Hybridization Chain Reaction-Enhanced Ultrasensitive Electrochemical Analysis of miRNAs with a Silver Nano-Reporter on a Gold Nanostructured Electrode Array
Abstract
1. Introduction
2. Experiments and Methods
2.1. Chemicals and Reagents
2.2. Fabrication of HCR-Based e-miRchip
2.3. Synthesis of AgNPs and AgNRs
2.4. GNEs Deposition
2.5. MiR-21 Detection
2.6. Electrochemical Measurement
2.7. Statistical Analysis
3. Results and Discussions
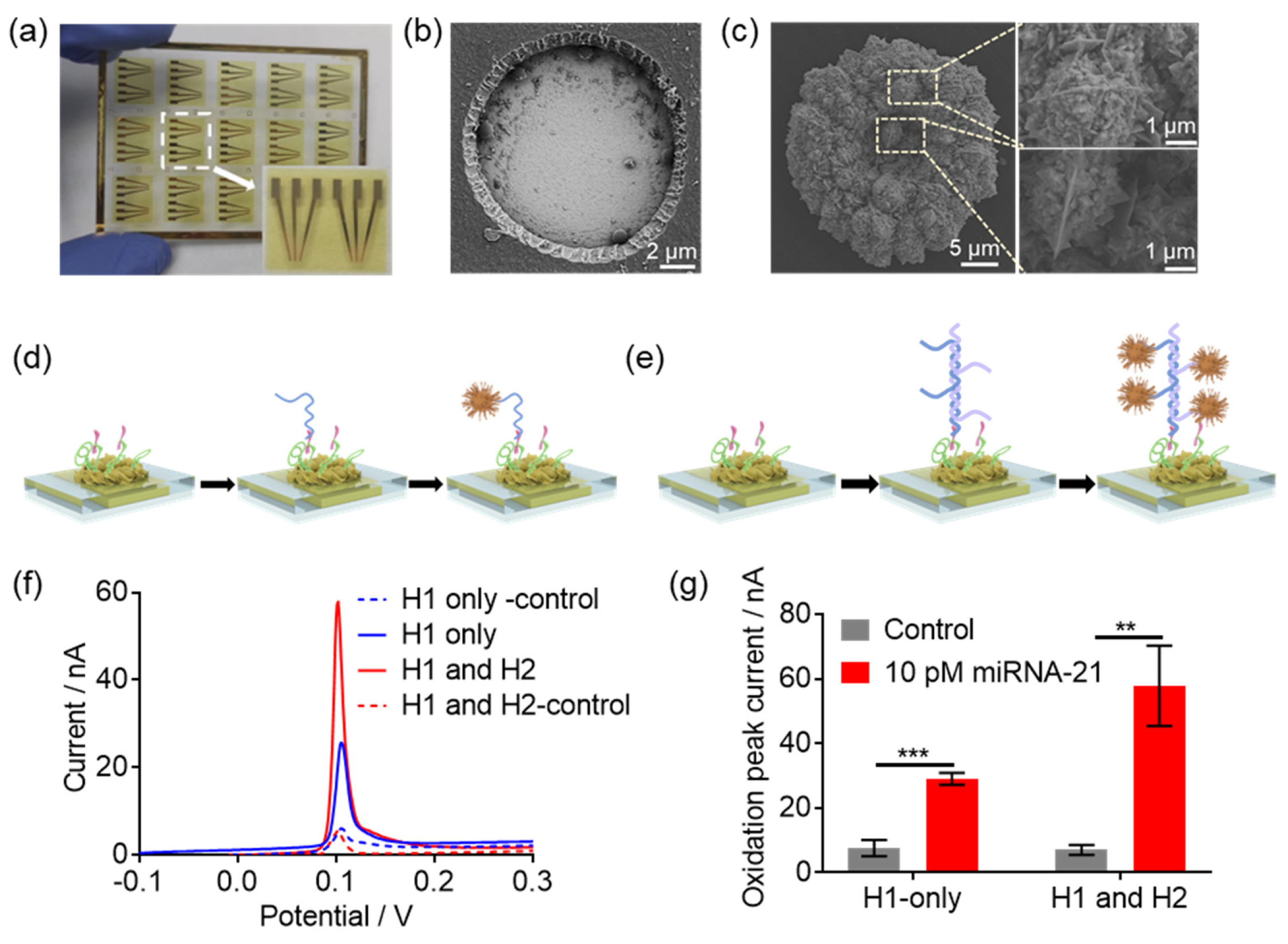
3.1. Design and Fabrication of HCR-Based e-miRchip
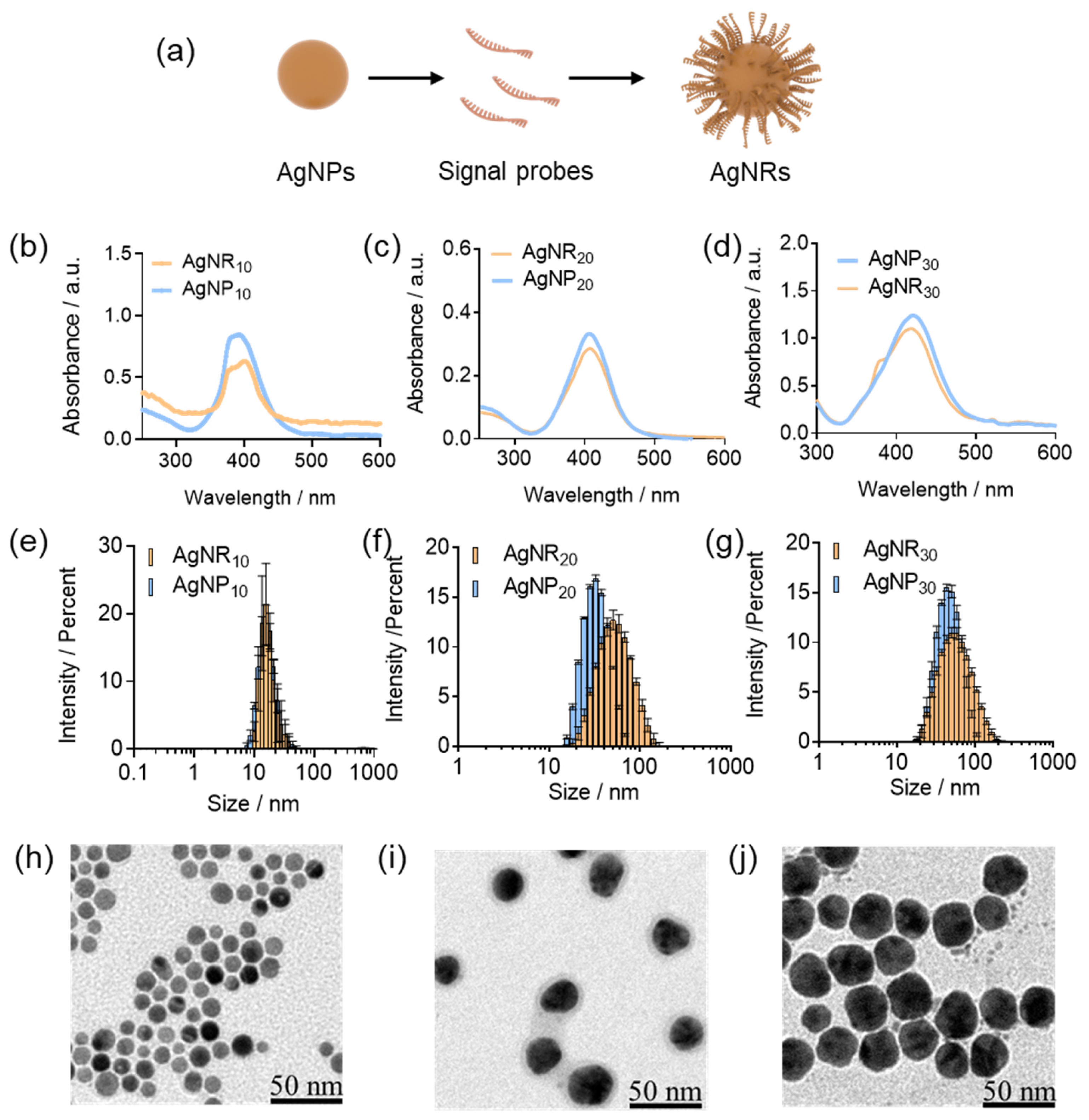
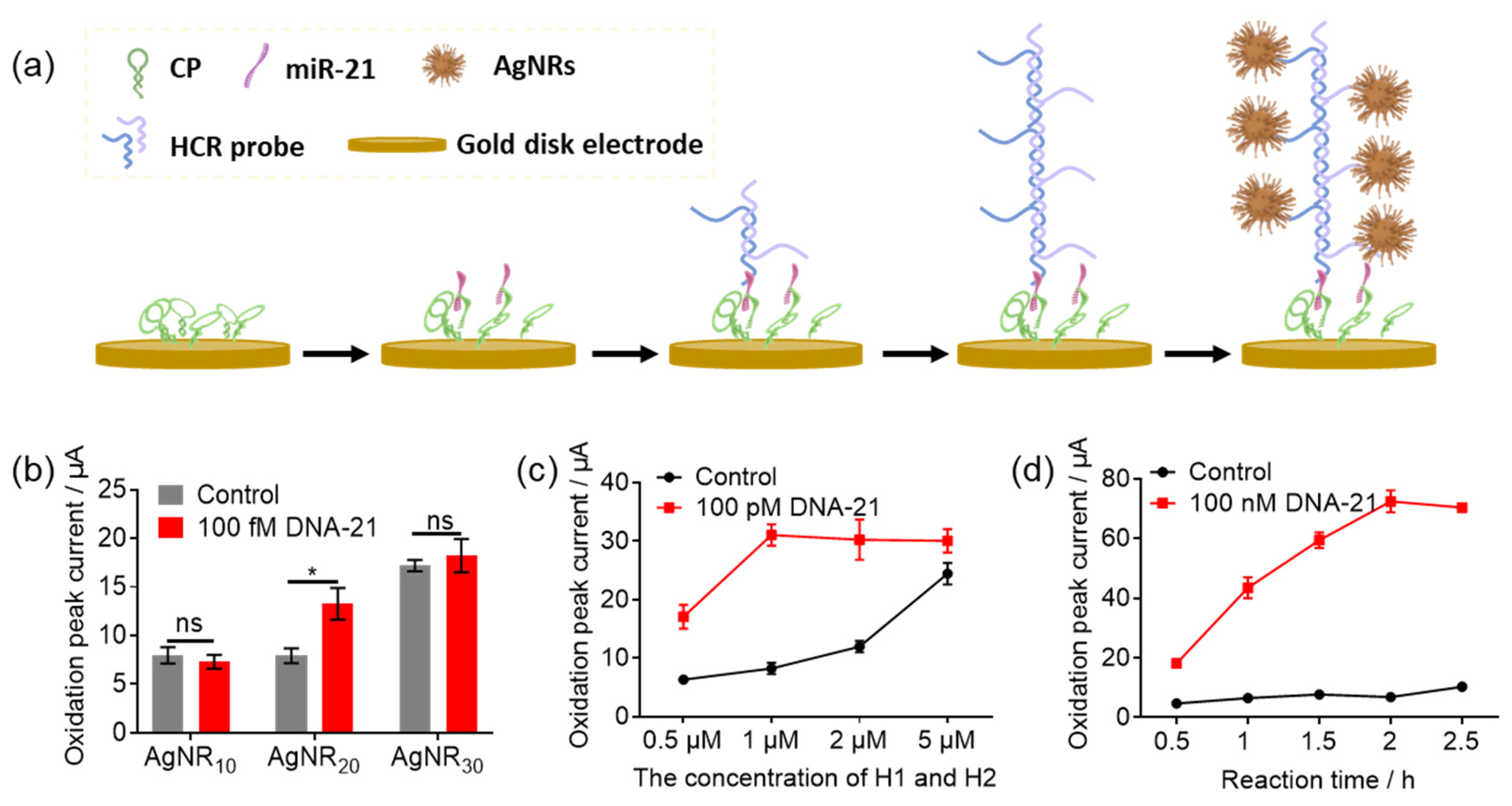
3.2. The Optimization of AgNR Sizes and HCR Conditions on Gold Disk Electrode
3.3. The Verification of HCR-Based e-miRchip in miR-21 Sensing
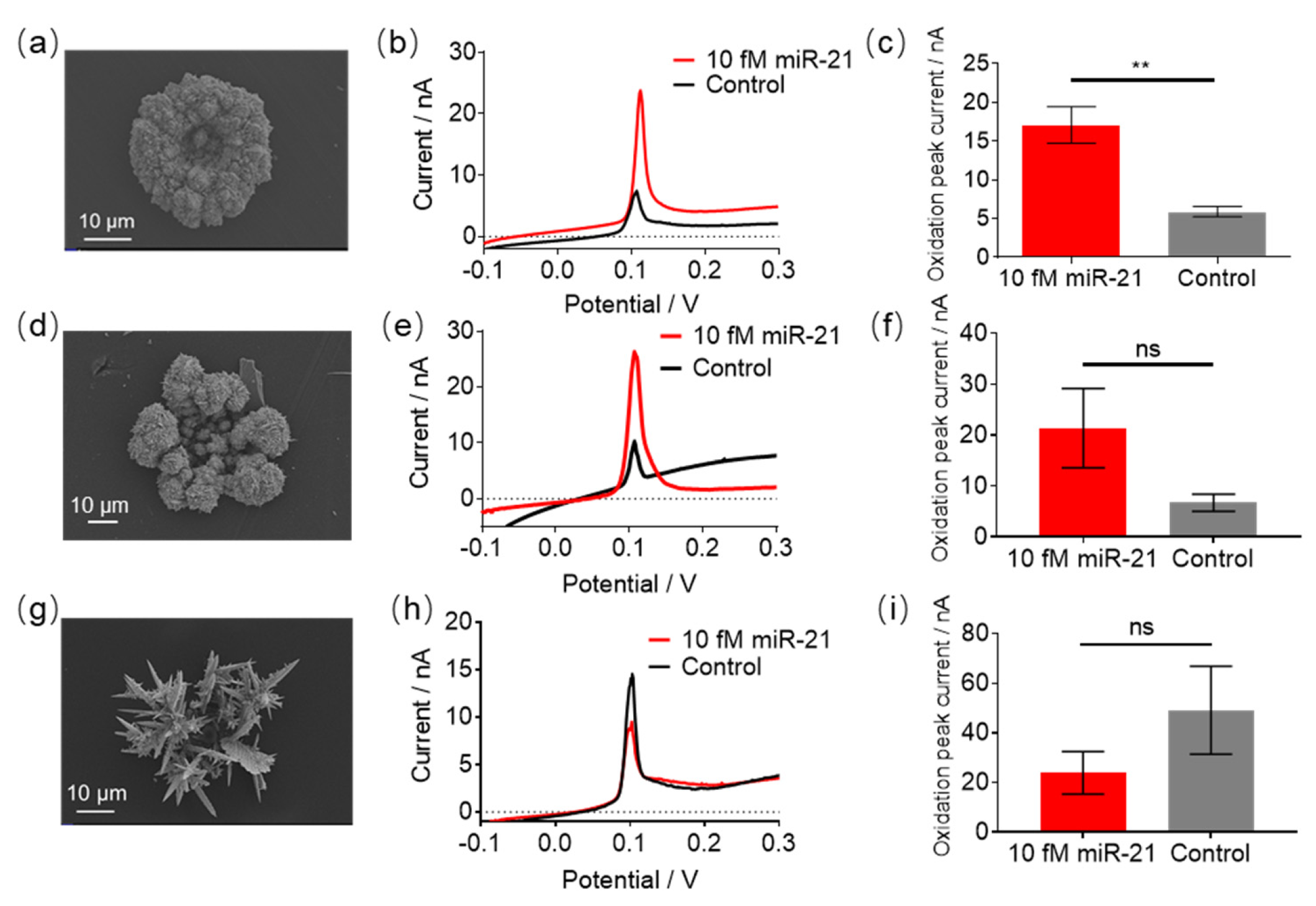
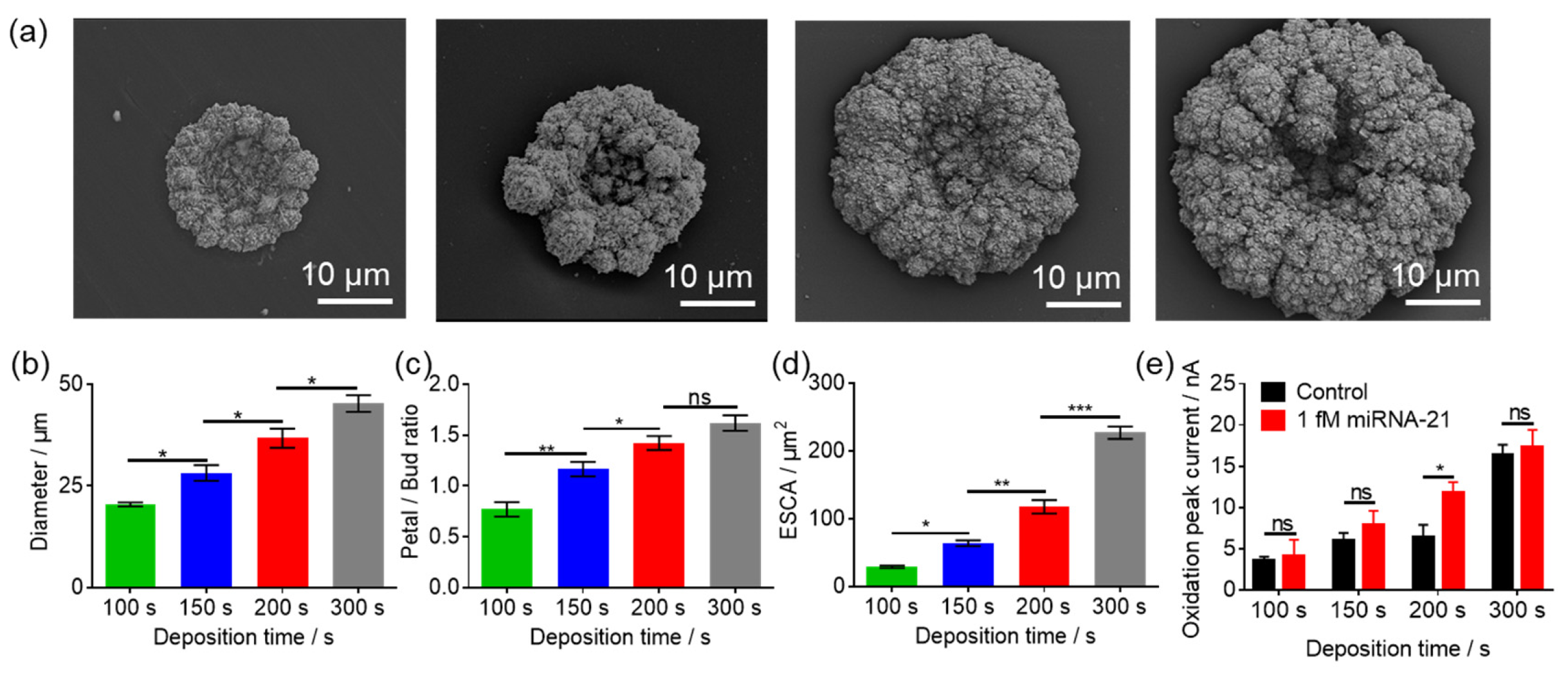
3.4. The Effect of GNEs Morphology and Size on miR-21 Detection
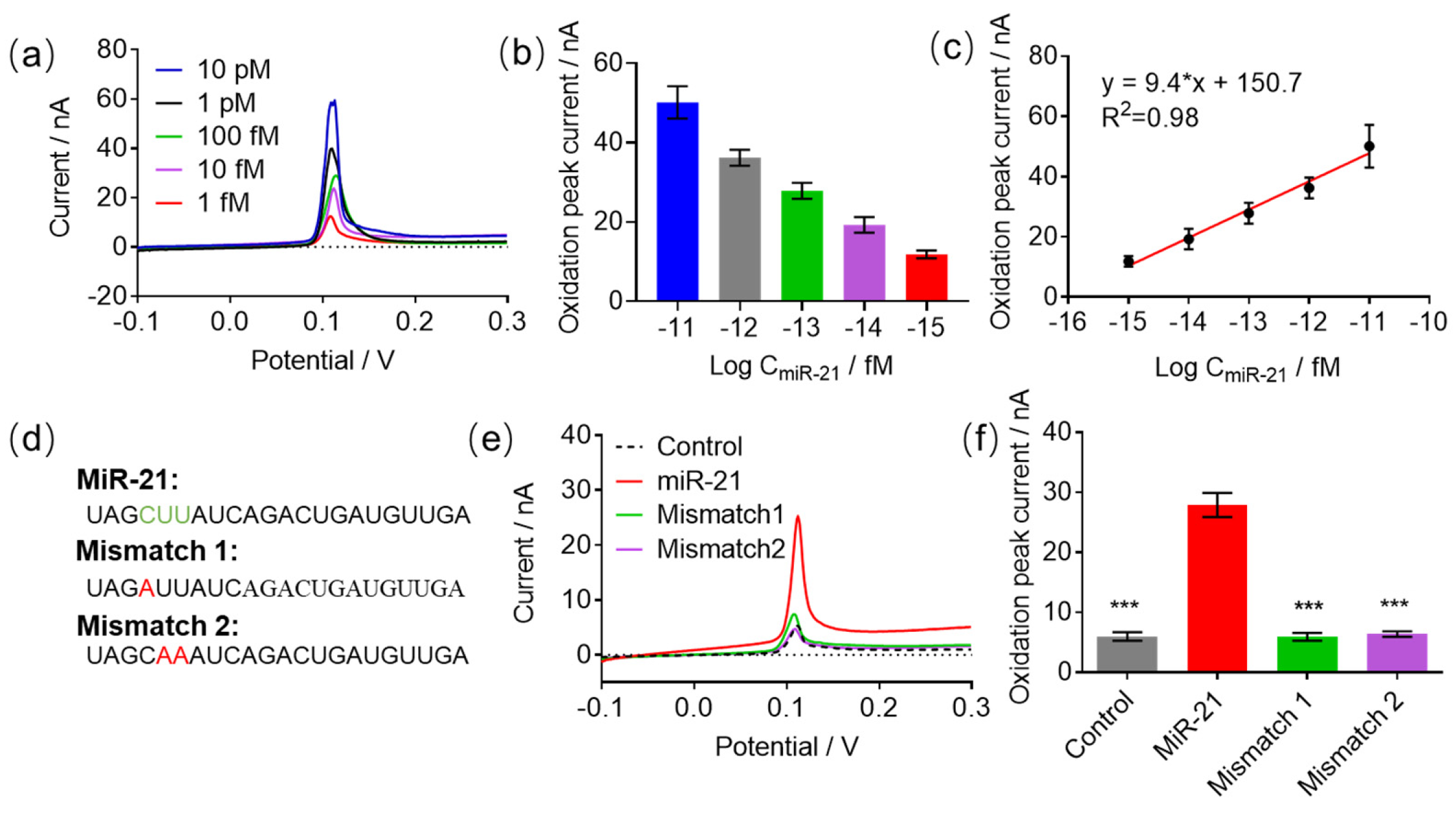
3.5. The Detection Performance of the HCR-Based e-miRchip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Zhang, Q.; Guo, S.; Wang, H. The role of microRNAs regulation of endoplasmic reticulum stress in ischemia-reperfusion injury: A review. Int. J. Bio Macromol. 2024, 283, 137566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.; Rao, H.; Cui, S.; Chen, G. MicroRNA gets a mightyaAward. Adv. Sci. 2025, 12, 2414625. [Google Scholar] [CrossRef] [PubMed]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Lewalle, P. Tumor suppressor miRNA in cancer cells and the tumor microenvironment: Mechanism of deregulation and clinical implications. Front. Oncol. 2021, 11, 708765. [Google Scholar] [CrossRef]
- Sidorova, E.A.; Zhernov, Y.V.; Antsupova, M.A.; Khadzhieva, K.R.; Izmailova, A.A.; Kraskevich, D.A.; Belova, E.V.; Simanovsky, A.A.; Shcherbakov, D.V.; Zabroda, N.N.; et al. The Role of Different Types of microRNA in the Pathogenesis of Breast and Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 1980. [Google Scholar] [CrossRef]
- Ouyang, T.L.; Liu, Z.Y.; Han, Z.Y.; Ge, Q.Y. MicroRNA Detection Specificity: Recent Advances and Future Perspective. Anal. Chem. 2019, 91, 3179–3186. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef]
- Li, B.; Lin, B.; Zeng, W.; Gu, Y.; Zhao, Y.; Liu, P. A fully integrated microfluidic cartridge for rapid and ultrasensitive nucleic acid detection from oropharyngeal swabs. Lab Chip 2025, 25, 454–464. [Google Scholar] [CrossRef]
- Du, Y.; Dong, S.J. Nucleic acid biosensors: Recent advances and perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, B.; Liu, B.; Li, X.; Jing, Y.; Li, Z.; Li, Z.; Sun, S. Aptamer-antibody sandwich immunosensor for electrochemical detection of FT4. Microchim. Acta 2025, 192, 105. [Google Scholar] [CrossRef]
- Wei, X.; Ye, M.; Jia, H.; Zhou, X.; Wang, Y.; Li, M.; Xue, C.; Xu, L.; Shen, Z. RCA-mediated tandem assembly of DNA molecular probes on lipid particles surface for efficient detection and imaging of intracellular miRNA. Biosens. Bioelectron. 2025, 271, 116975. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhou, M.X.; Ma, H.Q.; Gong, J.M.; Fung, S.Y.; Huang, X.; Yang, H. Silver nano-reporter enables simple and ultrasensitive profiling of microRNAs on a nanoflower-like microelectrode array on glass. J. Nanobiotechnol. 2022, 20, 456. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Deng, H.; Huang, M.; Li, H.; Yuan, R.; Wei, S. (Co, S)-Codoped BiOBr as a novel signal probe coupled with LAMP (H+)-pH responsive photoelectrochemistry biosensor for ultrasensitive detection of microRNA. Sensor. Actuat. B-Chem. 2025, 422, 136482. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Yan, T.; Feng, L.; He, N.; Tao, L.; Xu, L.-P.; Zhang, X. Low background electrochemical sensor based on HCR towards acute myocardial infarction-specific miRNA detection. Analyst 2025, 150, 362–370. [Google Scholar] [CrossRef]
- Deng, R.J.; Zhang, K.X.; Li, J.H. Isothermal amplification for microRNA detection: From the test tube to the cell. Acc. Chem. Res. 2017, 50, 1059–1068. [Google Scholar] [CrossRef]
- Ge, Z.L.; Lin, M.H.; Wang, P.; Pei, H.; Yan, J.; Sho, J.Y.; Huang, Q.; He, D.N.; Fan, C.H.; Zuo, X.L. Hybridization chain reaction amplification of microRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor. Anal. Chem. 2014, 86, 2124–2130. [Google Scholar] [CrossRef]
- Yang, C.Y.; Shi, K.; Dou, B.T.; Xiang, Y.; Chai, Y.Q.; Yuan, R. In Situ DNA-Templated Synthesis of Silver Nanoclusters.for Ultrasensitive and Label-Free Electrochemical Detection of MicroRNA. ACS Appl. Mater. Interfaces 2015, 7, 1188–1193. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y.G.; Yin, J. MicroRNA detection based on analyte triggered nanoparticle localization on a tetrahedral DNA modified electrode followed by hybridization chain reaction dual amplification. Chem. Commun. 2015, 51, 15629–15632. [Google Scholar] [CrossRef]
- Sage, A.T.; Besant, J.D.; Lam, B.; Sargent, E.H.; Kelley, S.O. Ultrasensitive electrochemical biomolecular detection using nanostructured microelectrodes. Acc. Chem. Res. 2014, 47, 2417–2425. [Google Scholar] [CrossRef]
- Mahshid, S.S.; Vallée-Bélisle, A.; Kelley, S.O. Biomolecular steric hindrance effects are enhanced on nanostructured microelectrodes. Anal. Chem. 2017, 89, 9751–9757. [Google Scholar] [CrossRef]
- De Luna, P.; Mahshid, S.S.; Das, J.; Luan, B.Q.; Sargent, E.H.; Kelley, S.O.; Zhou, R.H. High-Curvature nanostructuring enhances probe display for biomolecular detection. Nano Lett. 2017, 17, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, Y.; Zhu, D.; Chao, J.; Liu, X.F.; Wan, Y.; Su, Y.; Zuo, X.L.; Fan, C.H.; Wang, L.H. On-Electrode synthesis of shape-controlled hierarchical flower-like gold nanostructures for efficient interfacial DNA assembly and sensitive electrochemical sensing of microRNA. Small 2016, 12, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, L.; Fang, Z.C.; Sargent, E.H.; Kelley, S.O. Programming the detection limits of biosensors through controlled nanostructuring. Nat. Nanotechnol. 2009, 4, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.P.; Huang, H.D.; Chen, Y.; Liu, F.; Huang, C.Z.; Li, N. A distance-dependent metal-enhanced fluorescence sensing platform based on molecular beacon design. Biosens. Bioelectron. 2014, 52, 367–373. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G. Highly sensitive amperometric biosensor for determination of NADH and ethanol based on Au-Ag nanoparticles/poly(L-Cysteine)/reduced graphene oxide nanocomposite. Talanta 2017, 175, 382–389. [Google Scholar] [CrossRef]
- Tavallaie, R.; McCarroll, J.; Le Grand, M.; Ariotti, N.; Schuhmann, W.; Bakker, E.; Tilley, R.D.; Hibbert, D.B.; Kavallaris, M.; Gooding, J.J. Nucleic acid hybridization on an electrically reconfigurable network of gold-coated magnetic nanoparticles enables microRNA detection in blood. Nat. Nanotechnol. 2018, 13, 1066–1071. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical quantitation of DNA immobilized on gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef]
- Ding, X.J.; Cheng, W.; Li, Y.J.; Wu, J.L.; Li, X.M.; Cheng, Q.; Ding, S.J. An enzyme-free surface plasmon resonance biosensing strategy for detection of DNA and small molecule based on nonlinear hybridization chain reaction. Biosen. Bioelectron. 2017, 87, 345–351. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Figueroa-Miranda, G.; Wu, C.T.; Willbold, D.; Offenhäusser, A.; Mayer, D. Electrochemical dual-aptamer biosensors based on nanostructured multielectrode arrays for the detection of neuronal biomarkers. Nanoscale 2020, 12, 16501–16513. [Google Scholar] [CrossRef]
- Bin, X.M.; Sargent, E.H.; Kelley, S.O. Nanostructuring of sensors determines the efficiency of biomolecular capture. Anal. Chem. 2010, 82, 5928–5931. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, H.; Su, Y.; Liang, Y.; Shu, B.; Zhang, C. Electrochemical cloth-based DNA sensors (ECDSs): A new class of electrochemical gene sensors. Anal. Chem. 2020, 92, 7708–7716. [Google Scholar] [CrossRef]


| DNA/RNA | Sequences (5′-3′) |
|---|---|
| CP | ATAAGGTTTAGCTTATCAACATCAGTCTGATAAGCTAAACCTCCCC-C6-SH |
| MiR-21 | UAGCUUAUCAGACUGAUGUUGA |
| DNA-21 | TAGCTTATCAGACTGATGTTGA |
| Mismatch1 | UAGAUUAUCAGACUGAUGUUGA |
| Mismatch2 | UAGCAAAUCAGACUGAUGUUGA |
| H1 | TAAGCTAAACCTTATGTACATTTCGACGAATAAGGTTAACCACCTCCGATA |
| H2 | ATAAGGTTTAGCTTAAACCTTATTCGTCGAAGGCCACCTCCGATA |
| Signal probe | SH-C6-TTTATCGGAGGTGG |
| HCR-Based Amplification Method | LOD | Sensing Electrode | Multiplexed | Refs. |
|---|---|---|---|---|
| Tetrahedral DNA/avidin-HRP | 10 aM | bulk gold electrode | × | [16] |
| AgNCs/TAPNR | 0.6 fM | bulk gold electrode | × | [17] |
| AuNPs/AgNPs | 2 aM | bulk gold electrode | × | [18] |
| Au deposition/Exo I | 0.9 fM | bulk gold electrode | × | [14] |
| CdTe QDs/MWCNTs | 9 fM | SPE | × | [31] |
| AgNRs-GNEs | 0.9 fM | microaperture array | √ | current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ma, H.; Zhou, M.; Huang, X.; Gan, Y.; Yang, H. Hybridization Chain Reaction-Enhanced Ultrasensitive Electrochemical Analysis of miRNAs with a Silver Nano-Reporter on a Gold Nanostructured Electrode Array. J. Funct. Biomater. 2025, 16, 98. https://doi.org/10.3390/jfb16030098
Wang B, Ma H, Zhou M, Huang X, Gan Y, Yang H. Hybridization Chain Reaction-Enhanced Ultrasensitive Electrochemical Analysis of miRNAs with a Silver Nano-Reporter on a Gold Nanostructured Electrode Array. Journal of Functional Biomaterials. 2025; 16(3):98. https://doi.org/10.3390/jfb16030098
Chicago/Turabian StyleWang, Bin, Huiqiang Ma, Mingxing Zhou, Xian Huang, Ying Gan, and Hong Yang. 2025. "Hybridization Chain Reaction-Enhanced Ultrasensitive Electrochemical Analysis of miRNAs with a Silver Nano-Reporter on a Gold Nanostructured Electrode Array" Journal of Functional Biomaterials 16, no. 3: 98. https://doi.org/10.3390/jfb16030098
APA StyleWang, B., Ma, H., Zhou, M., Huang, X., Gan, Y., & Yang, H. (2025). Hybridization Chain Reaction-Enhanced Ultrasensitive Electrochemical Analysis of miRNAs with a Silver Nano-Reporter on a Gold Nanostructured Electrode Array. Journal of Functional Biomaterials, 16(3), 98. https://doi.org/10.3390/jfb16030098








