241
APA StyleFarjaminejad, S., Farjaminejad, R., & Garcia-Godoy, F. (2024). Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. Journal of Functional Biomaterials, 15(9), 241. https://doi.org/10.3390/jfb15090241








