Optimizing Flexor Digitorum Profundus Tendon Repair: A Narrative Review
Abstract
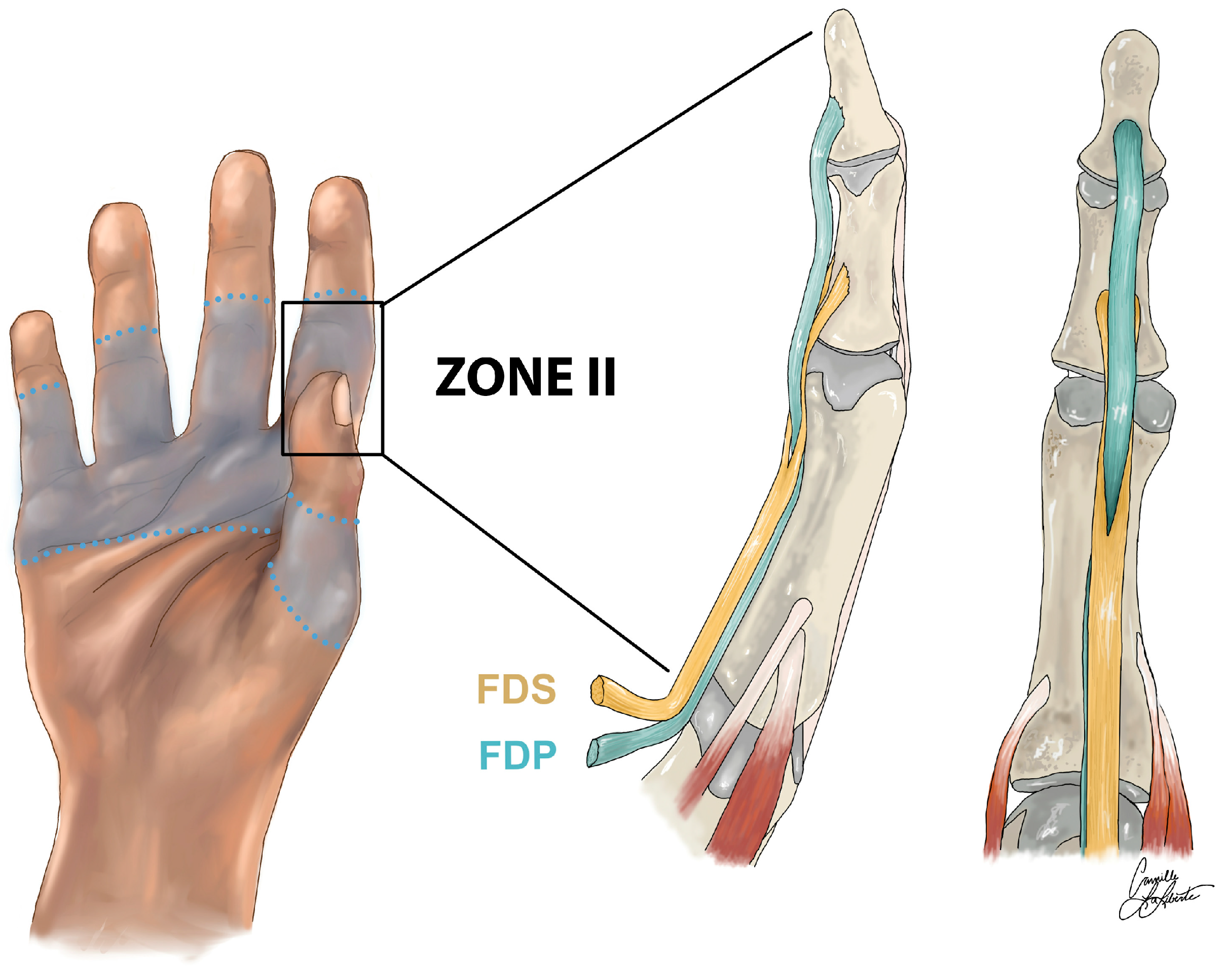
:1. Introduction
1.1. Biomaterials and Nanomaterials
1.1.1. Definition and Significance
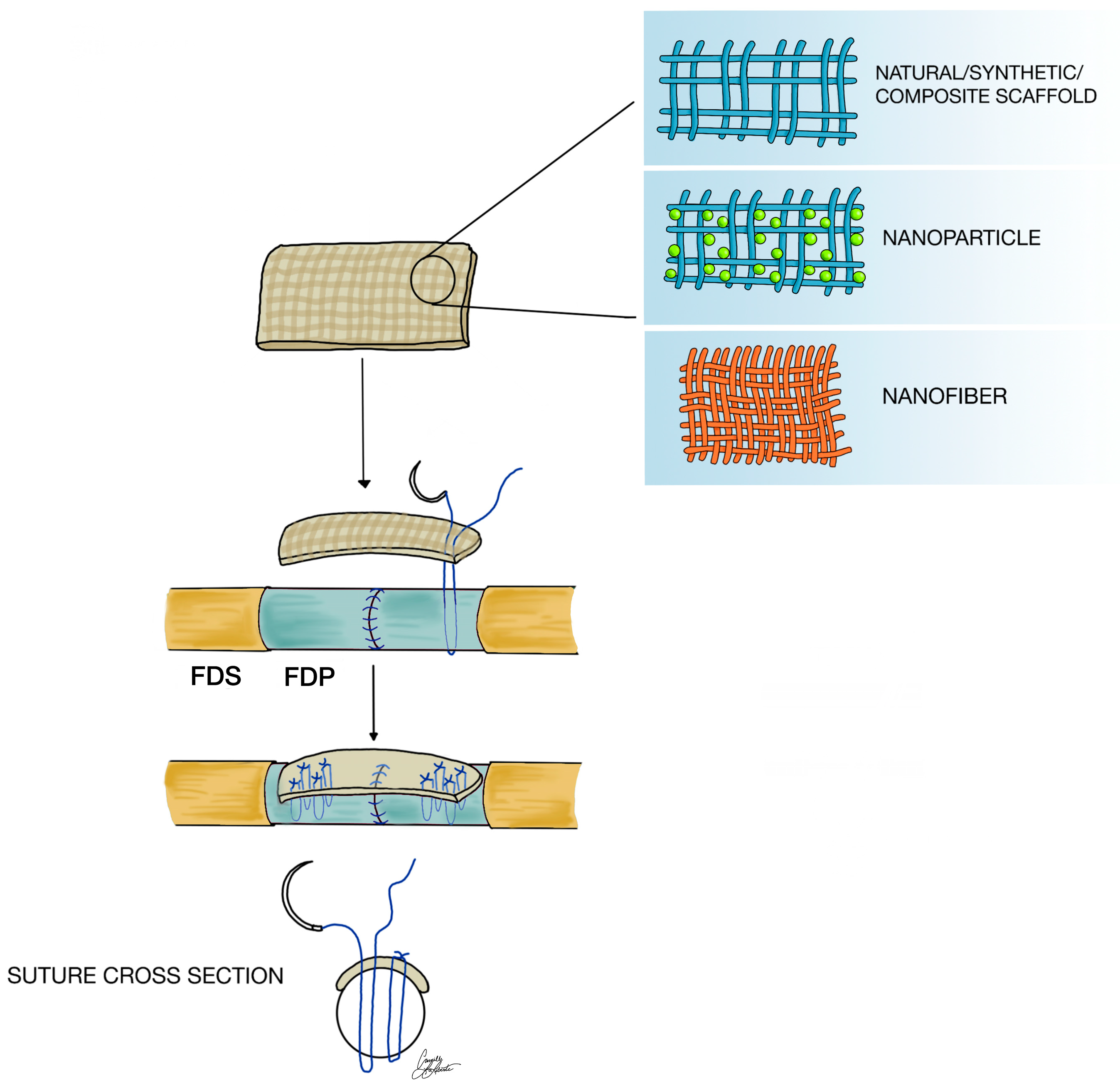
1.1.2. Application in Tendon Repair
2. Materials and Methods
3. Material Properties
3.1. Nanomaterials
3.2. Natural Materials
3.2.1. Silk Fibroin and Natural Silk
3.2.2. Collagen
3.3. Synthetic Materials
3.4. Composite Materials
4. Methods of Biomaterial Scaffold Fabrication
4.1. Electrospinning
4.2. Three-Dimensional Printing
4.3. Knitting and Weaving
4.4. Embroidery
4.5. Hybrid Techniques
5. Biomaterials and Nanomaterials in Tendon Repair
6. Discussion
6.1. Application in FDP Tendon Repair
6.1.1. Nanomaterials and Nanoparticles
6.1.2. Natural Materials
6.1.3. Synthetic Materials
6.1.4. Hybrid Materials
6.2. Best Material for FDP Tendon Repair
7. Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| FDP | Flexor digitorum profundus |
| FDS | Flexor digitorum superficialis |
| PCL | Polycaprolactone |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| PLGA | Polylactic-co-glycolic acid |
| SF | Silk fibroin |
| TE | Tissue engineering |
References
- Tobler-Ammann, B.C.; Beckmann-Fries, V.; Calcagni, M.; Kämpfen, A.; Schrepfer, L.; Vögelin, E. Outcomes of Primary Flexor Tendon Repairs in Zones 2 and 3: A Retrospective Cohort Study. J. Hand Surg. Glob. Online 2023, 5, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lung, B.E.; Burns, B. Anatomy, Shoulder and Upper Limb, Hand Flexor Digitorum Profundus Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tang, J.B.; Lalonde, D.; Harhaus, L.; Sadek, A.F.; Moriya, K.; Pan, Z.J. Flexor Tendon Repair: Recent Changes and Current Methods. J. Hand Surg. 2022, 47, 31–39. [Google Scholar] [CrossRef]
- Strickland, J.W. Flexor Tendon Injuries: I. Foundations of Treatment. J. Am. Acad. Orthop. Surg. 1995, 3, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Rawson, S.; Cartmell, S.; Wong, J. Suture Techniques for Tendon Repair; a Comparative Review. Muscles Ligaments Tendons J. 2013, 3, 220–228. [Google Scholar] [CrossRef]
- Liu, G.; Lv, G.; Liu, F. Suture Techniques in the Surgical Management of Flexor Tendon, Achilles Tendon and Cruciate Ligament Injuries: A Systematic Review. BMC Musculoskelet. Disord. 2024, 25, 1087. [Google Scholar] [CrossRef]
- Stevens, K.A.; Caruso, J.C.; Fallahi, A.-K.M.; Patiño, J.M. Flexor Tendon Lacerations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Xu, H.; Huang, X.; Guo, Z.; Zhou, H.; Jin, H.; Huang, X. Outcome of Surgical Repair and Rehabilitation of Flexor Tendon Injuries in Zone II of the Hand: Systematic Review and Meta-Analysis. J. Hand Surg. 2023, 48, 407.e1–407.e11. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.B. Rehabilitation after Flexor Tendon Repair and Others: A Safe and Efficient Protocol. J. Hand Surg. 2021, 46, 813–817. [Google Scholar] [CrossRef]
- Zeng, W.; Albano, N.J.; Sanchez, R.J.; Mccabe, R.; Vanderby, R.; Poore, S.O.; Dingle, A.M. Beyond the Core Suture: A New Approach to Tendon Repair. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3280. [Google Scholar] [CrossRef]
- Reisdorf, R.L.; Liu, H.; Bi, C.; Vrieze, A.M.; Moran, S.L.; Amadio, P.C.; Zhao, C. Carbodiimide-Derivatized Synovial Fluid for Tendon Graft Coating Improves Long-Term Functional Outcomes of Flexor Tendon Reconstruction. Plast. Reconstr. Surg. 2023, 152, 840e–849e. [Google Scholar] [CrossRef]
- Yaşar, B. Encircling Tendon Repair Site with Collagen Sheet in Flexor Zone 2: Retrospective Study. J. Orthop. Surg. 2023, 18, 793. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Chen, Y.; Mo, X.; Fan, C. Tenogenic Adipose-Derived Stem Cell Sheets with Nanoyarn Scaffolds for Tendon Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111506. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Z.; Xiang, L.; Zhao, Z.; Cui, W. Functional Biomaterials for Tendon/Ligament Repair and Regeneration. Regen. Biomater. 2022, 9, rbac062. [Google Scholar] [CrossRef]
- Hou, J.; Yang, R.; Vuong, I.; Li, F.; Kong, J.; Mao, H.-Q. Biomaterials Strategies to Balance Inflammation and Tenogenesis for Tendon Repair. Acta Biomater. 2021, 130, 1–16. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, J.G.; Park, K. Biomaterials for the Treatment of Tendon Injury. Tissue Eng. Regen. Med. 2019, 16, 467–477. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber-Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Parchi, P.D.; Vittorio, O.; Andreani, L.; Battistini, P.; Piolanti, N.; Marchetti, S.; Poggetti, A.; Lisanti, M. Nanoparticles for Tendon Healing and Regeneration: Literature Review. Front. Aging Neurosci. 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Hu, Y.; Fei, Y.; Liu, H.; Huang, Z.; Wang, C.; Ruan, D.; Heng, B.C.; Chen, W.; et al. Systematic Review of Silk Scaffolds in Musculoskeletal Tissue Engineering Applications in the Recent Decade. ACS Biomater. Sci. Eng. 2021, 7, 817–840. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in Vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in Tissue Engineering: Applications, Challenges and Prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Adjei-Sowah, E.; Chandrasiri, I.; Xiao, B.; Liu, Y.; Ackerman, J.E.; Soto, C.; Nichols, A.E.C.; Nolan, K.; Benoit, D.S.W.; Loiselle, A.E. Development of a Nanoparticle-Based Tendon-Targeting Drug Delivery System to Pharmacologically Modulate Tendon Healing. BioRxiv Prepr. Serv. Biol. 2023, 2023, 11.29.569204. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wang, Z.; Zheng, Z.; Ran, J.; Zhu, J.; Chen, W. A Collagen and Silk Scaffold for Improved Healing of the Tendon and Bone Interface in a Rabbit Model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 269–278. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, R.S.; al-Shaikh, R.; Lane, J.M. Collagen in Tendon, Ligament, and Bone Healing. A Current Review. Clin. Orthop. 1995, 318, 265–278. [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Bhavsar, D.; Shettko, D.; Tenenhaus, M. Encircling the Tendon Repair Site with Collagen-GAG Reduces the Formation of Postoperative Tendon Adhesions in a Chicken Flexor Tendon Model. J. Surg. Res. 2010, 159, 765–771. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Zhang, X.; Yang, L.; Zhang, J. Research Progress of Biodegradable Polymers in Repairing Achilles Tendon Injury. Front. Mater. 2022, 9, 815930. [Google Scholar] [CrossRef]
- Gomez-Romero, P.; Pokhriyal, A.; Rueda-García, D.; Bengoa, L.N.; González-Gil, R.M. Hybrid Materials: A Metareview. Chem. Mater. Publ. Am. Chem. Soc. 2024, 36, 8–27. [Google Scholar] [CrossRef]
- Arevalo, A.; Rao, S.; Willier, D.P.; Schrock, C.I.; Erickson, B.J.; Jack, R.A.; Cohen, S.B.; Ciccotti, M.G. Surgical Techniques and Clinical Outcomes for Medial Epicondylitis: A Systematic Review. Am. J. Sports Med. 2023, 51, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, F.; Akkus, O.; King, M.W. A Collagen/PLA Hybrid Scaffold Supports Tendon-Derived Cell Growth for Tendon Repair and Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2624–2635. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of Electrospun Scaffolds and Biological Polymers for Enhancing the Delivery and Efficacy of Mesenchymal Stem/Stromal Cell Therapies. Front. Biosci. Landmark Ed. 2024, 29, 228. [Google Scholar] [CrossRef]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Rinoldi, C.; Kijeńska-Gawrońska, E.; Khademhosseini, A.; Tamayol, A.; Swieszkowski, W. Fibrous Systems as Potential Solutions for Tendon and Ligament Repair, Healing, and Regeneration. Adv. Healthc. Mater. 2021, 10, e2001305. [Google Scholar] [CrossRef]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef]
- Hahn, J.; Schulze-Tanzil, G.; Schröpfer, M.; Meyer, M.; Gögele, C.; Hoyer, M.; Spickenheuer, A.; Heinrich, G.; Breier, A. Viscoelastic Behavior of Embroidered Scaffolds for ACL Tissue Engineering Made of PLA and P(LA-CL) After In Vitro Degradation. Int. J. Mol. Sci. 2019, 20, 4655. [Google Scholar] [CrossRef]
- Gögele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schröpfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-Lactide-Co-ε-Caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar] [CrossRef]
- von Witzleben, M.; Hahn, J.; Richter, R.F.; de Freitas, B.; Steyer, E.; Schütz, K.; Vater, C.; Bernhardt, A.; Elschner, C.; Gelinsky, M. Tailoring the Pore Design of Embroidered Structures by Melt Electrowriting to Enhance the Cell Alignment in Scaffold-Based Tendon Reconstruction. Biomater. Adv. 2024, 156, 213708. [Google Scholar] [CrossRef]
- Gögele, C.; Konrad, J.; Hahn, J.; Breier, A.; Schröpfer, M.; Meyer, M.; Merkel, R.; Hoffmann, B.; Schulze-Tanzil, G. Maintenance of Ligament Homeostasis of Spheroid-Colonized Embroidered and Functionalized Scaffolds after 3D Stretch. Int. J. Mol. Sci. 2021, 22, 8204. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Cámara-Torres, M.; Scopece, P.; Verga Falzacappa, E.; Patelli, A.; Moroni, L.; Mota, C. A Hybrid Additive Manufacturing Platform to Create Bulk and Surface Composition Gradients on Scaffolds for Tissue Regeneration. Nat. Commun. 2021, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chou, P.-Y.; Chen, Z.-Y.; Lin, F.-H. Electrospun Water-Borne Polyurethane Nanofibrous Membrane as a Barrier for Preventing Postoperative Peritendinous Adhesion. Int. J. Mol. Sci. 2019, 20, 1625. [Google Scholar] [CrossRef] [PubMed]
- Brebels, J.; Mignon, A. Polymer-Based Constructs for Flexor Tendon Repair: A Review. Polymers 2022, 14, 867. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Giavaresi, G.; Bellini, D.; Casagranda, V.; Pressato, D.; Fini, M. Evaluation of a New Collagen-Based Medical Device (ElastiCo®) for the Treatment of Acute Achilles Tendon Injury and Prevention of Peritendinous Adhesions: An in Vitro Biocompatibility and in Vivo Investigation. J. Tissue Eng. Regen. Med. 2020, 14, 1113–1125. [Google Scholar] [CrossRef]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Kotwal, P.P.; Ansari, M.T. Zone 2 Flexor Tendon Injuries: Venturing into the No Man’s Land. Indian J. Orthop. 2012, 46, 608–615. [Google Scholar] [CrossRef]
| Author | Journal | Medical Intervention | Specific Augmentation | Animal Models | Tendon Model |
|---|---|---|---|---|---|
| Tang et al. [14] | Regen Biomater | Biomaterial | Functional biomaterials | Human, rabbit, rat, sheep | Infraspinatus, dorsal myofascial, Achilles, supraspinatus tendon, subacromial deltoid bursa in patients with supraspinatus tendon tear |
| Zhang et al. [21] | ACS Biomater Sci Eng | Silk scaffold | Silk scaffold | Rabbit, rat, sheep | Achilles tendon |
| Mao et al. [22] | Front Bioeng Biotechnol | Biomaterial | Engineered scaffold | Rabbit, rat | Achilles, supraspinatus, infraspinatus, long digital extensor |
| Parchi et al. [20] (literary review) | FrontAging Neurosci | Nanomaterial | Nanomaterials | N/A | N/A |
| Material | Biocompatibility | Mechanical Strength | Degradation Rate | Integration with Suture Techniques | Effectiveness in Healing | Source |
|---|---|---|---|---|---|---|
| Nanofibers | High | Moderate | Controllable | Challenging | High | [20] |
| Nanoparticles | High | Low | Controllable | Moderate | High | [20] |
| Collagen | High | Low | Rapid | Moderate | High | [21,22] |
| Collagen | High | Low | Rapid | Moderate | High | [21,22] |
| Silk fibroin | High | High | Moderate | Good | High | [21] |
| PLA | Moderate | High | Predictable | Moderate | High | [8,22] |
| PGA | Moderate | High | Predictable | Moderate | High | [8,22] |
| PCL | Moderate | High | Slow | Moderate | High | [8,22] |
| PLGA | Moderate | High | Controllable | Moderate | High | [8,22] |
| Collagen–PCL | High | High | Controllable | Good | High | [8,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mereddy, R.R.; Zona, E.E.; LaLiberte, C.J.; Dingle, A.M. Optimizing Flexor Digitorum Profundus Tendon Repair: A Narrative Review. J. Funct. Biomater. 2025, 16, 97. https://doi.org/10.3390/jfb16030097
Mereddy RR, Zona EE, LaLiberte CJ, Dingle AM. Optimizing Flexor Digitorum Profundus Tendon Repair: A Narrative Review. Journal of Functional Biomaterials. 2025; 16(3):97. https://doi.org/10.3390/jfb16030097
Chicago/Turabian StyleMereddy, Rishith R., Emily E. Zona, Camille J. LaLiberte, and Aaron M. Dingle. 2025. "Optimizing Flexor Digitorum Profundus Tendon Repair: A Narrative Review" Journal of Functional Biomaterials 16, no. 3: 97. https://doi.org/10.3390/jfb16030097
APA StyleMereddy, R. R., Zona, E. E., LaLiberte, C. J., & Dingle, A. M. (2025). Optimizing Flexor Digitorum Profundus Tendon Repair: A Narrative Review. Journal of Functional Biomaterials, 16(3), 97. https://doi.org/10.3390/jfb16030097







