Impact of Calcium Lactate Pretreatment on Enamel Fluoride Uptake: A Comparative In Vitro Study of Different Fluoride Types and Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Enamel Slab Preparation
2.3. Extraction of Alkali-Soluble Fluoride
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barani-Sveçla, M.; Buleshkaj, S. Etiopathogenesis of Dental Caries. In Dentistry; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Hoxha, V. Etiopatogjeneza e Kariesit; Semundjet e Dhembit: Prishtina, Kosovo, 2019; pp. 54–59. [Google Scholar]
- Martinez-Mier, E.A.; Zandona, A.F. The Impact of Gender on Caries Prevalence and Risk Assessment. Dent. Clin. N. Am. 2013, 57, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Tarkkila, L.; Tiitinen, A. The menopause and oral health. Maturitas 2009, 63, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Ekstrand, K.; Thylstrup, A. Dental Plaque and Caries on Occlusal Surfaces of First Permanent Molars in Relation to Stage of Eruption. J. Dent. Res. 1989, 68, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J.M. The primary and mixed dentition, post-eruptive enamel maturation and dental caries: A review. Int. Dent. J. 2013, 63 (Suppl. S2), 3–13. [Google Scholar] [CrossRef]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological factors in dental caries enamel structure and the caries process in the dynamic process of demineralization and remineralization (part 2). J. Clin. Pediatr. Dent. 2004, 28, 119–124. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Koh, D.; Hsu, C.-Y.S. Salivary biomarkers for dental caries. Periodontology 2000 2016, 70, 128–141. [Google Scholar] [CrossRef]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofacial Res. 2016, 6, 67–76. [Google Scholar] [CrossRef]
- Reddy, K.V.; Nirupama, C.; Reddy, P.K.; Koppolu, P.; Alotaibi, D.H. Effect of iatrogenic factors on periodontal health: An epidemiological study. Saudi Dent. J. 2020, 32, 80–85. [Google Scholar] [CrossRef]
- Lazar, L.; Vlasa, A.; Beresescu, L.; Bud, A.; Lazar, A.P.; Matei, L.; Bud, E. White spot lesions (WSLs)-post-orthodontic occurrence, management and treatment alternatives: A narrative review. J. Clin. Med. 2023, 12, 1908. [Google Scholar] [CrossRef]
- Gal, J.-Y.; Bollinger, J.-C.; Tolosa, H.; Gache, N. Calcium carbonate solubility: A reappraisal of scale formation and inhibition. Talanta 1996, 43, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E.; Mangum, J.E.; Schneider, P.M. Pathophysiology of Demineralization, Part I: Attrition, Erosion, Abfraction, and Noncarious Cervical Lesions. Curr. Osteoporos. Rep. 2021, 20, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B. The Continuum of Dental Caries—Evidence for a Dynamic Disease Process. J. Dent. Res. 2004, 83 (Suppl. S1), C39–C42. [Google Scholar] [CrossRef] [PubMed]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Anatomy and physiology of the mineralized tissues: Role in the pathogenesis of osteoarthrosis. Osteoarthr. Cartil. 2004, 12, 20–30. [Google Scholar] [CrossRef]
- Hara, A.T.; Carvalho, J.C.; Zero, D.T. Causes of Dental Erosion: Extrinsic Factors. In Dental Erosion and Its Clinical Management; Amaechi, B., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Yan-Fang Ren, D.D.S. Dental Erosion: Etiology, Diagnosis and Prevention; ADA The Academy of Dental Therapeutic and Stomatology: Chicago, IL, USA, 2011. [Google Scholar]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Lussi, A. Understanding the chemistry of dental erosion. In Monographs in Oral Science; Karger Publishers: Basel, Switzerland, 2006; Volume 20, pp. 66–76. [Google Scholar] [CrossRef]
- Toumba, K.J.; Twetman, S.; Splieth, C.; Parnell, C.; van Loveren, C.; Lygidakis, N. Guidelines on the use of fluoride for caries prevention in children: An updated EAPD policy document. Eur. Arch. Paediatr. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Albahrani, M.M.; Alyahya, A.; Qudeimat, M.A.; Toumba, K.J. Salivary fluoride concentration following toothbrushing with and without rinsing: A randomised controlled trial. BMC Oral Health 2022, 22, 53. [Google Scholar] [CrossRef]
- Vogel, G.L. Oral Fluoride Reservoirs and the Prevention of Dental Caries. In Monographs in Oral Science; Karger Publishers: Basel, Switzerland, 2011; Volume 22, pp. 146–157. [Google Scholar] [CrossRef]
- Hong, Y.; Chow, L.; Brown, W. Basic Biological Sciences. J. Dent. Res. 1985, 64, 82–84. [Google Scholar] [CrossRef]
- Øgaard, B.; Rølla, G.; Arends, J.; Ten Cate, J.M. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Tenuta, L.; Schumacher, G.; Chow, L. A Calcium Prerinse Required to Form Calcium Fluoride in Plaque from a Sodium Fluoride Rinse. Caries Res. 2014, 48, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Ten Cate, J.M. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur. J. Oral Sci. 1997, 105, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Buchalla, W.; Lennon, A.M.; Trage, K.; Becker, K.; Attin, T. Enamel fluoride uptake following fluoride application and fluoride precipitation. Schweiz. Monatsschrift Zahnmed. 2007, 117, 118–122. [Google Scholar] [CrossRef]
- Mok, Y.; Hill, F.; Newman, H. Enamel Fluoride Uptake Affected by Site of Application: Comparing Sodium and Amine Fluorides. Caries Res. 1990, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, M.; Itthagarun, A.; King, N.M. Comparison of the remineralizing potential of child formula dentifrices. Int. J. Paediatr. Dent. 2011, 21, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Caslavska, V.; Moreno, E.; Brudevold, F. Determination of the calcium fluoride formed from in vitro exposure of human enamel to fluoride solutions. Arch. Oral Biol. 1975, 20, 333–339. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Microbiology of dental caries. J. Biol. Earth Sci. 2013, 3, M21–M24. [Google Scholar]
- Carey, C.M. Focus on Fluorides: Update on the Use of Fluoride for the Prevention of Dental Caries. J.Évid. Based Dent. Pract. 2014, 14, 95–102. [Google Scholar] [CrossRef]
- Ferizoli, B.; Cresswell-Boyes, A.J.; Anderson, P.; Lynch, R.J.M.; Hill, R.G. Effects of fluoride on in vitro hydroxyapatite demineralisation analysed by 19F MAS-NMR. Front. Dent. Med. 2023, 4, 1171827. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Li, C.; Luo, X.; Wang, Y. Fluoride contributes to the shaping of microbial community in high fluoride groundwater in Qiji County, Yuncheng City, China. Sci. Rep. 2019, 9, 14488. [Google Scholar] [CrossRef] [PubMed]
- Koo, H. Strategies to Enhance the Biological Effects of Fluoride on Dental Biofilms. Adv. Dent. Res. 2008, 20, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rošin-Grget, K.; Peroš, K.; Šutej, I. The cariostatic mechanisms of fluoride. Acta Medica Acad. 2013, 42, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E.; Clock, S.A.; Mota-Meira, M. Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol. Rev. 2003, 26, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, K.; Imazato, S.; Takahashi, Y.; Kiba, W.; Ebisu, S.; Takahashi, N. Fluoride released from glass-ionomer cement is responsible to inhibit the acid production of caries-related oral streptococci. Dent. Mater. 2009, 25, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Pessan, J.; Alves, K.; Ramires, I.; Taga, M.; Sampaio, F.; Whitford, G.; Buzalaf, M. Effects of Regular and Low-fluoride Dentifrices on Plaque Fluoride. J. Dent. Res. 2010, 89, 1106–1110. [Google Scholar] [CrossRef]
- Whitford, G.; Buzalaf, M.; Bijella, M.; Waller, J. Plaque Fluoride Concentrations in a Community without Water Fluoridation: Effects of Calcium and Use of a Fluoride or Placebo Dentifrice. Caries Res. 2005, 39, 100–107. [Google Scholar] [CrossRef]
- Whitford, G.M.; Wasdin, J.L.; Schafer, T.E.; Adair, S.M. Plaque Fluoride Concentrations Are Dependent on Plaque Calcium Concentrations. Caries Res. 2002, 36, 256–265. [Google Scholar] [CrossRef]
- Jullien, S. Prophylaxis of caries with fluoride for children under five years. BMC Pediatr. 2021, 21 (Suppl. S1), 351. [Google Scholar] [CrossRef]
- Spinola, M.d.S.; Tenuta, L.M.A. Calcium pretreatment enhances fluoride reactivity with enamel and dentine. Arch. Oral Biol. 2022, 134, 105338. [Google Scholar] [CrossRef]
- Stookey, G.K.; DePaola, P.F.; Featherstone, J.D.B.; Fejerskov, O.; Möller, I.J.; Rotberg, S.; Stephen, K.W.; Wefel, J.S. A Critical Review of the Relative Anticaries Efficacy of Sodium Fluoride and Sodium Monofluorophosphate Dentifrices. Caries Res. 1993, 27, 337–360. [Google Scholar] [CrossRef]
- Vogel, G.; Chow, L.; Carey, C. Calcium Pre-Rinse Greatly Increases Overnight Salivary Fluoride after a 228 ppm Fluoride Rinse. Caries Res. 2008, 42, 401–404. [Google Scholar] [CrossRef]
- Vogel, G.; Chow, L.; Carey, C.; Schumacher, G.; Takagi, S. Effect of a Calcium Prerinse on Salivary Fluoride after a 228-ppm Fluoride Rinse. Caries Res. 2006, 40, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Schumacher, G.; Chow, L.; Takagi, S.; Carey, C. Ca Pre-rinse Greatly Increases Plaque and Plaque Fluid F. J. Dent. Res. 2008, 87, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride Revolution and Dental Caries: Evolution of Policies for Global Use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Nayyer, M.; Gilani, M.; Aman, N.; Azad, A.A.; Shah, A.T.; Chaudhry, A.A.; Kaleem, M.; Khan, A.S. Comparative Fluoride Release and Antimicrobial Analysis of Commercial and Experimental Bioactive Glass/Nano-Oxide-Based Dentifrices. Eur. J. Dent. 2020, 14, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, R.M.; Jones, S. On the Relationship between the Rate of Salivary Flow and Salivary Fluoride Clearance. Caries Res. 2015, 49, 141–146. [Google Scholar] [CrossRef]
- Sezici, Y.L.; Yetkiner, E.; Yetkiner, A.A.; Eden, E.; Attin, R. Comparative evaluation of fluoride varnishes, self-assembling peptide-based remineralization agent, and enamel matrix protein derivative on artificial enamel remineralization in vitro. Prog. Orthod. 2021, 22, 4. [Google Scholar] [CrossRef]
- Batista, G.R.; Torres, C.R.G.; Sener, B.; Attin, T.; Wiegand, A. Artificial Saliva Formulations versus Human Saliva Pretreatment in Dental Erosion Experiments. Caries Res. 2016, 50, 78–86. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Tenuta, L.M.A.; Cury, A.A.D.B.; Nóbrega, D.F.; Budin, R.R.; de Queiroz, M.X.; Vogel, G.L.; Cury, J.A. Calcium Prerinse before Fluoride Rinse Reduces Enamel Demineralization: An in situ Caries Study. Caries Res. 2016, 50, 372–377. [Google Scholar] [CrossRef]
- Vogel, G.; Tenuta, L.; Schumacher, G.; Chow, L. No Calcium-Fluoride-Like Deposits Detected in Plaque Shortly after a Sodium Fluoride Mouthrinse. Caries Res. 2010, 44, 108–115. [Google Scholar] [CrossRef] [PubMed]
- van der Hoeven, J.; Schaeken, M.; Creugers, T. Effect of a Mouthrinse Containing Calcium Lactate on the Formation and Mineralization of Dental Plaque. Caries Res. 1989, 23, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.H.; Dorow, A.; Langenhorst, S.; Gintner, Z.; Bánóczy, J.; Gaengler, P. Effect of fluoride toothpastes on enamel demineralization. BMC Oral Health 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Lyaruu, D.M.; Tros, G.H.; Bronckers, A.L.; Wöltgens, J.H. Micro-PIXE (proton-induced X-ray emission) study of the effects of fluoride on mineral distribution patterns in enamel and dentin in the developing hamster tooth germ. Scanning Microsc. 1990, 4, 315–322. [Google Scholar] [PubMed]
- Chu, J.; Fox, J.; Higuchi, W.; Nash, W. Electron Probe Micro-analysis for Subsurface Demineralization and Remineralization of Dental Enamel. J. Dent. Res. 1989, 68, 26–31. [Google Scholar] [CrossRef]
- Hiraishi, N.; Sayed, M.; Hill, R.; Shimada, Y. Solid-state NMR spectroscopy measurement of fluoride reaction by bovine enamel and dentin treated with silver diammine fluoride. Dent. Mater. 2022, 38, 769–777. [Google Scholar] [CrossRef]
- Corpron, R.; More, F.; Mount, G. Comparison of Fluoride Profiles by SIMS with Mineral Density of Subsurface Enamel Lesions Treated Intra-orally with a Fluoride-releasing Device. J. Dent. Res. 1992, 71, 828–831. [Google Scholar] [CrossRef]

| Treatment Substance | Composition | Manufacturer | OT No. |
|---|---|---|---|
| Calcium lactate pentahydrate 150 mM | Calcium lactate pentahydrate—4.624 g Purifying water—100 mL | Farmalabor, GLJZ—Croatia | R2220462, 161022 |
| Sodium fluoride 500 ppm (226 ppm free F ion) | Sodium fluoride—0.05 g Purifying water—100 ml | Farmalabor, GLJZ—Croatia | R2119627, 161022 |
| Sodium fluoride 155 ppm (70 ppm free F ion) | Sodium fluoride—0.0155 g Purifying water—100 mL | Farmalabor, Hamapharma—Croatia | R2119627, 0070922 |
| Sodium Monofluorophosphate 500 ppm (65 ppm free F ion) | Sodium Monofluorophosphate—0.05 g Purifying water—100 mL | NEVIA (Magdis d.o.o), Hamapharma—Croatia | 100084372120200821, 0070922 |
| Sodium Monofluorophosphate 1720 ppm (226 ppm free F ion) | Sodium Monofluorophosphate—0.1720 g Purifying water—100 mL | NEVIA (Magdis d.o.o), Hamapharma—Croatia | 100084372120200821, 0070922 |
| Aminofluoride 500 ppm (75 ppm free F ion) | Aminofluoride— 0.05 g Purifying water—100 mL | Fagron, Hamapharma—Croatia | 104/22, 0070922 |
| Aminofluoride 1500 ppm (226 ppm free F ion) | Aminofluoride— 0.15 g Purifying water—100 mL | Fagron, Hamapharma—Croatia | 104/22, 0070922 |
| Group A | Group B | Group C | Group D | ||||
|---|---|---|---|---|---|---|---|
| NaF—a n = 10 | MFP—a n = 10 | AmF—a n = 10 | NaF—b n = 10 | MFP—b n = 10 | AF—b n = 10 | Ca n = 30 | Deionized Water n = 30 |
 |  |  |  |  |  |  |  |
| Calcium lactate pretreated for 5 min followed by 3 different fluoride solutions: (1–10) A NaF, (11–20) A-MFP, (21–30) A—AmF | Treated for 5 min with 3 different fluoride solutions as follows: (1–10) B- NaF, (11–20) B-MFP, (21–30) B—AmF | Treated with 150 mM calcium lactate solution | Treated with deionized water (negative control) | ||||
 |  |  |  | ||||
| Fluoride extraction was conducted by immersing the enamel slabs in a 1 M KOH solution for 24 h under agitation at room temperature. After the 24 h period, all extracts were buffered with TISAB III solution containing 1.0 M HCl | |||||||
 | |||||||
| The extracts were analyzed using a fluoride ion-specific electrode | |||||||
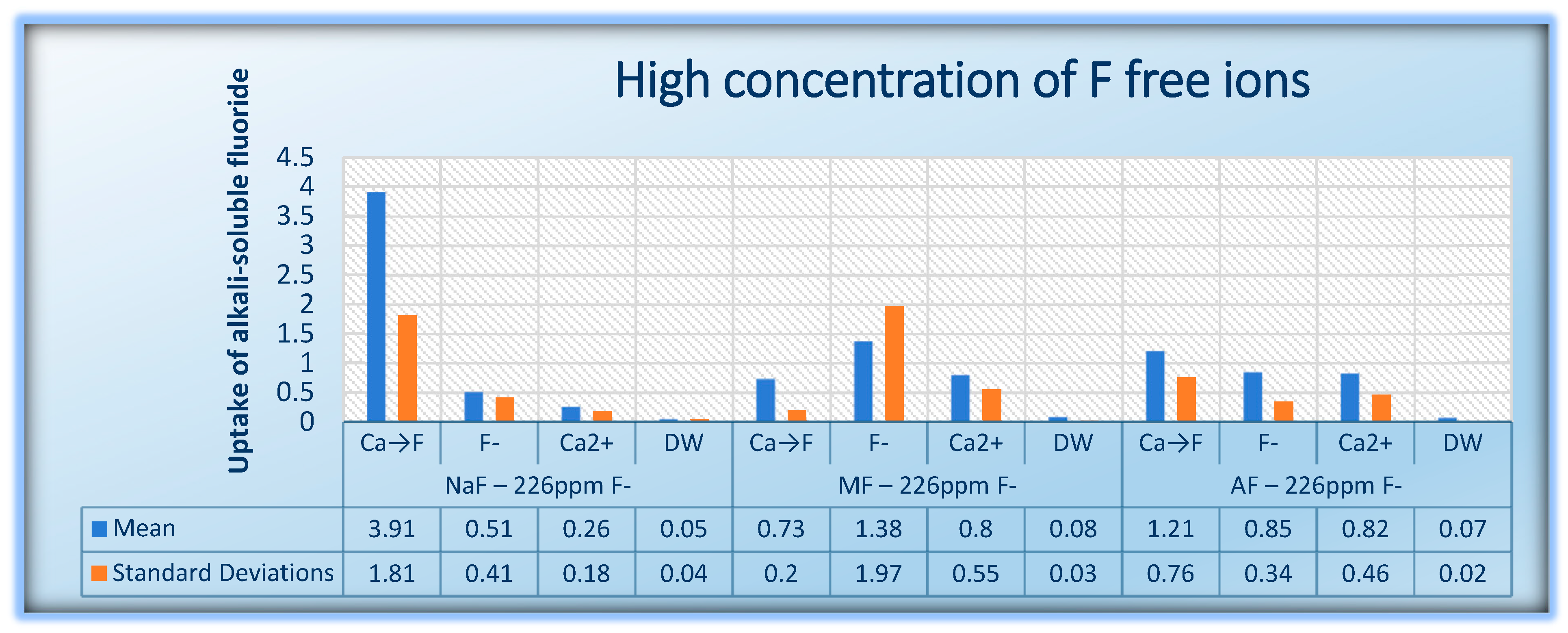
| 226 ppm Free F− Ion | NaF | MFP | AmF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca→F | F− | Ca2+ | DW | Ca→F | F− | Ca2+ | DW | Ca→F | F | Ca2+ | DW | |
| Median | 3.317 | 0.391 | 0.196 | 0.041 | 0.673 | 0.565 | 0.585 | 0.078 | 0.866 | 0.762 | 0.671 | 0.071 |
| Percentiles 25%/75% | 2.736 / 5.452 | 0.247 / 0.524 | 0.157 / 0.294 | 0.024 / 0.058 | 0.612 / 0.873 | 0.495 / 1.282 | 0.502 / 0.742 | 0.048 / 0.11 | 0.735 / 1.244 | 0.608 / 1.02 | 0.534 / 0.882 | 0.057 / 0.088 |
| NaF–70 ppm F− | MFP–65 ppm F− | AmF–75 ppm F− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca→F | F− | Ca2+ | DW | Ca→F | F− | Ca2+ | DW | Ca→F | F | Ca2+ | DW | |
| Median | 0.684 | 0.866 | 0.591 | 0.113 | 0.351 | 0.382 | 0.355 | 0.053 | 0.862 | 0.382 | 0.222 | 0.068 |
| Percentiles 25%/75% | 0.592 / 1.133 | 0.729 / 0.985 | 0.500 / 0.737 | 0.075 / 0.211 | 0.184 / 0.731 | 0.216 / 0.833 | 0.162 / 0.597 | 0.041 / 0.098 | 0.227 / 1.205 | 0.252 / 0.675 | 0.111 / 0.49 | 0.033 / 0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kullashi Spahija, F.; Sutej, I.; Basic, K.; Spahija, K.; Peros, K. Impact of Calcium Lactate Pretreatment on Enamel Fluoride Uptake: A Comparative In Vitro Study of Different Fluoride Types and Concentrations. J. Funct. Biomater. 2024, 15, 269. https://doi.org/10.3390/jfb15090269
Kullashi Spahija F, Sutej I, Basic K, Spahija K, Peros K. Impact of Calcium Lactate Pretreatment on Enamel Fluoride Uptake: A Comparative In Vitro Study of Different Fluoride Types and Concentrations. Journal of Functional Biomaterials. 2024; 15(9):269. https://doi.org/10.3390/jfb15090269
Chicago/Turabian StyleKullashi Spahija, Fjolla, Ivana Sutej, Kresimir Basic, Kreshnik Spahija, and Kristina Peros. 2024. "Impact of Calcium Lactate Pretreatment on Enamel Fluoride Uptake: A Comparative In Vitro Study of Different Fluoride Types and Concentrations" Journal of Functional Biomaterials 15, no. 9: 269. https://doi.org/10.3390/jfb15090269
APA StyleKullashi Spahija, F., Sutej, I., Basic, K., Spahija, K., & Peros, K. (2024). Impact of Calcium Lactate Pretreatment on Enamel Fluoride Uptake: A Comparative In Vitro Study of Different Fluoride Types and Concentrations. Journal of Functional Biomaterials, 15(9), 269. https://doi.org/10.3390/jfb15090269






